Abstract
Despite the widespread distribution of inhibitory synapses throughout the central nervous system, plasticity of inhibitory synapses related to associative learning has never been reported. In the cerebellum, the neural correlate of fear memory is provided by a long-term potentiation (LTP) of the excitatory synapse between the parallel fibers (PFs) and the Purkinje cell (PC). In this article, we provide evidence that inhibitory synapses in the cerebellar cortex also are affected by fear conditioning. Whole-cell patch-clamp recordings of spontaneous and miniature GABAergic events onto the PC show that the frequency but not the amplitude of these events is significantly greater up to 24 h after the conditioning. Adequate levels of excitation and inhibition are required to maintain the temporal fidelity of a neuronal network. Such fidelity can be evaluated by determining the time window for multiple input coincidence detection. We found that, after fear learning, PCs are able to integrate excitatory inputs with greater probability within short delays, but the width of the whole window is unchanged. Therefore, excitatory LTP provides a more effective detection, and inhibitory potentiation serves to maintain the time resolution of the system.
Keywords: cerebellum, associative learning, GABA inhibition, Purkinje cells, fear
Most investigations of long-term changes in synaptic transmission related to learning and memory processes have been carried out on excitatory synapses by using electrical stimulation to induce long-term potentiation (LTP). Several examples of behaviorally induced LTP have been described in the hippocampus (1–4), cerebellum (5), and amygdala (6, 7) after associative learning. Recently, long-term changes induced by electrical stimulation also have been observed at inhibitory synapses within several brain areas, including the cerebellum (8–10), hippocampus (11), brainstem (12), and lateral amygdala (13, 14). In addition, long-lasting plasticity of inhibitory synapses has been reported in vivo that mediates desensitization of the goldfish escape response (15). Inhibitory plasticity also can be induced in the developing Xenopus retinotectal system as a result of sensory experiences such as repetitive light stimuli (16). The existence of GABAergic synaptic plasticity induced in vivo by associative learning and its physiological role remains to be elucidated.
Integration of excitatory and inhibitory signals is a basic attribute of neuronal communication. A common feature of central neuronal circuits is that excitatory responses are truncated by incoming inhibition mediated by GABAergic interneurons (feed-forward inhibition, or FFI). Recent studies, both in the hippocampus and cerebellum, have shown that FFI plays a fundamental role in shaping the time window in which excitatory inputs can summate to reach the threshold for spike generation (17, 18). In fact, this time window is an indication of the temporal resolution for neuronal integration. Recently, it has been shown that the time window for multiple input coincidence detection by hippocampal neurons is unchanged after in vitro LTP only when both excitatory and inhibitory synapses are potentiated (19). Thus, the temporal fidelity for spike generation within a neuronal network requires adequate levels of excitation and inhibition to be maintained.
Several lines of experimental data demonstrate that, in addition to its major functional role in the regulation of fine motor control, the cerebellum is crucially involved in other important functions such as sensory-motor learning and memory, e.g., conditioned eye-blink responses (20–22).
Recent studies have shown that the cerebellum also is involved in fear-related memory (23, 24), and it is activated by a sensory stimulus predicting a painful stimulation after conditioning (25, 26). In addition, in the cerebellar cortex fear conditioning is accompanied by LTP of the excitatory synapses formed between parallel fibers (PFs) and Purkinje cells (PCs) (5). The aim of this work is to investigate in the same experimental paradigm possible long-term changes in the inhibitory synapses made on PCs by molecular layer interneurons. We provide evidence that learned fear is accompanied by a behaviorally induced LTP of GABAergic transmission. In addition, to check how the potentiation of both an excitatory and an inhibitory input to the same target neuron affects its temporal firing properties, we determined the time window where multiple coincident inputs can summate to reach the threshold for spike generation.
Results
Behavior.
Three groups of rats were studied: naïve animals (n = 15), which received no training; conditioned animals (n = 16), which received a series of pairings of conditioned stimuli (CS) and unconditioned stimuli (US); and unpaired control animals (n = 16), which received CS and US in a temporally uncorrelated manner (5). Fear retention was evaluated after 24 h by measuring freezing during the administration of the CS alone. In the fear-conditioned rats, the period of immobility expressed as percentage of the total time during the retrieval phase, was 91.17 ± 2.33% (mean ± SD). This time was significantly increased relative to the unpaired and naïve groups, which showed an amount of freezing, respectively, corresponding to 36.28 ± 4.41% and 29.73 ± 4.35% (one-way ANOVA, F(2,44) = 77.85, P < 0.001, Student–Neumann–Keuls post hoc) confirming previous observations (5, 27). Therefore, the increased freezing response observed in fear-conditioned rats was specifically caused by the processes underlying CS–US association, proving that the training session was effective to induce associative emotional learning.
Spontaneous and Miniature GABAergic Activity.
Cerebellar PCs exhibit sustained spontaneous inhibitory activity because of the massive GABAergic innervation from basket and stellate cells in the molecular layer and from neighboring PCs via their collaterals (28, 29).
We performed whole-cell voltage-clamp recordings on cerebellar slices from animals belonging to the three experimental groups (seven naïve rats, six unpaired, and five conditioned rats), with the aim of recording spontaneous and miniature inhibitory currents in PCs from vermal lobules V and VI. Previous work has shown that this is the site of convergence of acoustic and nociceptive sensory stimuli (30, 31), and it is related to the expression of emotional behavior (5, 32–34).
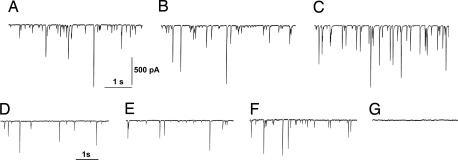
Fig. 1 A–C shows recordings made in the presence of the ionotropic glutamate receptor blocker kynurenic acid (1 mM) to isolate spontaneous GABAergic activity in the naïve, unpaired, and conditioned animals, whereas Fig. 1 D–F shows the recordings obtained by adding tetrodotoxin (TTX) (1 μM) to the bathing fluid to isolate the miniature GABAergic events. A peculiar feature of these events is that their current amplitude spans a wide range. As shown by Llano et al. (35), these events are the results of synchronized multivesicular release caused by random, ryanodine-sensitive intracellular Ca2+ transients in the presynaptic terminal. To evaluate the amplitude of both spontaneous and miniature GABAergic events, we constructed distribution histograms (Fig. 2). As previously reported (36), these histograms were heavily skewed. Therefore, we evaluated the median of the amplitude distribution for each cell and its average value for each group. Then we compared this parameter in the three behavioral groups by using the one-way ANOVA test (36).
Fig. 1.
Spontaneous and miniature GABAergic activity of PC neurons. (A) Patch-clamp recording of spontaneous GABAergic events from a PC of a naïve animal performed in voltage-clamp at a holding potential of −65 mV in the presence of kynurenic acid (1 mM). (B and C) Similar recordings from an unpaired animal (B) and from a conditioned subject (C). (D–F) Miniature GABAergic events recorded in the presence of 1 μM TTX from a naïve, unpaired, and conditioned animal, respectively. (G) Activity was blocked in the presence of 20 μM gabazine.
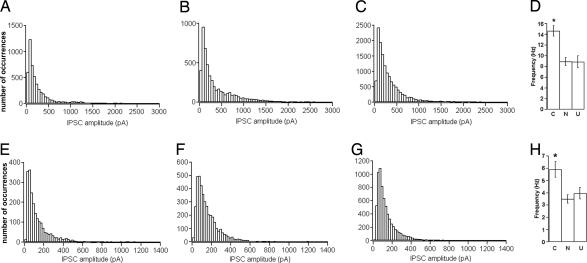
Fig. 2.
Amplitude and frequency distributions of spontaneous and miniature IPSPs. (A–C) Amplitude distributions of GABAergic spontaneous events in naïve, unpaired, and conditioned animals, respectively. (D) Mean (±SE) frequency values in the three groups. N, naïve; U, unpaired; C, conditioned. (E–G) Amplitude distributions of GABAergic miniature events in naïve unpaired and conditioned animals. (H) Mean (±SE) frequency values in the three groups.
In the absence of TTX (Fig. 2 A–C), the mean values of the medians were 199.4 ± 98.2 pA (naïve, n = 15 cells), 209.4 ± 67.1 pA (unpaired, n = 16 cells), and 245.6 ± 103.5 pA (conditioned, n = 15 cells). The one-way ANOVA test revealed no significant difference in the amplitude distributions among the three groups (F(2,43) = 0.682, P = 0.511). In the same experiments, after recording spontaneous activity, we added TTX to the external bath to record miniature GABAergic activity (Fig. 1 D–F). We found (Fig. 2 E–G) that the average median amplitude values were 102.9 ± 45.2 pA (naïve, n = 15), 105.5 ± 30.7 pA (unpaired, n = 16), and 103.4 ± 23.9 pA (conditioned, n = 15). Also in this case there was no significant difference among the three groups (one-way ANOVA, F(2,43) = 0.023, P = 0.976). The miniature activity was abolished in the presence of the GABAA receptor antagonist SR95531 (gabazine, 20 μM), thus confirming that recorded events were mediated by GABAA receptors (Fig. 1G).
As a next step, we evaluated the frequency of the GABAergic events. In the absence of TTX, the mean frequency values were 8.93 ± 2.85 Hz (naïve, n = 15), 8.89 ± 4.28 Hz (unpaired, n = 16), and 14.63 ± 3.84 Hz (conditioned, n = 15) (Fig. 2D). The one-way ANOVA test revealed a significant difference among the three groups (F(2,43) = 12.39, P < 0.001). The Tuckey post hoc test for multiple comparisons showed that in conditioned animals the frequency was significantly higher relative to the naïve and unpaired groups (P < 0.05). After TTX addition, the frequency values (Fig. 2H) were 3.49 ± 1.23 Hz, 3.96 ± 1.78 Hz, and 5.93 ± 2.36 Hz, respectively, for the naïve, unpaired, and conditioned groups. Statistical analysis on these data revealed that the mean frequency value of miniature GABAergic activity was significantly higher in conditioned animals relative to the naïve and unpaired (one-way ANOVA, F(2,43) = 6.575, P = 0.003, Tuckey post hoc test for multiple comparisons).
Altogether, these results demonstrate that fear conditioning induces a LTP of the GABAergic synapses onto the PCs. The locus of this long-term plastic change is presynaptic because the frequency but not the amplitude of GABAergic events was changed. This form of plasticity is specifically related to associative learning because it is not present in unpaired animals in which the same stimuli were delivered in an uncorrelated manner.
Time Window for Coincidence Detection.
The fact that fear learning is accompanied by an LTP of both the excitatory PF input (5) and of the inhibitory GABAergic synaptic input to PCs raises the question of the possible significance of this concomitant potentiation. The result of the integration of excitatory and inhibitory inputs on a neuron is the generation of a pattern of action potential discharge. It has been shown that plastic changes at the PF–PC synapse result in spike probability changes that can be detected as a change in the number of action potentials generated by the PC after PF stimulation (37). Because the axon of the PC is the only output from the cerebellar cortex, it is important to assess whether and how fear-induced long-term synaptic changes at both inhibitory and excitatory synapses of the PC integrate and influence its activity. To this aim, we performed whole-cell current-clamp recordings from PCs of cerebellar coronal slices (300 μm) from naïve and conditioned animals after activation of PFs with two stimulating electrodes (Fig. 3A). Slices were cut with a coronal orientation to preserve the integrity of PFs and the underlying FFI circuit.
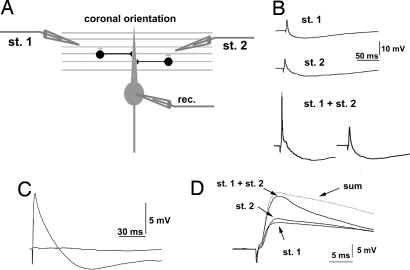
Fig. 3.
FFI and coincidence detection. (A) Recording configuration from PCs and FFI connection in coronal slices. The two simulating electrodes (st. 1 and st. 2) were placed at opposite sides relative to the recorded PC. (B) PSPs recorded in current-clamp mode. The upper two records show independent responses after subthreshold stimulation with st. 1 and st. 2. The lower two records show sample traces obtained with coincident stimulation (st. 1 plus st. 2, delay = 0 ms). The generation of a spike (Left) and a failure (Right) demonstrates the threshold for action potential generation. (C) Disynaptic activation of inhibition through PF stimulation. Bath application of 10 μM NBQX abolished both the excitatory and the inhibitory components of the PSP, demonstrating that the latter was disynaptically driven by PFs. (D) Stimulation of two independent PF beams. The two beams were considered independent when the amplitude of the PSP obtained with coincident subthreshold stimulation (st. 1 plus st. 2, delay = 0 ms) was at least 80% of the amplitude obtained by summing single PSPs (sum).
In a first set of experiments, we assessed the effectiveness of our experimental design in recruiting the FFI mechanism by PF stimulation (see Methods). Activation of a PF beam with each electrode elicited a brief excitatory postsynaptic potential (EPSP) followed by a slower inhibitory postsynaptic potential (IPSP) (ref. 28; Fig. 3B, upper two records). Application of the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) glutamate receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX) (10 μM; n = 5) abolished both the excitatory and the inhibitory PSPs (Fig. 3C), confirming that IPSP was evoked by feed-forward, disynaptic connections with PFs and not by direct stimulation of molecular layer interneurons (18). In each recorded cell, we verified that the two PF beams were independently activated by checking for linear summation of single postsynaptic responses when they were stimulated simultaneously below the threshold for spike generation (delay = 0). As shown in Fig. 3D, we compared the PSPs obtained by synchronous stimulation (stimulus 1 + stimulus 2) with the sum of the single PSP obtained with activation of each PF beam separately (stimulus 1 and stimulus 2). We analyzed those cells where the PSP peak value obtained with synchronous stimulation was at least 90% of the peak value obtained by the sum of single PSPs. To exclude that activation of one input could induce presynaptic refractoriness of the other, we considered those experiments in which the slope of the rising phase of the postsynaptic potential after simultaneous activation was at least 80% of the sum of the slopes of the two inputs alone, as previously done (38).
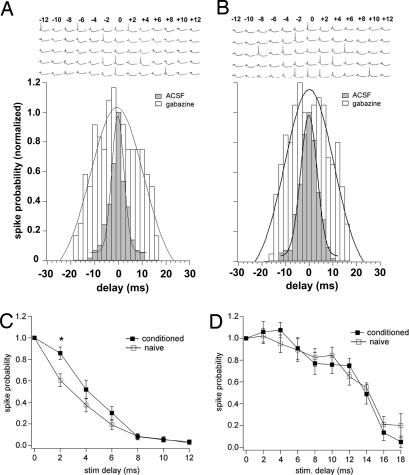
To determine the time window for integration in these conditions, we adjusted the stimulus intensity for each electrode to the threshold level for spike generation when the two pathways were stimulated simultaneously (Fig. 3B, lower traces) (17, 19). The threshold intensity was estimated by eliciting action potentials in 50% of the trials. Fig. 4 A and B (shaded columns) show the probability distribution for spike generation evaluated in naïve and conditioned rats by measuring the rate of spike occurrence as a function of the interstimulus delay. This rate decreased steeply when the two stimuli were delivered asynchronously. To detect possible differences between the two distributions we applied two types of analysis. First, we computed the Gaussian for each distribution (Fig. 4 A and B) in the form: P(x) = Ae−[(x−x0)/width]2, where x is the stimulation delay (in ms), x0 corresponds to delay = 0, and P is the spike probability (Fig. 4 A and B, shaded columns). Then we calculated the half-width value, which was 1.82 ± 0.33 ms for the naïve and 2.49 ± 0.19 ms for the conditioned group, respectively (mean ± SD). The t test showed that this parameter is significantly higher in the conditioned group compared with the naïve group (t = 4.68, P < 0.001). Sample traces of PSPs recorded with different interstimulus delays are shown in Fig. 4 A and B where recordings from cells from the naïve and the conditioned groups, respectively, are represented. To better characterize the hallmarks of the two distributions responsible for the observed difference in the Gaussian fits, we built one-sided probability distributions where each value reported in the y axis is the sum of the probabilities corresponding to equal but opposite delays for the naïve (n = 13, 8 animals) and conditioned (n = 13, 11 animals) groups (Fig. 4C) (19). The paired t test revealed that, for a delay of 2 ms, the spike probability in conditioned animals is significantly increased compared with the naïve (t = 4.014, P < 0.001). On the other hand, for interstimulus intervals ≥4 ms, we found no difference between the two groups (t = 0.25, P = 0.81). We conclude that, for short interstimulus intervals, the excitatory LTP prevails because the probability for spike generation is significantly increased, but the potentiation of inhibitory inputs onto the PC has a relevant effect in preventing this window from degradation. In fact, for delays longer than 4 ms, the probability distributions in conditioned and naïve animals are not significantly different.
Fig. 4.
Probability distributions as a function of stimulation delay. (A and B) Histograms are shown of the spike probability as a function of the interstimulus delay of the two PF beams from the naïve and conditioned groups, respectively. (Upper) Sample traces of PSPs recorded at different interstimulus delays for a naïve (A) and a conditioned (B) subject. Shaded bars represent the probability distributions obtained in ACSF (125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 1 mM MgCl2, 2 mM CaCl2, 26 mM NaHCO3, and 20 mM glucose), whereas unshaded bars represent the same distribution after adding 20 μM gabazine to the external bath. The probability distributions for the naïve and conditioned groups were normalized and were well fitted with a Gaussian. (C and D) One-sided distributions of the spike probability. Each value reported in the y axis is the average of the probabilities corresponding to equal but opposite delays for the naïve (filled squares) and conditioned (open squares) groups. The two graphs show the probability obtained in ACSF (C) and in the presence of gabazine (D).
Because the time window for coincidence detection in several structures is mainly determined by GABAergic synapses (17, 38), we evaluated the probability distribution in the presence of the GABAA receptor antagonist gabazine to assess the effect of GABAergic inputs on the coincidence detection after cerebellar associative learning. In the absence of inhibition, the stimulation intensity of each PF beam led to spike generation. Thus, we readjusted the stimulus intensities to both pathways to match the spike probability for synchronous stimulation observed in the absence of the blocker (17–19). Then we repeated the measurements previously performed without gabazine. Under these conditions, the synchronous activation of the two inputs elicited action potentials at longer interstimulus intervals up to 18 ms. The probability distributions obtained in the presence of gabazine for the naïve and conditioned groups still were fitted with a Gaussian (Fig. 4 A and B, unshaded columns). The half-width value for the naïve group was 7.89 ± 1.37 ms (n = 4, two animals) and 7.34 ± 1.16 ms (n = 5, three animals) for the conditioned group (mean ± SD). The one-sided probability distributions are represented in Fig. 4D. Neither the half-width value nor the one-sided probability distributions were significantly different in naïve and conditioned animals (t test on the half-width values: t = 1.31, P = 0.229, paired t test on the one-sided distributions: P > 0.05 for all of the delays). These results show that by blocking inhibition the change in spike coincidence detection observed in conditioned animals is abolished. In fact, in this condition, the probability distributions for spike generation versus interstimulus delay are much wider, and there is no difference between the two experimental groups. A similar result was found in the hippocampus (19). According to this model, in our system the increased probability observed in conditioned animals indicates that for short delays of PF activation the LTP of monosynaptic excitatory transmission prevails over the inhibitory LTP. Conversely, for longer delays the LTP of the FFI synapses plays the role of a barrage filter and cuts uncorrelated inputs, keeping the time resolution intact.
Discussion
We provide evidence that fear learning is associated with an increase of GABAergic synaptic transmission onto cerebellar PCs. This potentiation is long-lasting, because it is present 24 h after the training session and is specifically related to memory associative processes and because it is not present in unpaired and naïve control animals. In addition, the probability for excitatory inputs to summate and reach the threshold for spike generation in the PC is changed after emotional learning in such a way as to facilitate the summation of temporarily close related events. On the other hand, the presence of GABAergic potentiation prevents this temporal fidelity of signal processing from being degraded.
GABAergic Plasticity and Learned Fear.
To date, most studies on the cellular mechanisms of fear learning have focused on the amygdala (39, 40). After fear conditioning obtained by pairing an acoustic stimulus with an aversive one, there is a LTP of excitatory synapses between the thalamic auditory pathway and the lateral amygdala projection neurons (6, 7). In the same structure GABAergic activity also contributes to fear memorization. In fact, in mutant mice with a decreased GABAergic activity in the amygdala, fear conditioning resulted in greater and more persistent long-term fear memory (41) and in a generalization of conditioned fear to nonconditioned stimuli (42). In addition, in vitro, tetanic stimulation of the thalamic afferents evokes a LTP in GABAergic interneurons (13, 14). It has been reported that after fear conditioning, in vitro LTP of GABAergic activity in the projection neurons is significantly impaired with no concurrent influence on GABAergic baseline transmission (43). It has been suggested that such impairment may contribute to shift the balance toward excitatory transmission during fear learning.
In addition to the well established role in the conditioning of discrete motor responses (20–22), recent evidence demonstrates an important role of the cerebellum in aversive memory (34). Changes in heart rate induced by repeated pairing of an acoustic neutral stimulus with an aversive one are hampered by vermal lesion performed either before or after conditioning (22, 32). Similar results are observed in patients with medial cerebellar lesion (44). In addition, visual stimulation that signaled in advance of a painful stimulus is accompanied by an increased cerebellar activity (25, 26). It has been proposed that the amygdala and cerebellum are functionally interconnected during aversive learning (45, 46). A previous study showed that vermal electrical stimulation modulates amygdala activity (47). These effects may be mediated by indirect anatomical connections between the cerebellum and limbic areas as well as by the paleocerebellar projections to ascending catecholamine neurons of the locus coeruleus, ventral tegmental area, and periaqueductal gray (34). In line with these assumptions, a recent study in humans showed that cerebellar lesions are associated with a decrease in the activity of the amygdala as well as of the cingulate gyrus (48). More recently, we have reported that reactivated strong fear memories are affected by the combined but not independent amygdala and cerebellar blockade (49). These results demonstrate that under specific circumstances the cerebellum can support memory processes even in the absence of a crucial site for emotions like the amygdala.
In addition, an LTP of the PF to PC synapses has been induced by fear learning (5). Here we show that concomitant with the LTP in one of the excitatory pathway to the PCs there also is an LTP of the inhibitory ones. This is evidence of a behaviorally induced long-term plastic change of inhibitory synapses. This form of plasticity is presynaptically expressed as demonstrated by the increase of the frequency but not of the amplitude of the quantal events. Furthermore, the mechanism involved is likely to be an enhancement of GABA release independent from the synaptic transmission between PFs and molecular layer interneurons because this synapse was blocked with kynurenic acid in all of the experiments where GABAergic transmission was evaluated.
In the cerebellar cortex, the PFs run along the longitudinal axis of the folium and excite the PCs, which are located along the beam. They also excite two types of inhibitory interneurons: the stellate cells, which impinge mainly on the PCs located along the beam, and the basket cells which send their axons perpendicularly to the folium axis (28, 29). The former type of interneuron provides a mechanism of FFI. When a beam of PFs is stimulated, PCs show an excitatory response, which is truncated by the incoming stellate inhibition. By this mechanism, the inhibition contributes to sharpening the temporal pattern of the signal processing. On the other hand, the off-beam inhibition exerted by the basket cells provides a means to define the spatial pattern of the PC firing. By applying these concepts to our model, the simultaneous LTP of excitatory and inhibitory inputs should be seen as a mechanism of controlling the spatiotemporal pattern of PC firing.
Time Window for Coincidence Detection.
Recently, it has been shown that GABAergic synapses play a crucial role in shaping the time window for multiple coincidence detection, which represent the temporal fidelity of synapse integration (17, 19). In in vitro experiments this time window is preserved in hippocampal neurons only if LTP of excitatory synapses is accompanied by an LTP of the inhibitory ones and this form of plasticity ensures the maintenance of the temporal resolution of the neuronal circuit (19).
We found that, as a result of the associative learning, the probability of coincidence detection is significantly increased for interstimulus delays shorter than 4 ms. On the other hand the probability for time delays longer than 4 ms is not changed after fear learning. Therefore, although a higher probability of spike integration occurs within a longer time interval in conditioned animals, the probability for spike generation still is limited within the range of a few milliseconds, ensuring that the temporal fidelity of the network is maintained.
It previously has been reported that, in the amygdala, a specific mutation of the GABAB receptor subunit 1a leads to a nonassociative, NMDA receptor-independent form of presynaptic LTP at cortico-amygdala afferents. In this model, the balance between associative and nonassociative forms of LTP can be dynamically modulated by local inhibitory activity. In the same work, behavioral experiments have shown that genetic loss of this subunit results in a generalization of the conditioned fear consisting of an increased fear response to a CS not previously paired with the US (42). These data demonstrate that GABAergic manipulation can dramatically affect two associative phenomena occurring at considerably different time scales. Our results could explain such an impairment of the associative property with a degradation of the time resolution for coincidence detection.
The time window within which the activity of independent synaptic inputs must occur to trigger a spike is determined mainly by FFI inhibition as well as intrinsic membrane properties (17, 18, 50). Different conductances and passive membrane properties characterize the synaptic integration and firing patterns in several neuronal types (37, 51). However, recently, it has been shown that in our model of fear conditioning, intrinsic membrane properties are unmodified (27).
In conclusion, we found that both excitatory and inhibitory transmission are potentiated after learning and that the probability for coincidence detection is increased for small interstimulus intervals, whereas it is unchanged for longer delays. These results demonstrate that the presence of both forms of plasticity ensures a more effective coincidence detection without degrading the time resolution of the system. In addition, although these data do not establish a role for changes in inhibitory synaptic transmission in fear conditioning, they add further correlational evidence that cerebellar plasticity is involved in fear learning.
Methods
Behavior.
Young Wistar rats were randomly divided in three groups. The first group (conditioned) underwent Pavlovian fear conditioning. In the second group (unpaired), rats were administered CS and US in a temporally uncorrelated manner. The third group never left the home cage (naïve) [see supporting information (SI) Text I].
Slice Preparation.
Cerebellar parasagittal or coronal slices, 300 μm thick, were prepared from conditioned, unpaired, and naïve rats 24 h after the behavioral session following standard procedures (see SI Text II).
Electrophysiology Recordings.
Electrophysiological recordings from PCs somata were performed from the vermal part of the cerebellum. Evaluation of GABAergic spontaneous and miniature activity was obtained from whole-cell voltage-clamp recordings in parasagittal slices at holding potentials of −65 mV in the presence of the ionotropic glutamate receptor blocker kynurenic acid. The assessment of the time window for coincidence detection was performed with whole-cell current-clamp recordings in coronal slices (see SI Text III). Two different sets of PFs were stimulated with glass pipettes placed at a distance >150 μm from the recorded cell to avoid direct stimulation of interneuron axons (18). To maximize the probability of stimulating two independent PF beams, we placed the stimulation pipettes at opposite sides of the recorded PC (Fig. 3). To assess that our experimental protocol activated the inhibitory pathway via FFI, we verified that activation of PFs elicited an EPSP–IPSP sequence that was abolished by bath perfusion of the AMPA/kainate receptor antagonist NBQX (10 μM) (SI Text III; Fig. 3B). All of the chemicals were purchased from Tocris Cookson except for gabazine, which was purchased from Sigma–Aldrich.
The experimental plan was designed according to the European Community Council Directive of November 24, 1986 (86/609/EEC) for care and use of experimental animals and approved by the Bioethical Committee of the University of Turin.
Statistical Analysis.
See SI Text IV for details.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Prof. N. Hartell for revising the manuscript and A. Renna for excellent technical assistance. This work was supported by grants from the Italian Space Agency, Disorders of Motor and Cardiorespiratory Control, the Italian Ministry of University and Research, the Ministry of Health, European Community Contract no. 512039, Regione Piemonte, and the Compagnia San Paolo Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706342105/DC1.
References
- 1.Sacchetti B, Lorenzini CA, Baldi E, Bucherelli C, Roberto M, Tassoni G, Brunelli M. Eur J Neurosci. 2001;13:2291–2298. doi: 10.1046/j.0953-816x.2001.01609.x. [DOI] [PubMed] [Google Scholar]
- 2.Sacchetti B, Lorenzini CA, Baldi E, Bucherelli C, Roberto M, Tassoni G, Brunelli M. Eur J Neurosci. 2002;15:143–150. doi: 10.1046/j.0953-816x.2001.01844.x. [DOI] [PubMed] [Google Scholar]
- 3.Schimanski LA, Nguyen PV. Behav Neurosci. 2005;119:38–54. doi: 10.1037/0735-7044.119.1.38. [DOI] [PubMed] [Google Scholar]
- 4.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 5.Sacchetti B, Scelfo B, Tempia F, Strata P. Neuron. 2004;42:973–982. doi: 10.1016/j.neuron.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 6.McKernan MG, Shinnick-Gallagher P. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 7.Rogan MT, Staubli UV, LeDoux JE. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 8.Kano M. Jpn J Physiol. 1994;44(Suppl 2):S131–S136. [PubMed] [Google Scholar]
- 9.Llano I, Gerschenfeld HM. J Physiol. 1993;468:201–224. doi: 10.1113/jphysiol.1993.sp019767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitoma H, Konishi S. Neuroscience. 1999;88:871–883. doi: 10.1016/s0306-4522(98)00260-7. [DOI] [PubMed] [Google Scholar]
- 11.Otis TS, De Koninck Y, Mody I. Proc Natl Acad Sci USA. 1994;91:7698–7702. doi: 10.1073/pnas.91.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grabauskas G, Bradley RM. Neuroscience. 1999;94:1173–1182. doi: 10.1016/s0306-4522(99)00379-6. [DOI] [PubMed] [Google Scholar]
- 13.Mahanty NK, Sah P. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- 14.Bauer EP, LeDoux JE. J Neurosci. 2004;24:9507–9512. doi: 10.1523/JNEUROSCI.3567-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oda Y, Kawasaki K, Morita M, Korn H, Matsui H. Nature. 1998;394:182–185. doi: 10.1038/28172. [DOI] [PubMed] [Google Scholar]
- 16.Lien CC, Mu Y, Vargas-Caballero M, Poo MM. Nat Neurosci. 2006;9:372–380. doi: 10.1038/nn1649. [DOI] [PubMed] [Google Scholar]
- 17.Pouille F, Scanziani M. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- 18.Mittmann W, Koch U, Hausser M. J Physiol. 2005;563:369–378. doi: 10.1113/jphysiol.2004.075028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamsa K, Heeroma JH, Kullmann DM. Nat Neurosci. 2005;8:916–924. doi: 10.1038/nn1486. [DOI] [PubMed] [Google Scholar]
- 20.Kim JJ, Thompson RF. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- 21.Thompson RF. Annu Rev Psychol. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]
- 22.Weeks AC, Connor S, Hinchcliff R, LeBoutillier JC, Thompson RF, Petit TL. Learn Mem. 2007;14:385–389. doi: 10.1101/lm.348307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Supple WF, Jr, Leaton RN. Behav Neurosci. 1990;104:934–947. doi: 10.1037//0735-7044.104.6.934. [DOI] [PubMed] [Google Scholar]
- 24.Sacchetti B, Baldi E, Lorenzini CA, Bucherelli C. Proc Natl Acad Sci USA. 2002;99:8406–8411. doi: 10.1073/pnas.112660399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- 26.Ploghaus A, Tracey I, Clare S, Gati JS, Rawlins JN, Matthews PM. Proc Natl Acad Sci USA. 2000;97:9281–9286. doi: 10.1073/pnas.160266497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu L, Scelfo B, Tempia F, Sacchetti B, Strata P. Neuroscience. 2006;140:801–810. doi: 10.1016/j.neuroscience.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 28.Eccles JC, Sasaki K, Strata P. Exp Brain Res. 1967;3:81–94. doi: 10.1007/BF00234471. [DOI] [PubMed] [Google Scholar]
- 29.Eccles JC, Ito M, Szentágothai J. The Cerebellum as a Neuronal Machine. Berlin: Springer; 1967. [Google Scholar]
- 30.Snider RS, Stowell A. J Neurophysiol. 1944;7:331–358. [Google Scholar]
- 31.Huang CM, Liu G, Huang R. Brain Res. 1982;244:1–8. doi: 10.1016/0006-8993(82)90897-6. [DOI] [PubMed] [Google Scholar]
- 32.Supple WF, Jr, Sebastiani L, Kapp BS. NeuroReport. 1993;4:975–978. doi: 10.1097/00001756-199307000-00035. [DOI] [PubMed] [Google Scholar]
- 33.Sebastiani L, La NA, Paton JF, Ghelarducci B. Exp Brain Res. 1992;88:193–198. doi: 10.1007/BF02259141. [DOI] [PubMed] [Google Scholar]
- 34.Sacchetti B, Scelfo B, Strata P. Neuroscientist. 2005;11:217–227. doi: 10.1177/1073858405276428. [DOI] [PubMed] [Google Scholar]
- 35.Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- 36.Cooper EJ, Johnston GA, Edwards FA. J Physiol. 1999;521:437–449. doi: 10.1111/j.1469-7793.1999.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lev-Ram V, Mehta SB, Kleinfeld D, Tsien RY. Proc Natl Acad Sci USA. 2003;100:15989–15993. doi: 10.1073/pnas.2636935100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berger T, Luscher HR. Cereb Cortex. 2003;13:274–281. doi: 10.1093/cercor/13.3.274. [DOI] [PubMed] [Google Scholar]
- 39.Maren S, Quirk GJ. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 40.Phelps EA, LeDoux JE. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 41.Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hampton L, Battey JF, Dulac C, Kandel ER, Bolshakov VY. Cell. 2002;111:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- 42.Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der PH, Kaupmann K, Bettle B, Lüthi A. Nat Neurosci. 2006;9:1028–1035. doi: 10.1038/nn1732. [DOI] [PubMed] [Google Scholar]
- 43.Szinyei C, Narayanan RT, Pape HC. Eur J Neurosci. 2007;25:1205–1211. doi: 10.1111/j.1460-9568.2007.05349.x. [DOI] [PubMed] [Google Scholar]
- 44.Maschke M, Schugens M, Kindsvater K, Drepper J, Kolb FP, Diener HC, Daum I, Timmann D. J Neurol Neurosurg Psychiatry. 2002;72:116–118. doi: 10.1136/jnnp.72.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanton ME. Behav Brain Res. 2000;110:25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- 46.Lee T, Kim JJ. J Neurosci. 2004;24:3242–3250. doi: 10.1523/JNEUROSCI.5382-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heath CJ. Int Rev Physiol. 1978;17:193–237. [PubMed] [Google Scholar]
- 48.Turner BM, Paradiso S, Marvel CL, Pierson R, Boles Ponto LL, Hichwa RD, Robinson RG. Neuropsychologia. 2007;45:1331–1341. doi: 10.1016/j.neuropsychologia.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sacchetti B, Sacco T, Strata P. Eur J Neurosci. 2007;25:2875–2884. doi: 10.1111/j.1460-9568.2007.05508.x. [DOI] [PubMed] [Google Scholar]
- 50.Jaeger D, De SE, Bower JM. J Neurosci. 1997;17:91–106. doi: 10.1523/JNEUROSCI.17-01-00091.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stuart GJ, Hausser M. Nat Neurosci. 2001;4:63–71. doi: 10.1038/82910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.