Abstract
Striatal enkephalin and dynorphin opioid systems mediate reward and negative affect, respectively, relevant to addiction disorders. We examined polymorphisms of proenkephalin (PENK) and prodynorphin (PDYN) genes in relation to heroin abuse and gene expression in the human striatum and the relevance of genetic dopaminergic tone, critical for drug reward and striatal function. Heroin abuse was significantly associated with PENK polymorphic 3′ UTR dinucleotide (CA) repeats; 79% of subjects homozygous for the 79-bp allele were heroin abusers. Such individuals tended to express higher PENK mRNA than the 81-bp homozygotes, but PENK levels within the nucleus accumbens (NAc) shell were most strongly correlated to catecholamine-O-methyltransferase (COMT) genotype. Control Met/Met individuals expressed lower PENK mRNA than Val carriers, a pattern reversed in heroin users. Up-regulation of NAc PENK in Met/Met heroin abusers was accompanied by impaired tyrosine hydroxylase (TH) mRNA expression in mesolimbic dopamine neurons. In contrast to PENK, no association was detected between PDYN genotype (68-bp repeat element containing one to four copies of AP-1 binding sites in the promoter region) and heroin abuse, although there was a clear functional association with striatal PDYN mRNA expression: an increased number of inducible repeats (three and four) correlated with higher PDYN levels than adult or fetal subjects with noninducible (one and two) alleles. Moreover, PDYN expression was not related to COMT genotype. Altogether, the data suggest that dysfunction of the opioid reward system is significantly linked to opiate abuse vulnerability and that heroin use alters the apparent influence of heritable dopamine tone on mesolimbic PENK and TH function.
Keywords: catecholamine-O-methyltransferase, drug abuse, mu opioid receptor, nucleus accumbens, prodynorphin
Endogenous opioid neuropeptides derived from proenkephalin (PENK) and prodynorphin (PDYN) genes are key neurobiological substrates of addiction disorders, because they are highly implicated in reward, mood regulation, stress response, and motor function (1–3). Addiction vulnerability is often linked to individual reward sensitivity, but self-medication of underlying negative mood states has also been speculated. Genetic variability of endogenous enkephalin and dynorphin opioid systems, which mediate reward and dysphoria, respectively, may therefore be related to opioid abuse that has a strong heritability (4, 5). We recently observed a significant association between heroin abuse and an A118G SNP of the gene encoding the mu opioid receptor (MOR), which mediates enkephalin's actions (6). In contrast to the MOR gene, which is widely examined in substance abuse genetic studies (6–8), very limited investigations have been directed to the PENK gene despite its apparent importance to reward and hedonic state. Only a dinucleotide (CA) repeat polymorphism in the PENK 3′ UTR has been reported with potential relevance to opioid dependence (9).
Of the opioid neuropeptides, most genetic and molecular studies have been carried out on the PDYN gene. There is a polymorphic 68-bp repeat element of one to four copies that contains an AP-1 binding site in the PDYN promoter (10). In vitro evidence has implied that the polymorphism is functional with increased transcriptional activation with three or four, but not one or two, copies of the AP-1 repeats (10). Such allelic variations could influence gene expression and contribute to individual psychophysiological variability. Interestingly, increased numbers of the 68-bp repeats in the PDYN gene have recently been shown to be human-specific and driven by positive natural selection during evolution (11). Although several studies have implicated PDYN AP-1 polymorphism in substance abuse disorders, it is primarily in relation to psychostimulants (12, 13). There is still a large gap of knowledge regarding the relevance of the allelic variations of the PDYN promoter as related to the actual functional transcription of the PDYN mRNA in the human brain.
The present study directly examined PENK and PDYN polymorphisms in relation to heroin abuse and transcription levels in the postmortem human striatum. Given that striatal PENK and PDYN are localized to discrete medium spiny output pathways that are differentially regulated by prefrontal cortical activity and tonic dopamine levels (14), the impact of heritable prefrontal cortical dopamine tone to opioid neuropeptide mRNA expression was also explored by examining catecholamine-O-methyltransferase (COMT) polymorphism. COMT is a major enzyme for dopamine metabolism, particularly in the prefrontal cortex (15), and several human studies have linked polymorphism of the gene to cognition (16, 17) and downstream activity of midbrain dopamine function (18) and synthesis (19). As such, expression of tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine biosynthesis, was also examined.
Results
PENK, PDYN, and COMT genotypes were studied in our postmortem European Caucasian Hungarian and Swedish sample population (6) in relation to heroin abuse. Allele frequencies between the populations were similar, but the vast majority of subjects (90%) in this study were Hungarian. All genotypes conformed to Hardy–Weinberg equilibrium: P > 0.5.
PENK Genotype in Association with Heroin Abuse and PENK mRNA Expression.
DNA samples from control (n = 46) and heroin abuse (n = 76) individuals were amplified, and PCR products corresponding to PENK dinucleotide (CA) repeat alleles ranged from 77 (12 repeats) to 83 (15 repeats) bp in the population. Comparable with previous studies (9), the 79- and 81-bp repeat alleles were most common (54% and 46% frequency, respectively) and ≈45% of the total population were 79/81 heterozygotes; only five subjects had the 71 allele (four heroin and one control) and one had the 83-bp allele (heroin). Because of the few subjects with the minor alleles, ≤79-bp carriers were assigned to the 79 group and those with >79 alleles to the 81 subgroup. Examining the association between PENK polymorphism and heroin abuse revealed a significant genotype effect (χ2 = 8.490, P = 0.0143) with the 79 allele being more frequent (65.4%) in heroin users [supporting information (SI) Table 1]. Of individuals homozygous for the 79-bp allele, 79.4% were heroin abusers.
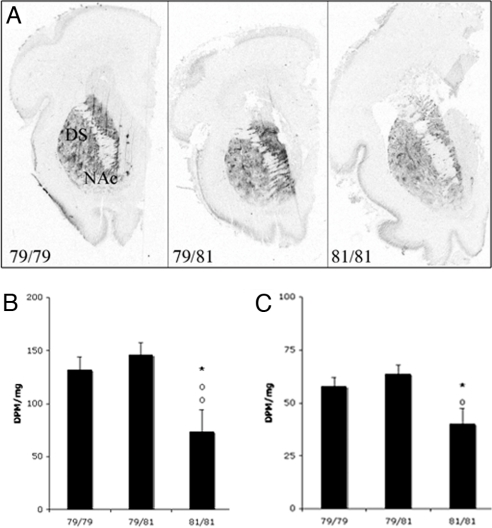
Molecular studies were carried out on a subpopulation of subjects. The PENK genotype distribution in the subpopulation was: 79/79 (n = 3, control; n = 18, heroin), 79/81 (n = 13, control; n = 10, heroin), and 81/81 (n = 4, control; n = 7, heroin). Because few 79/79 individuals were control subjects, that group could not be evaluated in the postmortem analyses. As described (6), in situ hybridization histochemistry showed that heroin abusers generally have reduced PENK mRNA expression levels in the striatum compared with controls presumably because of the effects of chronic drug intake. There was no significant difference between the PENK mRNA expression in the striatum [either the dorsal or mesolimbic ventral striatum or the nucleus accumbens (NAc)] of heroin users in association with the PENK 3′(CA)n repeat allele (because of significant variability particularly within the 79/79 group), although 81/81 subjects tended to have lower expression than the other genotypes (data not shown). To further explore the association between PENK mRNA levels and polymorphism, this relationship was also examined in another human population specifically focused on early development. The striatum was studied from midgestational (weeks 17–21) fetal samples. A significant main effect of PENK genotype (79/79, n = 20; 79/81, n = 22; 81/81, n = 7) was observed in relation to PENK mRNA such that 79-bp carriers had higher expression levels in both the dorsal striatum (F = 7.967, P = 0.0012, covaried for fetal weight) and NAc (F = 6.964, P = 0.0058; covaried for fetal weight) than the 81 bp (Fig. 1). Homozygotes with the 81 allele had significantly lower PENK mRNA expression than 79/79 homozygotes (P < 0.01) and heterozygous (P < 0.01) individuals in the NAc and significantly from 79/81 in the dorsal striatum (P < 0.05). PENK genotype was not significantly associated with the expression of PDYN mRNA expression in either the adult or fetal striatum.
Fig. 1.
Representative autoradiograms (A) and semiquantification (B and C) of PENK mRNA expression in the dorsal striatum (B) and NAc (C) of the human midgestational (weeks 17–21) fetus in relation to PENK genotype (79/79, 79/81, 81/81). Values are expressed as dpm/mg (mean ± SEM). *, P < 0.05, 81/81 vs. 79/79; ○, P < 0.01, difference between 81/81 vs. 79/81.
COMT Genotype Relevant to Mesolimbic PENK and TH mRNA Expression Levels.
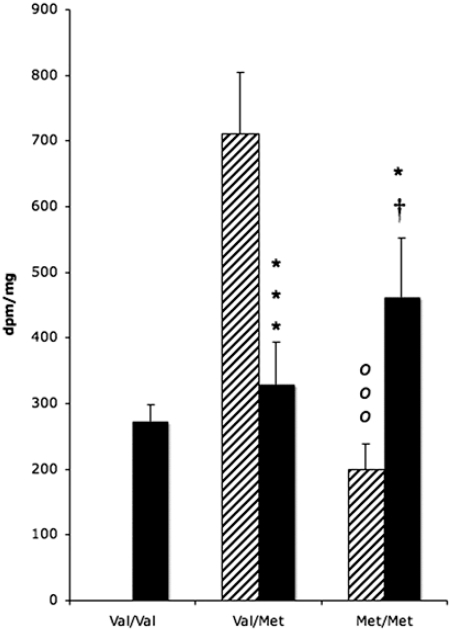
Because of apparent compensatory mechanisms on the PENK mRNA levels in the adult striatum, we examined the potential association with COMT Val-158–Met genotypes. Substitution of Val by Met reduces the thermostability and activity of the COMT catabolic enzyme, leading to increased prefrontal cortical dopamine levels (20) that should have downstream modulation of striatal cells (21). There was no significant association between COMT genotype and heroin abuse (χ2 = 0.830, P = 0.6603). Very few Val/Val control subjects (n = 2) were present in the current adult postmortem Caucasian population, thus this genotype could not be studied in the overall drug group analyses (control Val/Met, n = 9; control Met/Met, n = 6; heroin Val/Val, n = 7; heroin Val/Met, n = 17; heroin Met/Met, n = 8). A strong impact of COMT genotype was detected on PENK mRNA levels in the NAc shell, but no significance was observed in other striatal subregions. There was a significant genotype × drug group interaction in the NAc shell (P = 0.0003). Val/Met control subjects had significantly higher (≈3-fold) PENK mRNA expression than Met/Met individuals (P = 0.01; Fig. 2). An opposite pattern was evident in heroin abusers with PENK mRNA levels lower in Val/Met heroin users vs. controls (P = <0.05), but higher in Met/Met heroin users than controls (Fig. 2). In examining only the heroin group in relation to the three COMT genotypes, an increase in PENK mRNA expression with a greater Met allele load was apparent in the NAc such that Val/Val subjects expressed significantly lower mRNA levels than Met/Met individuals (P < 0.05).
Fig. 2.
PENK mRNA expression in the adult NAc shell of control (hatched bars) and heroin users (filled bars) in relation to COMT genotype. Values are expressed as dpm/mg (mean ± SEM). *, P < 0.05; ***, P < 0.001, heroin vs. control subjects; ooo, P < 0.001, difference between Val/Met and Met/Met genotypes in control subjects; †, P < 0.05, difference between Val/Val and Met/Met in heroin users.
To validate the relationship between the PENK expression and COMT genotype, the fetal specimens were evaluated. There was an apparent difference in the population frequency of the COMT allelic distribution because very few Met homozygous were present in the fetal specimens, which were predominantly (80%) of African-American descent. Nevertheless, there was a similar expression pattern to that observed in the adult control subjects such that individuals carrying the Met allele in the fetal specimens also expressed lower PENK mRNA levels than Val individuals in the NAc (68.75 ± 3.7 vs. 49.79 ± 3.0 for Val/Val and Val/Met groups, respectively; P = 0.0004, covaried for fetal weight), but no significant difference was found in the dorsal striatum.
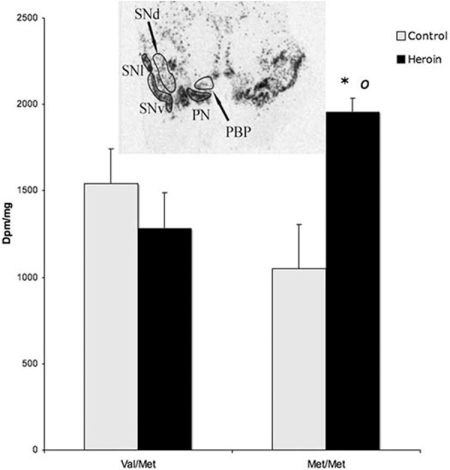
To determine whether there were COMT-related downstream alterations of dopamine-related events, TH mRNA expression, which is regulated by synaptic dopamine levels (22, 23), was studied in the adult midbrain of the control and heroin users. Five cell midbrain neuronal subgroups were examined: paranigral neurons (comprises the mesolimbic pathway), parabrachial pigmented nuclei and dorsal substantia nigra (constitutes the mesocortical circuit), and ventral and lateral substantia nigra (nigrostriatal pathway) (24). Midbrain samples were only available from 31 samples; because no Val/Val subjects were present in the controls, this genotype was excluded from the total analysis. COMT genotype midbrain samples were control Val/Met, n = 6; control Met/Met, n = 4; heroin Val/Met, n = 10; heroin Met/Met, n = 4. Consistent with the striatum, COMT-related association to TH mRNA expression was detected only in the mesolimbic subpopulation, namely the paranigralis nucleus (Fig. 3). In contrast to a tendency for decreased TH expression in normal Met/Met individuals, heroin subjects had a significant up-regulation of the gene (P = 0.0221).
Fig. 3.
Association of TH gene expression within the mesolimbic paranigralis nucleus (PN) with COMT genotypes in control (gray bars) and heroin users (black bars). Values are expressed as dpm/mg (mean ± SEM). *, P < 0.05, heroin vs. control subjects; ○, P < 0.05 difference between Val/Val and Met/Met genotypes in heroin users. (Inset) Autoradiogram of TH mRNA expression in the human midbrain. PBP, parabrachial pigmental nucleus; SNv, substantia nigra ventral part; SNl, substantia nigra lateral part; SNd, substantia nigra dorsal part; SCP, superior cerebellar peduncle; CP, cerebral peduncle.
PDYN Genotype in Association with Heroin Abuse and PDYN mRNA Expression.
PCR analysis of DNA samples studied from control and heroin abuse subjects for the PDYN genotyping revealed bands of 272, 340, 408, and 476 bp corresponding to the presence of one to four 68-bp repeats in different PDYN alleles consistent with previous investigations (10, 12). Based on previous in vitro studies (10) the samples were subdivided into three groups according to copy number of inducible or noninducible alleles. The first group were noninducible homozygotes (N/N), the second group, termed heterozygous (N/I), included noninducible (one or two) and inducible (three or four) repeats, and the third group was homozygous for inducible repeats (I/I). The frequency for the I alleles was 60% in the population. No significant difference was found in genotype distribution (χ2 = 1.181, P = 0.7577) and allele frequencies (χ2 = 3.946, P = 0.6841) between control and heroin users (SI Table 2).
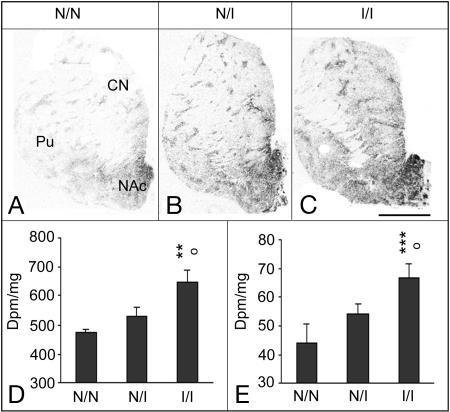
PDYN genotype distribution in the postmortem subpopulation examined was similar to the large population: I/I (n = 4 control, 5 heroin), I/N (n = 8 control, 17 heroin), and N/N (n = 9 control, n = 13 heroin). Statistical analyses showed a general association between the presence of the inducible or noninducible allele and the level of PDYN mRNA expression in the adult (Fig. 4) and fetal (Fig. 5) striatum. In adult subjects, there was a significant main effect of PDYN genotype and drug group [the heroin users had previously been shown to have reduced striatal PDYN mRNA expression (6)]. Significant PDYN genotype effect was detected in the NAc shell (F = 5.8269, P = 0.0059; Fig. 4D) and caudate nucleus (F = 8.9472 P = 0.0007; Fig. 4E); age and brain pH were covariates that influenced the mRNA levels. Post hoc analysis revealed that inducible homozygous I/I subjects had significantly higher PDYN mRNA expression than the other genotypes (NAc shell: N/N vs. I/I, P = 0.0039; N/I vs. I/I, P = 0.0103; caudate nucleus: N/N vs. I/I, P = 0.0009; N/I vs. I/I, P = 0.0115; N/N vs. N/I, P = 0.0972; Fig. 4). The PDYN gene expression pattern is very heterogenous in the striatum, and subregional analyses of the dorsal striatum showed generally comparable findings in the motor and associative subregions and in the patch and matrix compartments (data not shown). A significant genotype × drug group interaction (P = 0.0384) was detected in the putamen because of reduction of PDYN mRNA levels in this subregion only being most evident in heterozygote heroin users. PDYN mRNA expression levels were significantly related to PDYN genotype in control subjects: NAc shell, F = 6.379, P = 0.0153; NAc core, F = 9.6707, P = 0.0501; caudate nucleus, F = 7.109, P = 0.062; putamen, F = 17.243, P = 0.0001. Although PDYN expression was lower in heroin users than controls (6), heroin subjects still tended to show an association between increased PDYN mRNA levels with increasing number of the AP-1 repeats in the NAc core (F = 4.9129, P = 0.0261), NAc shell (F = 2.5387, P = 0.0999), and caudate nucleus (F = 5.283, P = 0.0031). The potential influence of the PDYN genotype on PENK mRNA expression was also assessed. The results clearly showed no association between the PDYN genotype on striatal PENK mRNA expression levels (P > 0.4) for all striatal subregions examined (data not shown).
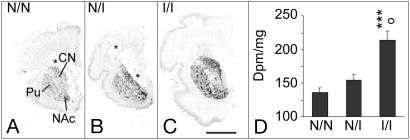
Fig. 4.
Association of PDYN gene expression with PDYN genotypes in the adult striatum. (A–C) Representative autoradiograms for subjects with homozygous N/N genotype (A), heterozygous I/N genotype (B), and homozygous I/I genotype (C). (Scale bar: 1 cm.) (D and E) Semiquantification of PDYN mRNA expression levels in the shell NAc (D) and associative caudate nucleus (CN; E). Values are expressed as dpm/mg (mean ± SEM). Pu, putamen; N, noninducible; I, inducible; N/N, subjects with one or two repeats; I/N, heterozygous subjects with one or two repeats and three or four repeats; I/I, homozygotes with three repeats and heterozygotes with three/four repeats. ○, P < 0.05, difference between N/I and I/I. **, P < 0.01 and ***, P < 0.001, difference between N/N and I/I.
Fig. 5.
Association of PDYN gene expression with PDYN genotypes in the human midgestational (week 17–21) fetal striatum. (A–C) Representative autoradiograms for subjects with homozygous N/N genotype (A), heterozygous I/N genotype (B), and homozygous I/I genotype (C). Pu, putamen; CN, caudate nucleus. (Scale bar: 1 cm.) (D) Semiquantification of PDYN mRNA expression levels in the fetal putamen. Values are expressed as dpm/mg (mean ± SEM). N, noninducible; I, inducible; N/N, subjects with one or two repeats; I/N, heterozygous subjects with one or two repeats and three or four repeats; I/I, homozygotes with three repeats and heterozygotes with three/four repeats. ○, P < 0.05, difference between N/I and I/I. ***, P < 0.001, difference between N/N and I/I.
COMT genotype was not significantly related to PDYN mRNA expression levels in either the dorsal striatum or NAc although PDYN levels in Met/Met individuals tended to be lower than Val/Met in the dorsal striatum and NAc core, a pattern similar for both control and heroin subjects.
PDYN mRNA expression was also examined in the fetal samples to validate the associations to PDYN genotype in early life. Only a subpopulation was examined because of the influence of several variables on the PDYN mRNA levels in the fetal analysis. The same pattern between PDYN genotype (I, n = 7; I/N, n = 7; N/N, n = 10) and striatal mRNA expression was apparent in the fetal subjects as in the adults (Fig. 5), but significance was detected only in the putamen (F = 10.5967, P = 0.0022; N/N vs. I/I, P = 0.0008; N/I vs. I/I, P = 0.0313; Fig. 5D).
Discussion
The current study revealed distinct contributions of the PDYN and PENK polymorphisms to striatal function in the human brain and heroin abuse vulnerability with enhanced sensitivity of mesolimbic PENK transcription in relation to heritable dopamine tone that is dysregulated in Met/Met heroin users. In contrast to the cis-regulatory PDYN polymorphism, variations of the PENK 3′(CA)n repeat were significantly associated with heroin abuse. Approximately 80% of the 79/79 homozygous subjects in our study population were heroin abusers. These findings support a strong relationship between the PENK/MOR reward-related system and opioid abuse susceptibility considering that we also recently observed a significant association between heroin use and the A118G MOR gene (OPMR1) SNP in this European Caucasian population (6). There was no significant interaction between the PENK and OPMR1 genotypes in the current population (data not shown), suggesting that each contributes independently to heroin abuse vulnerability, although impacting similar neurobiological networks.
The functional relevance of the 3′ UTR is an active area of research where accumulating evidence has shown it to be an important structural component of the gene that can modulate e.g., gene expression, mRNA stability, and translation (25). The 3′ UTR PENK dinucleotide repeat polymorphism appears to be of functional relevance to gene transcription as evidenced in the fetal brain where the 79 allele was associated with higher striatal PENK mRNA expression than the 81 homozygotes. Although a similar pattern was apparent in the adult brain, it was evident that other factors influenced the PENK mRNA levels. It is feasible that linkage disequilibrium with another as-yet-unidentified functional variant of the PENK or other genes more directly conferred the vulnerability to heroin abuse. Increased NAc PENK mRNA levels has been associated with elevated heroin self-administration (26), but enhanced NAc PENK transcription alone does not appear to directly correlate to opiate abuse vulnerability because 79/81 heterozygotes also had elevated PENK mRNA levels compared with 79 homozygous subjects, although the frequency of the 79/81 genotype was similar in control and heroin subjects. Further studies are required to fully reveal the specific association between the PENK gene, transcription and heroin abuse risk, and the contribution of other neurotransmitter systems to the apparent complex regulation of PENK.
An important finding was that heritable dopamine tone, as related to COMT polymorphism, influenced PENK mRNA levels within the NAc shell and depended on the history of heroin use. Based on the existing concept of the prefrontal cortical tonic-phasic dopamine regulation of the NAc (21) and anatomical organization (27), increased prefrontal cortex dopamine levels in Met/Met individuals would be associated with increased tonic, but decreased phasic, NAc dopamine levels. Increased tonic dopamine that acts primarily through D2 receptors specifically diminish the responsiveness of striatopallidal neurons (28). Our findings in control subjects are thus consistent with this concept given the strong PENK mRNA reduction in Met subjects and the opposite alterations in individuals carrying the Val allele who, based on the tonic-phasic model, would be predicted to have reduced tonic NAc dopamine levels. TH, which is well established to be regulated by synaptic dopamine levels particularly via D2 receptors (22, 23), was also associated with COMT genotype as observed (19); increased stimulation of D2 receptors in Met/Met individuals would predict feedback reduction of TH mRNA, a trend observed in control subjects. It should be noted, however, that most animal models have failed to detect significant association between COMT depletion and striatal dopamine levels (29). Such studies only examined the dorsal striatum where we also found no significant association with the opioid peptide mRNA levels. The alterations in relation to the COMT genotype were specifically within the ventral striatum in our population. That the apparent influence of COMT genotype on PENK was localized to the NAc shell is important because this striatal subregion is most linked with drug reward and the hedonic state mediated by both dopamine and enkephalinergic systems (30, 31). Moreover, COMT polymorphism was only significantly related to TH mRNA expression within the paranigralis dopamine neuronal populations that directly innervate the NAc underlining the continuum of disturbance along the mesolimbic circuit. Interestingly, in vivo imaging of MOR binding sites also revealed significant association with COMT genotype in the NAc, not dorsal striatum (32).
An opposite association pattern between COMT genotype and expression level was evident in heroin users as compared with control subjects. Heroin users generally express low striatal PENK transcription levels (6), but it was only evident in the Val/Met subjects, whereas Met homozygotes heroin users had increased PENK mRNA expression compared with their genotype controls. Although COMT genotype was not directly associated with heroin abuse and there was no interaction between the PENK and COMT genes, COMT polymorphism strongly influenced NAc PENK mRNA expression, emphasizing that critical networks of gene interaction contribute to striatal function. Previous in vivo studies have observed an inverted u-shaped response curve regarding COMT such that increased dopamine after amphetamine administration improves cognitive function in Val subjects, but impairs cognition in Met individuals who already have an elevated dopamine tone (33). Such a compensatory switch in relation to the COMT genotype appears also relevant for PENK transcription and NAc function and for TH mRNA expression.
Another key aspect of the current study was providing evidence that tandem 68-bp repeats containing AP-1 binding sites in the PDYN promoter are associated with inducibility of the PDYN mRNA expression in the human striatum that primarily constitutes the major “direct” striatonigral output pathway. The fact that this association is evident in early stages of development in normal subjects and in the adult brain emphasizes the enduring individual nature of the PDYN transcriptional regulation despite expected diverse influences throughout life. Even heroin use in adult subjects, which generally attenuates striatal PDYN mRNA expression (6), failed to affect the association between PDYN allelic variation and mRNA levels expressed in the striatum, highlighting the important regulation of the cis-regulatory polymorphism on its transcription. Similar to our results, no association was detected between heroin abuse and PDYN polymorphism in larger sample size investigations in Caucasian populations (10, 34). Together, these findings strongly suggest that, in contrast to the PENK and OPMR1 A118G polymorphism, genetic variants of the PDYN AP-1 promoter do not confer risk for vulnerability to heroin abuse in Caucasian populations. Ray et al. (34) did observe a weak association between PDYN polymorphism and opioid dependence in African-Americans, which underscores the potential relevance of ethnicity in regard to the PDYN genotype. Although the PDYN genotype was not associated with heroin abuse, PDYN plays an important role in various brain functions, and inherited features of this gene may be of critical importance in the individual predisposition to normal and pathological reactions of the CNS.
Limitations of this postmortem human investigation include the small sample size, lack of detailed medical and drug histories (although the subjects were documented heroin abusers), potential stratification even within this homogenous population, and the potential for haplotype rather than genotype analyses to provide stronger associations to the outcome variables. For example, COMT haplotype has been shown to influence COMT function to a greater extent than the COMT Val-158–Met genotype (35). Further studies in larger populations in which haplotype analyses can be better evaluated are clearly required to replicate and extend the current findings. Nevertheless, COMT genotype was significantly associated with specific neurobiological features that emphasize that genotype has important functional relevance. Moreover, although there were apparent ethnic differences in the allelic distribution of the COMT polymorphism, the neurobiological correlations to genotype were the same irrespective of population. In addition, the fact that the same general pattern of increasing PDYN transcript levels with increasing inducible alleles was evident in various striatal subregions irrespective of heroin use, and that a similar pattern was detected in fetal subjects, strongly suggests that the PDYN promoter polymorphism is functionally associated with PDYN transcription in the human striatum. The ability to detect significant polymorphic associations of heroin abuse with both the PENK and OPMR1 genes, which are critical for the rewarding actions of opiates, implies that it is possible to dissociate systems linked with heroin vulnerability in the current population. The lack of association between the PDYN genotype and PENK mRNA expression and vice versa underscores the neurobiological specificity of the polymorphisms.
In summary, association of polymorphic disturbances within the enkephalin system, PENK and OPMR1, with heroin abuse provides strong evidence that impairment of enkephalin neurotransmission is tightly related to opiate abuse vulnerability. Although COMT genotype on its own was not associated with heroin abuse, apparent mesolimbic dopamine tone specifically contributes to the PENK mRNA expression in the human NAc shell highly relevant to reward. The clear functional relevance of the PDYN promoter polymorphism in regulating the striatal PDYN mRNA expression suggests that striatal PDYN transcription is not linked with opiate abuse risk, but may have important significance to other neuropsychiatric conditions in which basal ganglia function or negative mood states are important features of the disorder.
Materials and Methods
Adult Human Brains.
Human brain samples examined in this study were part of a heroin abuse postmortem collection that has been described (6). Briefly, brains from apparent heroin overdose and normal control adult Caucasian subjects without head trauma were collected at autopsy within 24 h after death at the Department of Forensic Medicine, Semmelweis University, and the National Institute of Forensic Medicine, Karolinska Institutet under local ethical approved guidelines. All cases were assessed for common drugs of abuse (including alcohol) and therapeutic agents. Subjects included in the heroin group died from apparent heroin overdose as verified by toxicology and had physical signs of heroin use such as needle track marks, and most had a history of heroin abuse as ascertained by medical records and police and/or family reports. The heroin group represented a unique drug abuse population given that they were predominant heroin users with no methadone treatment. The control group, predominant cause of death being mycocardial infarction, had negative toxicology for opiates or other illicit drugs of abuse. Positive alcohol was evident in very few cases in which ethanol concentrations were similar to the limited alcohol-positive subjects identified in the heroin group. Nicotine toxicology was not conducted, but high tobacco use is frequent in the general population from which the subjects were examined. We studied 127 subjects: 46 controls (37 males, 9 females; average age 36.8 ± 1.98) and 81 heroin users (67 males, 14 females; average age 25.7 ± 0.61). Of these, 53 specimens were used for postmortem brain analyses (see ref. 6): controls, 16 male, 3 females, average age 23.4 ± 0.3 years, average brain pH 6.72 ± 0.05; heroin, 30 males, 4 females, average age 223.45 ± 0.33, average brain pH 6.55 ± 0.04. Immediately after autopsy, the brains were cut in 1.5-cm slabs, frozen, and kept at −70°C. Coronal 20-μm sections (Microm HM560; Microm International) were quickly mounted onto Superfrost plus-glass (Brain Research Laboratories) and then kept at −30°C. All procedures were carried out blinded to the subject group.
Fetal Brains.
A total of 49 postmortem human fetal brain samples were studied from cases collected to evaluate developmental exposure to cannabis (36). The cannabis-exposed (n = 24) and nonexposed (n = 25) group were ≈80% African-American: average age 20.2 ± 0.3 and 20.4 ± 0.3 weeks, male/female: 13/11 and 13/12, postmortem interval 7.9 ± 0.70 and 9.6 ± 0.75 h, respectively. Fetal brains were lightly fixed with 1% paraformaldehyde and frozen in isopentane at −40°C. Coronal sections (20 μm thick) were processed by using the same procedure as for the adult tissue.
Genotyping.
DNA was purified from cerebellar tissue by using DNeasy columns (Qiagen). For PDYN, DNA (100 ng) was amplified by sense and antisense primers (5′-CCTGTGTATGGAGAGGCTGAGT and 5′-GCGGTTAGGTAGAGTTGTCAGATT), and PCR products were resolved on a 2.5% agarose gel. For PENK, the polymorphic area was amplified by sense and antisense primers (5′- TAATAAAGGAGCCAGCTATG and 5′-ACATCTGATGTAAATGCAAGT (6-FAM labeled) (37), and PCR products were analyzed by capillary electrophoresis DNA analyzer ABI3730 (Applied Biosystems). COMT genotypes were determined by NIaIII restriction of PCR products generated by DNA amplification with primers Comt1 (5′-CTCATCACCATCGAGATCAA) and Comt2 (5′-CCAGGTCTGACAACGGGTCA) (32). Every analyzed set of PCR products included fragment size markers in the experiment, and genotyping results were always double-checked to provide genotyping accuracy.
In Situ Hybridization Histochemistry.
The PENK riboprobe was an EcoRI/Pvu 792-bp fragment complementary to the full coding region of the PENK human gene (38). The PDYN probe contained the 5′ region of the gene inserted (GenBank accession no. NM_024411, bases 215/−72) (39). In situ hybridization is described in SI Text. Briefly, brain sections were hybridized with 20 × 103 cpm/μl riboprobe solution. Optical density values measurements were taken from film images of the NAc and associative and motor divisions of the caudate nucleus and putamen based on the functional organization of the primate striatum (40). Optical density values were converted to dpm/mg by reference to coexposed C14 standards (American Radiolabeled Chemicals).
Statistics.
General linear stepwise regression analysis was used to evaluate genotype group differences with covariates: e.g., age, postmortem interval, brain pH, and sex. Other variables evaluated for adult subjects were ethanol toxicology, drug group (control vs. heroin) and brain freezer storage time; and for fetal specimens maternal drug use was evaluated. Post hoc analysis regarding genotype was performed by using Fisher's least-squares difference test. Association between genotypes and vulnerability to drug abuse and Hardy–Weinberg equilibrium was analyzed by χ2 tests. Significance was set at P < 0.05, and trends were considered for P < 0.10. See more detail in SI Text.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Mrs. Alexandra Tylec for technical assistance and Elisabeth Berg (Department of Learning, Informatics, Management, and Ethics, Medical Statistic Division, Karolinska Institutet) for statistical guidance. This study was supported by National Institutes of Health/National Institute on Drug Abuse Grant DA15446, Hungarian Scientific Research Fund Grant T32227, and Swedish Science Council Grant 11252. M.C.H. was supported by the Swedish Institute and Hungarian Scholarship Board.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710902105/DC1.
References
- 1.Hauser KF, et al. Pathobiology of dynorphins in trauma and disease. Front Biosci. 2005;10:216–235. doi: 10.2741/1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelley AE, et al. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 3.Drolet G, et al. Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- 4.Goldman D, Oroszi G, Ducci F. The genetics of addictions: Uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 5.Tsuang MT, et al. Co-occurrence of abuse of different drugs in men: The role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- 6.Drakenberg K, et al. Mu opioid receptor A118G polymorphism in association with striatal opioid neuropeptide gene expression in heroin abusers. Proc Natl Acad Sci USA. 2006;103:7883–7888. doi: 10.1073/pnas.0600871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bart G, et al. Substantial attributable risk related to a functional mu-opioid receptor gene polymorphism in association with heroin addiction in central Sweden. Mol Psychiatry. 2004;9:547–549. doi: 10.1038/sj.mp.4001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oslin DW, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuro psycho pharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- 9.Comings DE, et al. The proenkephalin gene (PENK) and opioid dependence. NeuroReport. 1999;10:1133–1135. doi: 10.1097/00001756-199904060-00042. [DOI] [PubMed] [Google Scholar]
- 10.Zimprich A, et al. An allelic variation in the human prodynorphin gene promoter alters stimulus-induced expression. J Neurochem. 2000;74:472–477. doi: 10.1046/j.1471-4159.2000.740472.x. [DOI] [PubMed] [Google Scholar]
- 11.Rockman MV, et al. Ancient and recent positive selection transformed opioid cis-regulation in humans. PLoS Biol. 2005;3:e387. doi: 10.1371/journal.pbio.0030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahl JP, et al. Confirmation of the association between a polymorphism in the promoter region of the prodynorphin gene and cocaine dependence. Am J Med Genet B Neuropsychiatr Genet. 2005;139:106–108. doi: 10.1002/ajmg.b.30238. [DOI] [PubMed] [Google Scholar]
- 13.Chen AC, et al. Potentially functional polymorphism in the promoter region of prodynorphin gene may be associated with protection against cocaine dependence or abuse. Am J Med Genet. 2002;114:429–435. doi: 10.1002/ajmg.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. J Neurol. 2000;247(Suppl 5):V1–V15. doi: 10.1007/pl00007778. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto M, et al. Catechol O-methyltransferase mRNA expression in human and rat brain: Evidence for a role in cortical neuronal function. Neuroscience. 2003;116:127–137. doi: 10.1016/s0306-4522(02)00556-0. [DOI] [PubMed] [Google Scholar]
- 16.Egan MF, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnett JH, Jones PB, Robbins TW, Muller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: A meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- 18.Meyer-Lindenberg A, et al. Midbrain dopamine and prefrontal function in humans: Interaction and modulation by COMT genotype. Nat Neurosci. 2005;8:594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- 19.Akil M, et al. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci. 2003;23:2008–2013. doi: 10.1523/JNEUROSCI.23-06-02008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lachman HM, et al. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: Relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- 22.Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. Ann NY Acad Sci. 1990;604:323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- 23.O'Hara CM, Uhland-Smith A, O'Malley KL, Todd RD. Inhibition of dopamine synthesis by dopamine D2 and D3 but not D4 receptors. J Pharmacol Exp Ther. 1996;277:186–192. [PubMed] [Google Scholar]
- 24.Haber SN, Fudge JL. The primate substantia nigra and VTA: Integrative circuitry and function. Crit Rev Neurobiol. 1997;11:323–342. doi: 10.1615/critrevneurobiol.v11.i4.40. [DOI] [PubMed] [Google Scholar]
- 25.Wang GJ, Yang P, Xie HG. Gene variants in noncoding regions and their possible consequences. Pharmacogenomics. 2006;7:203–209. doi: 10.2217/14622416.7.2.203. [DOI] [PubMed] [Google Scholar]
- 26.Ellgren M, Spano SM, Hurd YL. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology. 2007;32:607–615. doi: 10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- 27.Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: Implications for schizophrenia. Physiol Behav. 2002;77:513–517. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- 28.Surmeier DJ, et al. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Yavich L, et al. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci. 2007;27:10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelley AE. Ventral striatal control of appetitive motivation: Role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Nicola SM, Taha SA, Kim SW, Fields HL. Nucleus accumbens dopamine release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience. 2005;135:1025–1033. doi: 10.1016/j.neuroscience.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 32.Zubieta JK, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 33.Mattay VS, et al. Catechol O-methyltransferase Val158-Met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray R, et al. A functional prodynorphin promoter polymorphism and opioid dependence. Psychiatr Genet. 2005;15:295–298. doi: 10.1097/00041444-200512000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Diatchenko L, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, et al. In utero marijuana exposure associated with abnormal amygdala dopamine D2 gene expression in the human fetus. Biol Psychiatry. 2004;56:909–915. doi: 10.1016/j.biopsych.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Weber JL, May PE. Dinucleotide repeat polymorphism at the PENK locus. Nucleic Acids Res. 1990;18:2200. [PMC free article] [PubMed] [Google Scholar]
- 38.Noda M, et al. Isolation and structural organization of the human preproenkephalin gene. Nature. 1982;297:431–434. doi: 10.1038/297431a0. [DOI] [PubMed] [Google Scholar]
- 39.Nikoshkov A, et al. Prodynorphin transcripts and proteins differentially expressed and regulated in the adult human brain. FASEB J. 2005;19:1543–1545. doi: 10.1096/fj.05-3743fje. [DOI] [PubMed] [Google Scholar]
- 40.Parent A, Côté P-Y, Lavoie B. Chemical anatomy of primate basal ganglia. Prog Neurobiol. 1995;46:131–197. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.