Abstract
An inborn predisposition to attend to biological motion has long been theorized, but had so far been demonstrated only in one animal species (the domestic chicken). In particular, no preference for biological motion was reported for human infants of <3 months of age. We tested 2-day-old babies' discrimination after familiarization and their spontaneous preferences for biological vs. nonbiological point-light animations. Newborns were shown to be able to discriminate between two different patterns of motion (Exp. 1) and, when first exposed to them, selectively preferred to look at the biological motion display (Exp. 2). This preference was also orientation-dependent: newborns looked longer at upright displays than upside-down displays (Exp. 3). These data support the hypothesis that detection of biological motion is an intrinsic capacity of the visual system, which is presumably part of an evolutionarily ancient and nonspecies-specific system predisposing animals to preferentially attend to other animals.
Many adaptive responses to natural objects depend on the way such objects move about. This is particularly true for the movement of other animals, the detection of which is critical for any adaptive behavior. The movement of living beings, vertebrates in particular, is typically nonrigid, although it is at the same time characterized by a number of constraints caused by the articulated structure of the animal body. This information allows the human observer to extract the configural invariants, which are processed extremely effectively (1–4). Interestingly, even extremely sparse information, such as that provided by only a dozen of points of light placed on the joints of the moving animal, suffice to produce the vivid and compelling perception of coordinated animal locomotion. The perception induced by this pattern of motion, namely biological motion, and the typical display used for its investigation, the point-light animation, were first described in 1973 by Johansson (4). Ever since, perception of biological motion has become one of the classical topics in vision research.
Point-light displays are invaluable experimental tools to separate, and therefore to investigate, information concerning motion from any other type of visual information. The presence of animated motion in such displays is almost instantaneously detected by the visual system. Adults, for example, need as little as 100 ms to identify a point-light human walker. The locomotion of four-legged animals can also be promptly recognized by human observers, who can identify the different animals by their typical pattern of motion (5, 6).
Several animal species are also able to discriminate and even to specifically respond to point-light displays depicting motion of conspecifics (cats, ref. 7; pigeons, refs. 8 and 9; chicks, ref. 10; monkeys, ref. 11; apes, ref. 12; dolphins, ref. 13).
To the human observer a variety of adjunctive information is also conveyed by point-light animations such as the nature of the action, the gender, and even the identity of the actor and one's own identity (14–16). Such information cannot be extracted by the observer from any given single static frame of the animation, which appears as a plain set of unconnected and meaningless points, meaning that a very complex structure-from-motion analysis is rapidly carried out by the visual system, extracting and integrating form to motion. This perception is rather robust as it is affected little by perturbations such as reverse transformation (playing the animation backwards) and changes in the velocity of the animation (playing it faster or slower than normal) (17) or by masking elements (18). Moreover, it seems that form extraction does not fully explain the processes underlying biological motion detection, as, for example, scrambled biological motion displays are still perceived as being biological (19, 20), although no known animal species can be identified in such displays.
Explanations of how observers extract form and action from these displays fall into two classes: event-from-form and event-from-dynamics theories. Event-from-form explanations suggest that visual processes first extract form and then determine action. The event-from-dynamics explanations are based on temporal information for action and argue that the most useful information is that about dynamics, the force acting on objects. Experiences of events are claimed to be necessary for perception according to both theories (21).
Although it has impressive resilience to perturbations, biological motion perception is dramatically affected by display inversion. Similarly to what is known to occur for face recognition, whenever the point-light displays are presented upside-down performance drops dramatically in almost all visual tasks (17, 18, 21, 22). Prior knowledge concerning display inversion does not seem to be sufficient for recognition of inverted biological motion (17). Also, when walking-on-hands was represented rather than walking-on-foot, either inverted or upright, subjects were less accurate at detecting walking-on-hands when the display was turned upside down than when it was upright so that the orientation of gravity, and not form, seemed crucial for detection (21). Overall, a perceptual rather than knowledge-based origin for the inversion effect seemed the most reasonable explanation for these data. Scrambled biological motion displays, for example, were shown to retain information about the direction of motion (i.e., of the walking) as long as they were presented upright, such information was lost after inversion of the scrambled animation (19).
Sensitivity to biological motion and the inversion effect has been investigated from a developmental perspective in our species, to clarify the ontogenesis of the processes involved. For example, perception of biological motion is readily available to 3- to 4-year-old children (6), although such perception is disrupted after display inversion in 6-year-old children and adults (17). These data are in agreement with previous studies carried out with infants. By 3 months of age, infants discriminate both point-light displays of biomechanical motion from displays of identical absolute motion with scrambled spatial relations and upright vs. inverted point light moving displays (23). By 4 months of age, infants exhibited a visual preference for upright over upside-down point light figures (24). Furthermore, 5-month-old infants discriminate a locally rigid point light walker display from one in which local rigidity is perturbed, but only as long as it is presented upright (25). So, orientation specificity in perception of biological motion seems to emerge quite early during perceptual development, possibly as early as sensitivity to actual upright displays. Nevertheless, 2-month-old infants did not show any preference for biological motion displays (24), and such a finding seemed to support the idea that perception of locomotion in point-light displays required either some visual experience or some maturation of the visual structures. Only one study tested newborns (26) and reported a preference for a single point-light that moved according to kinematic specification of dynamics described as nonbiological (27).
To summarize, to our knowledge no study has investigated sensitivity to Johansson's biological motion displays or the effect of the inversion of such displays at birth. Previously, the data available on totally naïve subjects came from newly hatched domestic chicks. Visually naïve chicks, on their first exposure to point-light animations depicting either a walking adult hen or a nonbiological motion stimulus, were shown to prefer the biological to the nonbiological motion (20). Chicks were also shown to prefer biological motion displays even when these displays depicted an animal of a different species, i.e., a walking cat, which constitutes a potential predator to the young chick. The walking cat was preferred to any nonbiological motion displays used in comparison, but not to the walking hen, supporting the conclusion that sensitivity to biological motion would depend on general mechanisms for the detection and extraction of invariants in biological motion displays and would not therefore be limited by species-specific constraints. Data from naïve chicks also provide evidence that the inversion effect would be independent from experience, which was interpreted as caused by the fact that gravity may constitute a predisposed additive parameter playing a crucial role in biological motion perception (28).
This interpretation is in line with previous hypothesis suggesting that the visual system's sensitivity to dynamic information and the forces associated with moving objects might reflect either unlearned intrinsic constraints of the visual system (29) or a predisposition embodied in the architecture of all animal neural systems responding to legged vertebrates (1). Recently, it has been suggested that mechanisms similar to those found in visually inexperienced newborn chicks could be at work in human babies (2).
The present study investigated the origin of the sensitivity to biological motion in humans at birth. The same animations used to test the newborn chicks in previous studies (20, 28) were used with 2-day-old newborns, because previous comparative studies suggest that a general mechanism is at work, which should not be based on species-specific cues. Moreover, the use of hen-walking animations rather than human-walker animations ruled out the, potentially remote, possibility that newborns may have had any previous experience with the kind of motion depicted in the stimuli used. Three experiments were carried out, each on a separate group of newborns. If sensitivity to biological motion is experience independent, then we expected babies to discriminate biological from nonbiological patterns and to exhibit a spontaneous preference for the biological stimulus. Moreover, we also investigated the origins of the above-described effect of display inversion by testing whether a preference for upright rather than upside-down biological motion displays is present at birth, and is therefore experience-independent.
Results
Exp. 1.
Exp. 1 tested whether newborns were capable of discriminating after exposure a pattern of 13 moving elements representing a walking hen (the biological motion stimulus) from the same pattern of elements moving in a random manner (the nonbiological motion stimulus). Eighteen 1- to 3-day-old newborns, aged (mean ± SEM) 44 ± 9 h (range: 10–130 h) were tested by using an infant-control visual habituation technique.
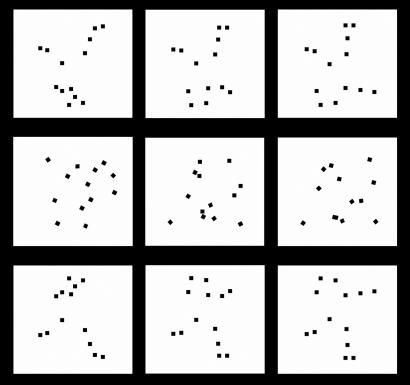
The stimuli were the same animation sequences used to test newborn chicks in previous studies (20–28). The walking-hen animation sequence was originally obtained by locating 13 points of light on the main joints of the digitalized image of a video recording of a real walking hen. Twenty-three frames were required to cover an animal's entire step sequence. The digitalized sequence was looped and projected onto a computer screen after substraction of the translation component [supporting information (SI) Movie 1]. The random motion animation sequence, in which the same set of 13 points of light moved in arbitrary directions, was originally obtained by using the function “random movement and rotation” in the software program Macromedia Director MX (version 9.0). The points of light in this display could move randomly within a window corresponding to the area of the walking-hen display; once they reached the edge of the defined window, they would not disappear, but rather would turn around and head back. The overall characteristics of the motion matched those portrayed in the walking-hen sequence in the sense that each point of light was associated with a different velocity, corresponding to the average velocity of each of the 13 points of light of the hen animation (SI Movie 2; for a detailed description of how the stimuli were produced see the original study in ref. 20). For the present study, the original sequences were modified, using the software program Macromedia Director MX (version 10.1), according to the standard stimuli format used in previous literature on newborns' perception (30). In the modified animations (Fig. 1), sets of 13 black elements [0.4 candelas (cd)/m2] were moving on a white background (103 cd/m2) rather than the usual pattern of point-lights moving on a black background. Each black element was square-shaped and was composed of 16 × 16 pixels on a 1,024 × 768-pixel resolution screen. Each element measured 0.6 × 0.6 cm on the screen with the actual visual angle measuring 1.2° at a viewing distance of 30 cm. The average velocity of the 13 elements in the stimuli was in the range of 122 pixels per s (9.15°/s): lowest velocity 24 pixels per s (1.8°/s) and highest velocity 221 pixels per s (16.6°/s). The original average velocity (20) was reduced (ratio = 4/7) to better match the characteristics of the immature visual system at birth (31). Each set of elements occupied a window of 480 × 437 pixels; the actual visual angle of the window measured 33.6° (width) and 32.7° (height) at a viewing distance of 30 cm.
Fig. 1.
Three sample frames taken from the animation sequences used in the study: the biological motion stimulus (i.e., the walking hen) (Top), the nonbiological motion stimulus (random motion) (Middle), and the inverted biological motion display (upside-down walking hen) (Bottom). Squares indicate the point-lights.
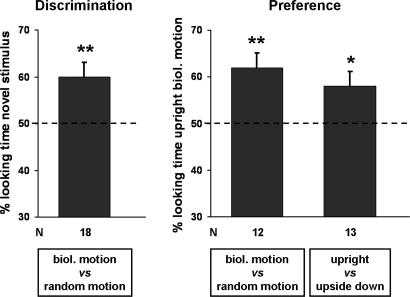
Half of the newborns were habituated to the biological motion and half to the nonbiological motion. All newborns reached the habituation criterion. The average total fixation time to habituate to the biological and nonbiological stimuli was, respectively, (mean ± SEM) 41.31 ± 2.10 s and (mean ± SEM) 50.6 ± 6.38 s. A t test for independent samples revealed that the difference between total fixation times in the two groups (i.e., newborns habituated to the biological motion and newborns habituated to the nonbiological motion) was not significant, t16 = 1.51, P = 0.15, two-tailed. After habituation, newborns' visual preference was probed for the habituation stimulus vs. the novel stimulus. Newborns looked longer at the novel stimulus (mean ± SEM = 27.26 ± 1.55 s) than at the familiar stimulus (mean ± SEM = 18.34 ± 1.34 s). To test whether newborns were able to recognize the stimulus to which they had been habituated, a novelty preference score (percentage) was computed for each newborn. To determine whether novelty preference scores were significantly different from chance level (i.e., 50%) a one-sample t test was applied. The mean preference for the novel stimulus (mean ± SEM = 60 ± 3.07%) was significantly higher than chance, t17 = 3.11, P = 0.006, two-tailed. A t test for independent samples was run to compare the mean novelty preference scores of the newborns habituated to the biological stimulus (mean ± SEM = 61.25 ± 2.77%) with the mean novelty preference score of the newborns habituated to the nonbiological stimulus (mean ± SEM = 58.29 ± 5.2%). The comparison did not reach statistical significance, t16 = 0.47, P = 0.65, two-tailed.
This outcome demonstrates that newborns discriminate between a biological motion animation (i.e., the walking hen) and a nonbiological motion animation sequence (i.e., the random motion). However, this result tells us nothing about possible predispositions of the human system at birth to preferentially attend to biological motion stimuli.
Exp. 2.
Exp. 2 tested newborns' spontaneous preference for biological vs. nonbiological motion. Using an infant-control preferential looking technique, 12 newborns aged (mean ± SEM) 29.42 ± 4 h (range: 10–55 h) were presented with the same biological and nonbiological motion stimuli used in Exp. 1.
The average total fixation time was (mean ± SEM) 66 ± 8.77 s for the biological motion stimulus and (mean ± SEM) 37 ± 4.08 s for the nonbiological motion stimulus. To determine whether fixation times toward the biological motion stimulus significantly differed from chance level (50%), fixation times were transformed into percentages. The percentage of total fixation time newborns spent looking at the biological motion stimulus was (mean ± SEM) 62 ± 3.18% and differed significantly from chance level (one-sample t test: t11 = 3.57, P = 0.004, two-tailed). Eight of 12 subjects preferred the walking hen to the random motion. A correlation between the age of the newborns (h) and preference score was not significant (r = 0.01, P = 0.97). Overall, the results of Exp. 2 favor the idea that naïve newborns show a preference for biological motion, hence such a preference would be largely intrinsic rather than acquired over time, i.e., through experience.
Exp. 3.
Exp. 3 tested for the presence at birth of the biological motion inversion effect. Thirteen newborns aged (mean ± SEM) 50 ± 4 h (range: 17–88 h) were presented with a canonical (upright) or an inverted (upside down) biological motion animation sequence picturing a walking hen (the same animation also used as biological motion stimulus in Exps. 1 and 2) (SI Movie 3).
As in the previous experiment, fixation times were registered in a spontaneous preferential looking task. Fixation times were (mean ± SEM) 41.69 ± 4.38 s and (mean ± SEM) 29.58 ± 3.3 s to the upright and the upside-down sequence, respectively. The percentage of time newborns spent looking at the upright sequence was (mean ± SEM) 58 ± 3.32% and differed from chance level, i.e., 50% (one-sample t test, t12 = 2.43, P = 0.032, two-tailed). Eleven of 13 participants preferred to look at the upright walking hen rather than at the inverted walking hen. The correlation between the age of newborns (h) and the preference score was not significant (r = 0.39, P = 0.2). Results revealed that newborns showed a significant preference for the upright animation sequence.
Results of the three experiments are represented in Fig. 2.
Fig. 2.
Results of the three experiments, expressed as the percentage of time (mean ± SEM) spent looking at the biological motion stimulus. Dashed lines indicate chance level. *, P < 0.05; **, P < 0.01.
Discussion
To date, one of the main unresolved issues in the study of the perception of biological motion concerns the ultimate nature (inborn vs. acquired through experience) of its well established special status in visual perception. Johansson (4) suggested that detection of biological motion could be an intrinsic capacity of the visual system. Similarly, the nature of the so-called inversion effect has also been questioned. So far, developmental studies have been unable to unequivocally address this issue in human subjects because results obtained with infants could always be accounted for by either innate or learning mechanisms. A final answer to such an issue can be obtained only from truly naïve newborns.
In the present study, we showed that newborn babies are able to discriminate between two different point-light displays depicting either biological motion or nonbiological (random) motion and they manifest a spontaneous preference for the biological motion display even if it depicts an unfamiliar shape such as a walking hen. Even more interestingly, the results demonstrated that the preference for biological motion was orientation-specific. Newborns were shown to prefer upright compared with inverted biological motion displays.
Overall, such data disentangle the question about the role of previous experience in perceiving biological motion. Newborns' performance cannot be accounted for on the basis of information acquired through learning for two reasons: the very young age of our subjects and the biological motion stimulus did not represent a familiar shape (such as a human body). It is highly unlikely that newborns, at the time of the test, had experienced any human walking, and it can certainly be excluded that they had any previous experience of walking hens.
Of course, we cannot exclude a possible role of fetal experience with vestibular motion cues and postnatal experience with vestibular, motor, and visual cues in influencing the sensitivity to low-level features of biological motion. Unfortunately, it would be actually impossible to rule out such stimuli in normal subjects. Moreover, for stronger claims on sensitivity to gravity it would be important to understand, and separately test, the role of vestibular information independently from the orientation of the retinal image (this could be obtained, for example, by placing the observer horizontally and presenting the stimuli on the ceiling).
Results of Exps. 1 and 2 are consistent in demonstrating an inborn sensitivity to the dynamic features of point-light displays. Such dynamic information suffices for the discrimination and exhibition of a spontaneous preference for one of the two displays. Such sensitivity must reflect unlearned intrinsic constraints of the visual system (29).
However, the sensitivity to dynamic features per se cannot explain the preference for the upright compared with the inverted display. Exp. 3 rules out the possibility that sensitivity to dynamic information would be limited to local information because the local dynamic relationships between the kinematics of the point-lights are in fact identical in the upright and inverted displays. Nevertheless, a selective preference was found for the upright display.
As already stated, our babies could not rely on information acquired through learning to discern the point-light displays. Such information, if available, may explain our findings as it would have resulted in a stored representation of, most likely upright, walking humans/animals. In the absence of any previous experience, the hypothesis that the inversion effect depends on the fact that the inverted shape would not match an experience-based template can be ruled out. Therefore, babies either relied on some unlearned representation of an upright walker, which would necessarily be general enough to allow for the detection of human and other animal walkers, or, alternatively, the visual system uses some general and local dynamic information to constrain the organization of biological motion displays. Such general dynamic information would permit differentiation between an upright and an inverted version of the same biological motion point-light display. The best candidate as a possible dynamic constraint applying to biomechanical motions would be gravity: violation of a gravitational constraint would result in failure at discriminating such displays (32). This hypothesis, already put forward to explain previous results on the inversion effect with infants, had not been tested before with newborns to our knowledge.
A more specific, and not necessarily contrasting, hypothesis (1, 2) would involve the presence of a visual filter selectively tuned to the characteristic motion of the limbs of an animal in locomotion. Such a mechanism would provide a general detection system for the presence of terrestrial vertebrates (a so-called life detector). This last hypothesis could find support in our data because it involves selective detection of the motion of limbs (our biological motion stimuli were bipedal, much like humans, although representing a species from a different animal class). Of course, further research is necessary to demonstrate that our data depended on the selective detection of the hen's legs.
The results of Exp. 3 have been taken as evidence of a very early origin of sensitivity to gravitational constraints. Although newborn babies had not been tested before to our knowledge, the current literature on the understanding of the effects of gravity on the motion of physical objects reports negative evidence for infants (33, 34) and animals, i.e., adult and young chimpanzees (35). Nevertheless, some basic perceptual ability, such as the discrimination of direction of motion, was shown to be present in 5-month-old infants (33). Overall, previous studies on our species suggest that sensitivity to certain effects of gravity develops gradually during infancy. It should be pointed out that the tasks used in those studies required the subjects to respond to or predict the future location of a moving object. It may be that the preference for the correct gravitational constraints within the motion of a living being found in our work preexists and may even constitute a prerequisite for the later development of the ability to make inferences according to such constraints.
Our findings conflict with previous results reported for newborns (26), using stimuli that were not obtained from the animation of a real animal while walking, but that rather depicted the motion of one single point-light that was described as biological or nonbiological on the basis of the kinematic specification of dynamics, according to laws of natural motion relating curvature and tangential velocity (27).
Our findings also conflict with Fox and McDaniel (24), who reported no evidence for discrimination of biological motion in 2-month-old babies. A possible explanation for the lack of any preference found in their work could be the difference in the procedure. A forced-choice preferential looking technique was used with rather short trials (on average, 15 s each). Previous studies on face perception have demonstrated that most failures to find a preference until 4 months have been attributed to short trials of a fixed length such as 30 s or even less (36).
Overall, the present data are consistent with the existence in humans, at birth, of a predisposed and experience-independent perceptual mechanism for the detection and analysis of biological motion. However, further empirical evidence is necessary to finally establish that the preference for biological motion is instrinsic and specific. For instance, the specificity of the sensitivity toward biological motion patterns could be assessed by contrasting biological motion with other types of nonbiological motion, such as rigid object motion, whereas the role of local information may be tested by using phase or spatially scrambled biological motion. If confirmed, such a predisposed mechanism would enable newborns to detect, and preferentially attend to, the movement of biologically relevant signals in his/her environment. Moreover, data coming from nonhuman species suggest that this system is phylogenetically remote; thus, it would not necessarily be tuned to be species-specific.
This conclusion is compatible with the Human First hypothesis (37), which posits either that humans identify objects and separate conspecifics by using their different properties or that detection of kind properties, guiding distinction between the two different classes of animate/inanimate, may be a feature of the system rendered directly available by the architecture of the brain very early in life. Empirical evidence on infant perception supports this conclusion by showing that the ability to discriminate a wide array of properties is not limited to basic low-level stimulus features but rather extends to complex properties that could allow newborns to uniquely single out conspecifics (30, 38, 39,). The results of the present study suggest that biological motion could be one of the perceptual properties that allows humans to distinguish living creatures from other objects and identify conspecifics from birth.
Methods
Full-term newborns were selected to participate in the study from the maternity ward of the Pediatric Clinic of the University of Padua. A total of 60 newborns participated in the experiments. Seventeen newborns were not used because of position bias (during the test phases they looked >80% in one direction; n = 7) or they changed their state during testing (n = 10). The final sample consisted of 43 newborns (18 males and 25 females). Their postnatal age ranged from 10 to 130 h (mean ± SEM = 42 ± 4 h). All of them met the screening criteria of normal delivery, had a birth weight between 2,620 and 4,290 g, and had an Apgar score between 9 and 10 at 5 min. Newborns were tested only if awake and in an alert state, and after the parents had provided informed consent. All experimental procedures have been licensed by the Responsible Office of the Dipartimento di Pediatria, Università degli Studi di Padova.
The stimuli were presented on two adjacent 19-inch Acer liquid crystal display (4:3) computer monitors (refresh rate = 60 Hz) at a distance of 30 cm from the newborn. Plain white curtains were drawn on both sides of the newborn to prevent interference from irrelevant distractors. A red flickering light-emitting diode (LED) was located between the two monitors to attract the newborns' attention. The LED subtended ≈2° of visual angle and, when turned on, blinked at a rate of 300 ms on and 300 ms off. Above the monitors, a video camera recorded the newborns' eye movements to monitor their looking behavior on-line and to allow off-line coding of their fixation.
The newborn sat on an experimenter's lap in front of the two monitors. The experimenter holding the baby was naïve to the hypothesis being tested and the stimuli being presented and was instructed to fix his/her gaze on a camera located on the ceiling throughout the experimental session. Testing began with the onset of the central flickering LED. As soon as the newborn's gaze was properly aligned with the LED, the sequence of trials was started by a second experimenter who watched the newborn's eyes through the video camera and pressed a key on the computer keyboard that automatically turned off the central LED and activated the onset of the stimuli. Two different techniques were used: an infant-control visual habituation technique (Exp. 1) and an infant-control preferential looking technique (Exps. 2 and 3). In the visual habituation technique, the habituation criterion was established by recording the duration of individual fixations. The observer recorded the duration of each fixation on the stimuli by pressing a push button that was connected to the computer. Because the stimulus was presented on the left and the right side during the habituation phase, the amount of looking time was recorded irrespective of the side. A look-away criterion of 2 s was used to determine the end of each fixation. To be sure that this criterion was strictly respected, the software automatically compacted two consecutive fixations that were not separated by a time interval of at least 2 s. The habituation phase was terminated when the habituation criterion was reached, that is when from the fourth fixation the sum of any three consecutive fixations was 50% or less than the total of the first three (40). When the habituation criterion was reached, the stimuli were automatically turned off and the central flickering LED was turned on. As soon as the newborn's gaze was realigned to the LED, the preference test phase began. Each newborn was given two paired presentations of the test stimuli in which the position of the stimuli was reversed. During each presentation, the newborn was presented with the familiar stimulus paired with a novel stimulus. The initial left-right order of presentation was counterbalanced across participants. The central LED flickered between the first and second presentations but did not flicker while the test stimuli were shown. A presentation lasted until each stimulus had been fixated on at least once and a total of 20 s of a looking fixation had been accumulated. The experimenter recorded the duration of the newborns' fixations on each stimulus by pressing two different keys depending on whether the newborn looked at the right or the left. Videotapes of the newborn's eye movements throughout the test phase were subsequently coded by a different observer unaware of the stimuli presented (it was not possible for the scorer to recognize the stimuli from the corneal reflection). The mean estimated reliability between coders was 0.90 (Pearson correlation, P < 0.001). In the preferential looking technique, the procedure used was identical to that used in the test phase of the visual habituation, with the exception that each trial ended when the newborn did not fixate on the display for at least 10 s. The mean estimated reliability between on-line and off-line coding of newborns' fixation times was 0.91 (Pearson correlation, P < 0.001) in Exp. 2 and 0.87 (Pearson correlation, P = 0.005) in Exp. 3.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Mark Johnson for reading and commenting on the article; Dr. Beatrice Dalla Barba and the nursing staff at the pediatric clinic of the University of Padua for their collaboration; Elisa Di Giorgio and Lara Bardi for assistance with infant testing; and the babies who took part in the study and their parents.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707021105/DC1.
References
- 1.Troje NF, Westhoff C. Curr Biol. 2006;16:821–824. doi: 10.1016/j.cub.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Johnson M. Curr Biol. 2006;16:R376–R377. doi: 10.1016/j.cub.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Johansson G. Psychol Res. 1976;38:379–393. doi: 10.1007/BF00309043. [DOI] [PubMed] [Google Scholar]
- 4.Johansson G. Percept Psychophys. 1973;14:201–211. [Google Scholar]
- 5.Mather G, West S. Perception. 1993;2:759–766. doi: 10.1068/p220759. [DOI] [PubMed] [Google Scholar]
- 6.Mitkin A, Pavlova MA. Psychologishe Beiträge. 1990;32:28–35. [Google Scholar]
- 7.Blake R. Psychol Sci. 1993;4:54–57. [Google Scholar]
- 8.Omori E, Watanabe S. Int J Comp Psychol. 1996;9:92. [Google Scholar]
- 9.Dittrich WH, Lea SEG, Barrett J, Gurr PR. J Exp Anim Behav. 1998;70:281–299. doi: 10.1901/jeab.1998.70-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regolin L, Tommasi G, Vallortigara G. Anim Cognit. 2000;3:53–60. [Google Scholar]
- 11.Siegel RM, Andersen RA. Nature. 1988;331:259–261. doi: 10.1038/331259a0. [DOI] [PubMed] [Google Scholar]
- 12.Tomonaga M. Psychologia. 2001;44:46–59. [Google Scholar]
- 13.Herman LM, Morel-Samuels P, Pack AA. J Exp Psychol Gen. 1990;119:215–230. doi: 10.1037//0096-3445.119.2.215. [DOI] [PubMed] [Google Scholar]
- 14.Rusenon S, Frykholm G. J Exp Psychol Hum Percept Perform. 1981;7:733–740. doi: 10.1037//0096-1523.7.4.733. [DOI] [PubMed] [Google Scholar]
- 15.Cutting JE, Kozlowski LT. Bull Psychonomic Soc. 1997;9:353–356. [Google Scholar]
- 16.Kazlowski LT, Cutting JE. Percept Psychophys. 1977;21:575–580. [Google Scholar]
- 17.Pavlova M, Sokolov A. Percept Psychophys. 2000;62:889–899. doi: 10.3758/bf03212075. [DOI] [PubMed] [Google Scholar]
- 18.Bertenthal BI, Pinto J. Psychol Sci. 1994;5:221–225. [Google Scholar]
- 19.Troje NF. J Vis. 2004;4:227a. [Google Scholar]
- 20.Vallortigara G, Regolin L, Marconato F. PLoS Biol. 2005;3:1312–1316. doi: 10.1371/journal.pbio.0030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shipley TF. Psychol Sci. 2003;14:377–380. doi: 10.1111/1467-9280.24471. [DOI] [PubMed] [Google Scholar]
- 22.Sumi S. Perception. 1984;13:283–286. doi: 10.1068/p130283. [DOI] [PubMed] [Google Scholar]
- 23.Bertenthal BI, Proffitt DR, Cutting JE. J Exp Child Psychol. 1984;37:213–230. doi: 10.1016/0022-0965(84)90001-8. [DOI] [PubMed] [Google Scholar]
- 24.Fox R, McDaniel C. Science. 1982;218:486–487. doi: 10.1126/science.7123249. [DOI] [PubMed] [Google Scholar]
- 25.Bertenthal BI, Proffitt DR, Kramer SJ, Spetner NB. Dev Psychol. 1987;23:171–178. [Google Scholar]
- 26.Méary D, Kitromilides E, Mazens K, Graff C, Gentaz E. PLoS One. 2007;1:e186. doi: 10.1371/journal.pone.0000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viviani P, Stucchi N. J Exp Psychol Hum Percept Perform. 1992;18:603–623. doi: 10.1037//0096-1523.18.3.603. [DOI] [PubMed] [Google Scholar]
- 28.Vallortigara G, Regolin L. Curr Biol. 2006;16:R279–R280. doi: 10.1016/j.cub.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 29.Shepard RN, Hurwitz S. Cognition. 1984;18:161–193. doi: 10.1016/0010-0277(84)90024-6. [DOI] [PubMed] [Google Scholar]
- 30.Morton J, Johnson MH. Biology and Cognitive Development. Oxford, UK: Blackwell; 1991. [Google Scholar]
- 31.Atkinson J, Braddick O. In: Infant Development. Slater A, Bremner G, editors. Hillsdale, NJ: Erlbaum; 1989. pp. 7–41. [Google Scholar]
- 32.Bertenthal BI, Proffitt DR, Kramer SJ. J Exp Psychol Hum Percept Perform. 1987;13:577–585. doi: 10.1037//0096-1523.13.4.577. [DOI] [PubMed] [Google Scholar]
- 33.Kim IK, Spelke ES. J Exp Psychol Hum Percept Perform. 1992;18:385–393. doi: 10.1037//0096-1523.18.2.385. [DOI] [PubMed] [Google Scholar]
- 34.Kim IK, Spelke ES. Dev Sci. 1999;2:339–362. [Google Scholar]
- 35.Tomonaga M, Imura T, Mizuno Y, Tanaka M. Dev Sci. 2007;10:411–421. doi: 10.1111/j.1467-7687.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 36.Maurer D. In: Social Perception in Infancy. Field TM, Fox N, editors. Ablex, NJ: Norwood; 1985. pp. 73–100. [Google Scholar]
- 37.Bonatti L, Frot E, Zangl R, Mehler J. Cognit Psychol. 2002;44:388–426. doi: 10.1006/cogp.2002.0779. [DOI] [PubMed] [Google Scholar]
- 38.Bertoncini J, Moralis J, Bijeliac-Babic R, McAdams S, Peretz I, Mehler J. Brain Lang. 1989;37:591–605. doi: 10.1016/0093-934x(89)90113-2. [DOI] [PubMed] [Google Scholar]
- 39.Meltzoff AN, Kuhl PK. In: The Development of Intersensory Perception: Comparative Perspectives. David JL, editor. Hillsdale, NJ: Erlbaum; 1994. pp. 335–369. [Google Scholar]
- 40.Slater AM, Morison V, Rose D. Infant Behav Dev. 1984;7:183–200. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.