Abstract
Clinically, the overlap of gastroduodenal symptoms, such as visceral pain or hypersensitivity, is often observed in functional gastrointestinal disorders. The underlying mechanism may be related to intraspinal neuronal processing of noxious convergent inputs from the stomach and the intestine. The purpose of this study was to examine whether single low thoracic (T9-T10) spinal neurons responded to both gastric and duodenal mechanical stimulation. Extracellular potentials of single T9-T10 spinal neurons were recorded in pentobarbital anesthetized, paralyzed, and ventilated male rats. Graded gastric distensions (GD, 20, 40, 60 mmHg, 20s) were induced by air inflation of a latex balloon surgically placed in the stomach. Graded duodenal distensions (DD, 0.2, 0.4, 0.6 ml, 20s) were produced by water inflation of a latex balloon placed into the duodenum. Of 70 deeper (depth from dorsal surface of spinal cord: 0.3–1.2 mm) spinal neurons responsive to noxious GD (≥ 40 mmHg), 44(63%) also responded to noxious DD (≥ 0.4 ml). Similarly, 13/17 (76%) superficial neurons (depth < 0.3 mm) responded to both GD and DD. Of 57 gastroduodenal convergent neurons, 41 (72%) had excitatory and 6 had inhibitory responses to both GD and DD; the remaining neurons exhibited multiple patterns of excitation and inhibition. 43/57 (75%) gastroduodenal convergent neurons had low-threshold (≤ 20 mmHg) responses to GD, whereas 42/57 (74%) of these neurons had high-threshold (≥ 0.4 ml) responses to DD. In addition, 34/40 (85%) gastroduodenal convergent neurons had somatic receptive fields on the back, flank, and medial/lateral abdominal areas. These results suggested that superficial and deeper T9-T10 spinal neurons received innocuous and/or noxious convergent inputs from mechanical stimulation of the stomach and duodenum. Gastroduodenal convergent spinal neurons might contribute to intraspinal sensory transmission for cross-organ afferent-afferent communication between the stomach and duodenum and play a role in visceral nociception and reflexes.
Keywords: Visceral pain, spinal visceral afferents, stomach, duodenum, visceral reflex, spinal cord
1. Introduction
Mechanical and chemical stimulations by food in gastric and duodenal lumens produce a number of sensory and motor regulatory events associated with gastrointestinal digestive and absorptive functions. For example, duodenal distension markedly inhibits gastric motility, causing gastric relaxation, as a negative feedback regulation, which plays an important role in accommodation and control of gastric emptying in various species (Azpiroz and Malagelada, 1990; De Ponti et al., 1987; 1989; Holzer and Raybould, 1992). The enterogastric reflex is mediated by both vagal and spinal capsaicin-sensitive afferents and involves various neurotransmitter systems, including a nonadrenergic and noncholinergic vagal pathway, NO released within prevertebral ganglia by gastric afferent fibers, and activation of 5-HT3 receptors on extrinsic duodenal afferents (De Ponti et al., 1987; 1989; Glise and Abrahamsson, 1980; Holzer and Raybould, 1992; Quinson et al., 2000; Raybould et al., 2003). Another example of gastroduodenal interaction is overlap in the clinical presentation of visceral pain and/or viscerosomatic referred hyperalgesia originating in stomach or duodenum of patients with peptic ulcer disease (Texter, 1987; Werdmuller et al., 1997). Gastric and duodenal pain is frequently experienced as unpleasant pressure, fullness, burning, aching and colic. Sites of pain are predominantly located on the epigastric, retrosternal and periumbilical areas, and pain also is often referred to the right upper quadrant of the abdominal wall and middle back. The symptomatic similarity and overlap of pain characteristics from stomach and duodenum often influences clinicians’ differential diagnosis for diseases of the upper gastrointestinal tract, for example, it is impossible to diagnose peptic ulcer disease only on the basis of clinical presentation without endoscopic examination (Texter, 1987; Werdmuller et al., 1997). Spinal processing for sensory-motor and sensory-sensory interactions between stomach and duodenum has not been explored. The stomach and duodenum are innervated with afferent vagal fibers that project via nodose ganglia to the nucleus of the solitary tract (NTS) in the medulla, and by splanchnic nerves that project via dorsal root ganglia to thoracic and upper lumbar spinal cord segments. It has been shown electrophysiologically that vagal afferent volleys evoked by gastric and duodenal mechanical and/or chemical stimuli converge onto single NTS neurons in rats, which is considered a neuronal substrate for afferent information interaction between the stomach and duodenum (Zhang et al., 1995). The aim of the present study in rats was to determine electrophysiologic characteristics of single thoracic (T9-T10) spinal neurons that receive convergent visceral inputs from stomach and duodenum. Preliminary results of this study have been presented previously in an abstract (Qin et al., 2007a).
2. Results
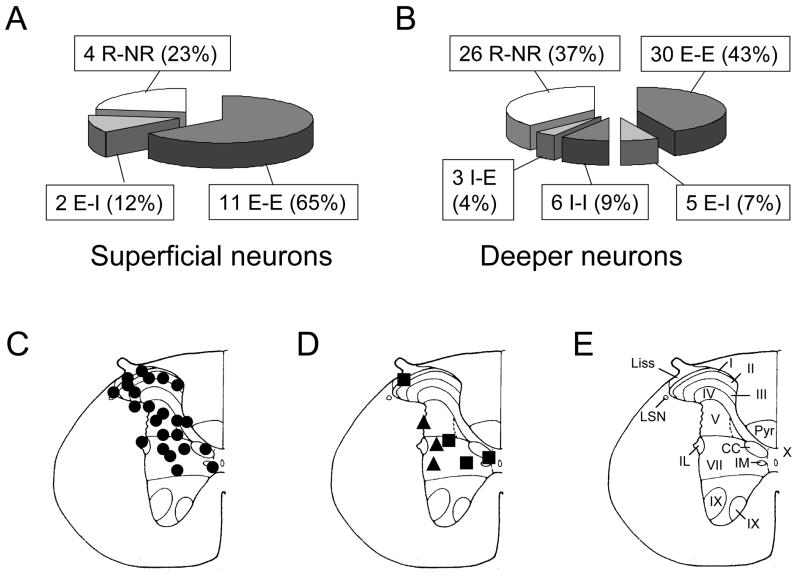
Of 87 spinal neurons responding to noxious GD (≥40 mmHg, 20 s), noxious DD (≥0.4 ml, 20 s) altered the activity of 57 (66%) neurons and did not affect the activity of 30 (33%) neurons. A majority (48/57; 85%) of the spinal gastroduodenal neurons had spontaneous activity (>0.5 imp/s) and the remainder had no or low background activity (≤0.5 imp/s). Noxious DD affected activity of 13/17 (76%) superficial spinal neurons (depth <0.3 mm from dorsal surface of spinal cord) responding to noxious GD, whereas DD altered activity of 44/70 (63%) neurons in deeper laminae of spinal cord (depth 0.3–1.2 mm). A comparison of proportions of superficial and deeper neurons responding to gastric and duodenal stimuli is shown in Fig. 1A and B. Electrolytic lesions of recording sites for some spinal gastroduodenal convergent neurons responding to both GD and DD were verified histologically (Fig. 1C–E). Neurons responding to both GD and DD were primarily located in laminae I, II, III, V, VII and X of gray matter in T9-T10 spinal cord.
Fig. 1.
Recording sites and response patterns of low thoracic (T9-T10) spinal neurons to gastric distension (GD) and duodenal distension (DD). A, B: Comparison of superficial and deeper spinal gastroduodenal convergent neurons. E, excitatory response. I, inhibitory response. R, response. NR, no response. First response is to GD. Second response is to DD. C: Locations of spinal neurons responding to both GD and DD. The black circles represent neurons excited by both GD and DD. D: The black squares represent neurons excited by GD but inhibited by DD. The triangles represent neurons inhibited by GD but excited by DD. E: Schematic drawing of the T10 spinal segment (Molander et al. 1984). I-X indicates laminae; Liss, Liss’s tract; LSN, lateral spinal nucleus; Pyr, pyramidal tract; IL, intermediolateral nucleus. IM, intermediomedial nucleus. CC, column of Clarke.
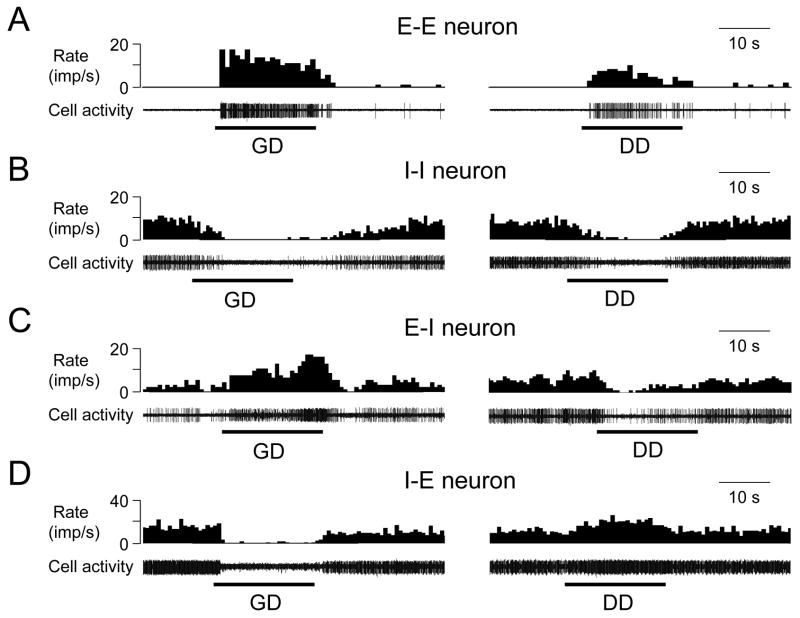
Multiple patterns of excitatory and inhibitory responses to GD and DD were observed. Of 57 gastroduodenal convergent neurons, 41 (72%) neurons had excitatory and 6 neurons had inhibitory responses to both GD and DD; the remaining neurons exhibited either excitatory/inhibitory or inhibitory/excitatory patterns. The proportions of spinal neurons with different response patterns to GD and DD are shown in Fig. 1A, B, and examples of these neurons are shown in Fig. 2. Statistical analyses of the characteristics of neuronal excitatory and inhibitory responses to GD and DD are summarized in Table 1. Maximal excitatory responses to noxious GD in gastroduodenal convergent neurons were significantly greater than to noxious DD (19.2±1.8 vs 13.1±1.3 imp/s, P<0.01). Mean latency to excitatory responses was significantly longer to noxious DD than to noxious GD (3.4±0.3 s vs 1.9±0.2 s, P<0.01).
Fig. 2.
Convergent afferent input patterns of thoracic (T9-T10) spinal neurons responding to both GD and DD. A: A spinal neuron excited by both GD (60 mmHg, 20 s) and DD (0.4 ml, 20s). B: A spinal neuron inhibited by both GD and DD. C: A spinal neuron excited by GD but inhibited by DD. D: a spinal neuron inhibited by GD and excited by DD.
Table 1.
Characteristics of spontaneous activity and responses of spinal gastroduodenal convergent neurons to noxious GD (40 mmHg, 20s) and DD (0.4 ml, 20s).
| Stimuli | Response | n | Spontaneous activity (imp/s) | Latency (s) (imp/s) | E-Response(imp/s) | I-responses(imp/s) | Duration (s) |
|---|---|---|---|---|---|---|---|
| GD | E | 48 | 7.7±1.1 | 1.9±0.2 | 19.2±1.8 | / | 39.6±3.3 |
| I | 9 | 10.3±1.8 | 1.9±0.4 | / | 9.1±2.0 | 39.8±6.9 | |
|
| |||||||
| DD | E | 45 | 8.3±1.1 | 3.4±0.3 * | 13.1±1.3 * | / | 32.3±2.6 |
| I | 12 | 9.0±1.8 | 2.6±0.5 | / | 5.7±0.7 | 38.4±4.5 | |
E, excitatory. I, inhibitory.
P<0.01 compared with corresponding activity of spinal neuronal responses to GD.
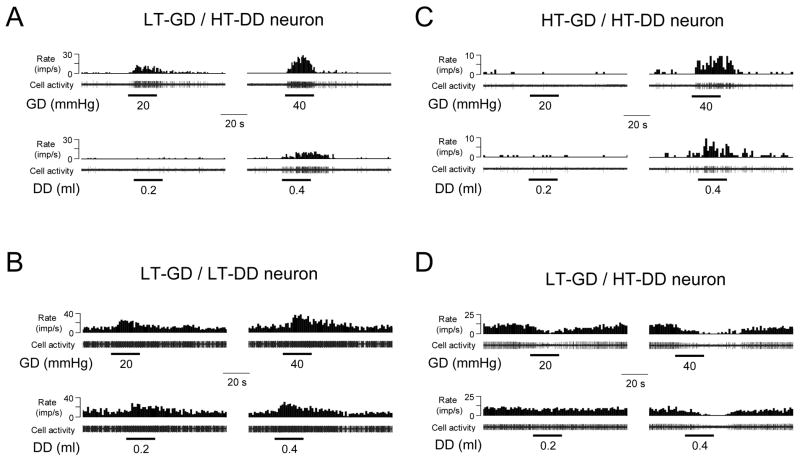
Based on the intragastric pressure that produced a neuronal response, spinal neurons responding to GD were divided into the following two subgroups: low-threshold (LT) neurons responded to intragastric pressure ≤20 mmHg; high-threshold (HT) neurons responded to ≥40 mmHg pressure of GD (Qin et al., 2007b). Furthermore, spinal neuronal responses to DD also were classified as LT and HT neurons that were responsive to ≤0.2 ml or ≥0.4 ml of DD (Qin et al., 2007c), respectively. Examples of LT and HT responses of gastroduodenal convergent neurons to GD and DD are shown in Fig. 3. A comparison of LT and HT response patterns of gastroduodenal convergent neurons to GD or DD is shown in Table 2. Of 57 gastroduodenal convergent neurons, 43/57 (75%) neurons had LT responses to GD and 14 neurons had HT responses to GD. In contrast, 42/57 (74%) of the gastroduodenal convergent neurons had HT responses to DD and 15 neurons had LT responses to DD. Thus, the patterns of response thresholds of these neurons to GD and DD was significantly different (P<0.01).
Fig. 3.
Responses of spinal neurons to graded visceral stimuli. A: A spinal neuron with low-threshold excitatory response to GD but high-threshold excitatory response to DD. B: A spinal neuron with low-threshold excitatory responses to both GD and DD. C: A spinal neuron with high-threshold excitatory responses to both GD and DD. D: a spinal neuron with low-threshold inhibitory response to GD but high-threshold inhibitory response to DD.
Table 2.
Comparison of low- and high-threshold responses of spinal gastroduodenal convergent neurons to GD and DD.
| E-response to DD | I-response to DD | ||||
|---|---|---|---|---|---|
| LT | HT | LT | HT | ||
| E-response to GD | LT | 10 | 23 | 0 | 4 |
| HT | 3 | 6 | 0 | 2 | |
| I-response to GD | LT | 0 | 3 | 2 | 1 |
| HT | 0 | 0 | 0 | 3 | |
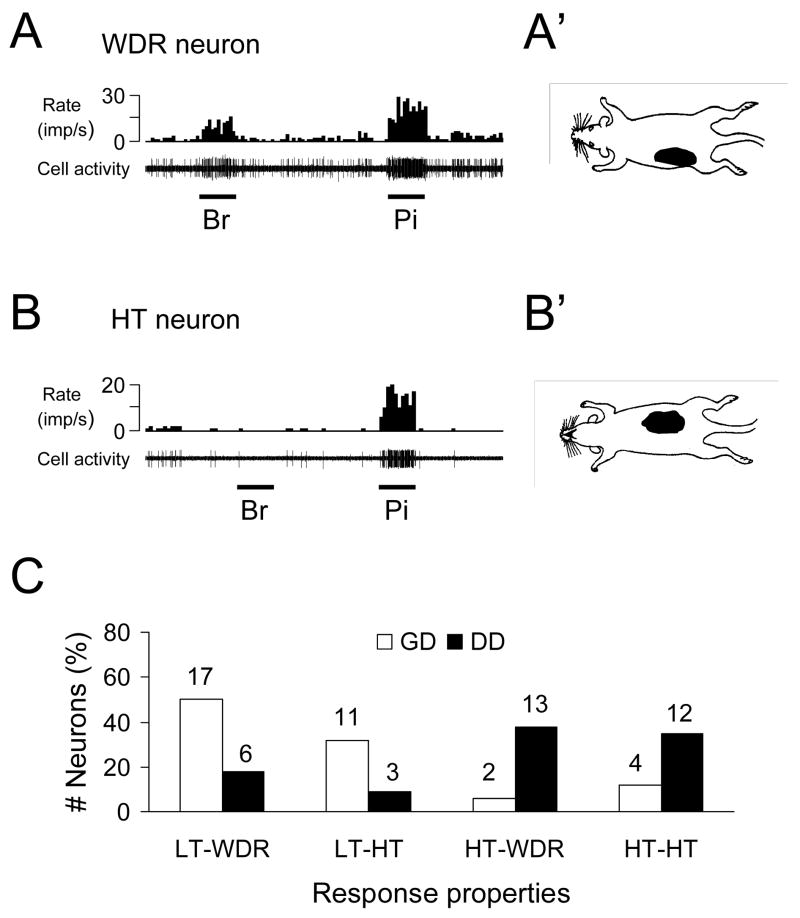
Of 40 viscerovisceral convergent neurons, 34 (85%) neurons also responded to stimulation of somatic receptive fields. Somatic receptive fields were generally located on lower chest, middle and lower back, flank, medial/lateral abdominal areas. Nineteen viscerosomatic convergent neurons were classified as WDR, 15 neurons were HT, and no LT neuron was found. Figure 4A and B show examples of the response of viscerosomatic convergent neurons to somatic stimuli. Furthermore, a comparison of response properties of spinal neurons to visceral and somatic is shown in Fig. 4C. LT neurons responding to GD were more frequently classified as WDR neurons with somatic input; while HT neurons responding to DD was more likely to be HT neurons with somatic input.
Fig. 4.
Characteristics of somatic receptive fields of gastroduodenal convergent neurons in lower thoracic (T9-T10) spinal cord. A: A spinal neuron with wide dynamic range (WDR) responses to brush (Br) and pinch (Pi) of somatic fields and location of somatic receptive field (right panel). B: A spinal neuron with high-threshold (HT) responses to somatic stimulation and location of somatic receptive field. C: Comparison between somatic and visceral inputs of spinal neurons with convergent inputs from stomach and duodenum. LT, low-threshold response to visceral or somatic stimulus. HT, high-threshold response to visceral or somatic stimulus. WDR, wide dynamic range response to somatic stimulus.
3. Discussion
In the present study in rats, T9-T10 spinal segments were selected for recording spinal neurons that responded to GD and DD, because these segments receive primary visceral afferent inputs from splanchnic nerve fibers innervating the stomach and duodenum (Holzer et al., 2005; Neuhuber and Niedrle, 1979; Ozaki and Gebhart, 2001; Qin et al., 2007b, c; Schicho et al., 2005; Schuligoi et al., 1996). This overlap in sensory projections from stomach and duodenum to T9-T10 spinal cord provided more opportunities to study gastroduodenal afferent convergent neurons than in other segments. As shown in the present study, gastroduodenal afferent convergent neurons were located in laminae I, II, III, V, VII and X in the gray matter of T9-T10 spinal cord. This observation generally agreed with previous studies, in which repeated noxious GD or DD induces neuronal staining of c-fos-like immunoreactive neurons bilaterally in superficial and deeper laminae predominately at the lower thoracic segments of the rat spinal cord (DeLeo et al., 1991; Traub et al., 1996). The regional distribution of gastroduodenal afferent convergent neurons also was consistent with recent investigations, in which spinal neuronal responses either to GD or DD are characterized in rats (Qin et al., 2007b, c).
Sixty-six percent of T9-T10 spinal neurons with gastric mechanical input also responded to noxious DD in the present study. Of gastroduodenal responsive neurons, the majority (72%) of neurons were excited by both GD and DD and the remaining neurons were inhibited or had biphasic response patterns. These results are different from a previous observation in NTS, in which 58% (11/19) of gastroduodenal convergent neurons are excited by both GD and DD, and the remainder are inhibited or have biphasic responses to these two visceral stimuli (Zhang et al., 1995). This difference might represent different central processing for convergent inputs from those visceral organs in spinal cord and NTS. The different characteristics of spinal and vagal primary afferent fibers originating from those visceral structures might also play a role in observed differences of central neuronal responsiveness visceral stimuli (Ozaki et al., 1999; Ozaki and Gebhart, 2001). Furthermore, excitatory responses to noxious GD in gastroduodenal convergent neurons were significantly greater than to noxious DD in the present study. This finding indicated that GD might activate more mechanical receptors in the gastric wall as well as spinal afferent fibers than DD did in duodenal wall, because the volume and mass are different for these two hollow visceral structures. In the present study, based on excitatory responses to graded GD or DD, neurons were divided into LT and HT subgroups. Results showed that 75% of gastroduodenal convergent neurons had LT responses and the remaining neurons had HT responses to GD. In contrast, 74% of the gastroduodenal convergent neurons had HT responses and the remaining neurons had LT responses to DD. Presumably, HT spinal neurons play an important role in intraspinal processing associated with visceral nociception, whereas LT spinal neurons might be relevant to nonpainful sensations, such as fullness and nausea. Therefore, spinal gastroduodenal convergent neurons observed in the present study were more likely to process innocuous gastric input and noxious duodenal afferent information.
It also should be noted that mean latency to excitatory responses produced by DD was significantly longer than to GD. This difference might be explained by organization of visceral afferent fibers originating from stomach and duodenum to T9-T10 spinal cord. Small spinal visceral afferents (A-delta and C fibers) receive information for mechanical strength stimuli from the gastric and duodenal walls. Therefore, spinal gastroduodenal convergent neurons might receive more mechanical afferent impulses traveling in A-delta afferent fibers than in C fibers innervating the stomach; whereas more volleys from C-fibers than A-delta fibers originating from the duodenum might be transmitted to these spinal neurons. In support of this possibility, 81% of single splanchnic afferents responding to GD in rats are classified as A-delta fibers with mean conduction velocity of 7.6 m/s, whereas 19% of afferents are C-fibers with mean conduction velocity of 1.2 m/s (Ozaki and Gebhart, 2001). No comparable data for duodenal afferents are available in the literature. A recent study in opossums shows that 12% of splanchnic afferent fibers respond to DD and all are identified as slowly adapting receptors (Schloithe et al., 2006). However, these investigators did not measure the conduction velocity for splanchnic afferent fibers originating from the duodenum. In addition, other differences such as distension rates of the air balloon for GD and water balloon for DD, compliance of those visceral organs as well as peripheral receptive field properties of the stomach and duodenum also may influence the latency to the onset of the spinal neuronal responses.
The stomach and duodenum receive dual innervation from vagal and spinal visceral afferent fibers. Traditionally, it is believed that vagal afferents from stomach and duodenum may play a role in conveying digestive information, such as absorption, secretion, and storage; whereas, nociceptive information mainly travels via the splanchnic sympathetic afferent nerves to the spinal cord. Gastroduodenal convergent spinal neurons characterized in the present study likely are involved in intraspinal sensory transmission for cross-organ afferent-afferent communication between the stomach and duodenum and, thus, might contribute to visceral nociception and viscerovisceral reflexes. For example, these neurons could provide an intraspinal connection or integrator for the enterogastric motility-inhibitory reflex (Azpiroz and Malagelada, 1990; De Ponti et al., 1987; 1989; Holzer and Raybould, 1992). These neurons also might be important in mediating nociceptive sensations and reflex alterations in gastric motility induced by gastrointestinal diseases. Delayed gastrointestinal transit and overlap of visceral pain and viscerosomatic referred hypersensitivity in response to peptic ulcers, gastritis, as well as input from the ileus after abdominal surgery or during intestinal obstruction have been noted (Werdmuller et al., 1997; Texter, 1987).
4. Experimental procedures
This study was performed in 19 male Sprague-Dawley rats (Charles River Inc. 350–450 g). Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center. Animals were initially anesthetized with intraperitoneal injection of sodium pentobarbital (60 mg/kg). The left cervical jugular vein was cannulated for intravenous infusion of pentobarbital (15–25 mg/kg/hr) throughout the experiments. The right carotid artery was intubated for continuous blood pressure monitoring during the experiments. During an experiment, the average arterial blood pressure was kept at >80 mmHg. After a tracheotomy, the trachea was cannulated for artificial ventilation using a constant-volume pump (55–60 strokes/min, 3.0–5.0 ml stroke volume). Pancuronium bromide (initial dose 0.4 mg/kg) was administered intravenously and supplemental doses (0.2 mg/kg) were given as needed throughout the experiments to provide and maintain muscle relaxation in the animals. Rectal temperature was kept between 36.7 and 37.3°C by a servo-controlled heating blanket and overhead infrared lamps.
The procedures to induce gastric distension (GD) or duodenal distension (DD) were the same as in previous studies from this laboratory (Qin et al., 2003; 2007b, c). For inducing GD, after midline laparotomy to expose the stomach, gastric contents were gently removed through a small incision in the fundus wall. A latex balloon (3–4 cm in length) attached to polyethylene tubing (PE-240) with 3–5 small holes near the tip was inserted into the gastric cavity through the incision and fixed on the edge of the incision by a ligature. The air balloon had a greater volume than that of the stomach and provided no resistance to inflation when the stomach was distended. Noxious GD (>40 mm Hg, 20 s) was employed as a search stimulus for examining neuronal responses. GD or DD were applied with 3 min intervals between distensions. Pressures were monitored continuously via a pressure sphygmomanometer. Neurons that responded to GD were examined with distension pressures of 20, 40, 60 mmHg, 20 s. This range for GD is considered to provide innocuous to noxious mechanical stimuli (Ozaki et al., 1999; Ozaki and Gebhart, 2001; Traub et al., 1996).
Neurons that responded to GD also were examined for effects of DD. A small incision was made at intestinal wall where 2–3 cm was distally far away from the pylorus of the stomach. The duodenal contents in duodenal cavity between pylorus and incision were removed gently through the small incision. A latex balloon (1.0 cm in length) attached to PE-240 tubing with 2–3 small holes near the tip was inserted through the incision into the duodenal segment between incision and the pylorus of the stomach. The duodenal catheter was fixed at the edge of the incision with a ligature. Duodenal distensions (DD) were produced by injecting warm water (0.2, 0.4 ml) through a catheter over 2–5 seconds (s); distension was maintained for 20 s. Measurements of the pressure of duodenum showed that 0.2 ml volume of duodenal distension was 22.7±1.3 mmHg (n=3) and 0.4 ml was 30.4±5.2 mmHg. The range selected for DD was based on previous studies, in which 0.2 ml of DD often was perceived as innocuous, whereas ≥0.4 ml caused significant passive avoidance behavior and pseudoaffective cardiovascular responses in rats and were believed to be noxious (Nijsen et al., 2003; Qin et al., 2007c; Stam et al., 2004). Therefore, to find the maximal number of spinal neurons with duodenal input, noxious DD (0.4 ml, 20s) was used as a search stimulus. During the procedures, special care was taken not to damage blood vessels and nerve branches around the stomach and duodenum.
A laminectomy was performed to expose the T9-T10 spinal segments. Animals were mounted in a stereotaxic headholder and stabilized with clamps attached to L1-L2 and T5-T7 vertebral processes. Dura mater was carefully removed and the dorsal surface of spinal cord was covered with warm agar (3–4% in saline) to improve recording stability. Carbon-filament glass microelectrodes were used for extracellular recordings of action potentials of single T9-T10 spinal neurons (depth: 0–1.2 mm, lateral from the midline: 0.5–1.5 mm) in either the left or right side of the spinal cord. Superficial neurons were recorded within 0–0.3 mm and deeper neurons within 0.31–1.2 mm from the dorsal surface of the spinal cord. Extracellular potentials were fed into a window discriminator, displayed on an oscilloscope, and stored in a computer with Spike 3 data acquisition software (CED, Cambridge) for off-line analyses. Neuronal activity was measured using rate histograms (1 s/bin). Spontaneous activity of neurons was determined by counting activity for 10 s before GD or DD to obtain impulses per second (imp/s). Neuronal responses (imp/s) during GD or DD were defined as increases or decreases ≥ 20% in maximal activity compared to spontaneous activity. For neurons with a firing rate less than 5 Hz, response activity was considered valid only if it increased or decreased by 1 Hz. Statistical comparisons were made using Student’s paired or unpaired t-test and the Chi-square analysis. Data are presented as means ± S.E and P<0.05 was considered statistically significant.
Somatic receptive fields of spinal neurons with gastric and/or duodenal inputs were examined for responses to innocuous brushing with a camel-hair brush, light pressure with a blunt stick, and noxious pinching of skin with a blunt forceps. Neurons were classified as follows: low-threshold (LT) neurons responded to hair movement and/or light pressure; high-threshold (HT) neurons responded only to noxious pinching of the somatic field; wide dynamic range (WDR) neurons responded to innocuous stimuli and also had greater responses to noxious pinch of somatic fields. Outlines and descriptions of receptive fields were recorded manually for all neurons examined.
An electrolytic lesion (50μA DC, 20 s) was made to mark the recording site after neurons were studied. At the end of experiments, animals were euthanized with an intravenous overdose of pentobarbital (200 mg/kg). The lower thoracic spinal cord was removed and placed in 10% buffered formalin solution. Frozen sections (55–60μm) of the spinal cord were viewed to find lesion sites where the neuronal recordings had been made. Locations were drawn on cross sections from the cytoarchitectonic scheme of rat spinal cord (Molander et al., 1984).
Acknowledgments
The authors would like to thank Dr. M. J. Chandler for helpful comments and D. Holston for her excellent technical assistance. We also appreciate Dr. S. H. Liu for histological examination of recording sites in spinal cord. This study was partially supported by a grant from National Institutes of Health (DK063733, Dr. J.D.Z. Chen; HL075524, Dr. R.D. Foreman).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azpiroz F, Malagelada JR. Perception and reflex relaxation of the stomach in response to gut distention. Gastroenterol. 1990;98:1193–1198. doi: 10.1016/0016-5085(90)90333-v. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Coombs DW, McCarthy LE. Differential c-fos-like protein expression in mechanically versus chemically induced visceral nociception. Brain Res Mol Brain Res. 1991;11:167–170. doi: 10.1016/0169-328x(91)90118-h. [DOI] [PubMed] [Google Scholar]
- De Ponti F, Azpiroz F, Malagelada JR. Reflex gastric relaxation in response to distention of the duodenum. Am J Physiol. 1987;252:G595–G601. doi: 10.1152/ajpgi.1987.252.5.G595. [DOI] [PubMed] [Google Scholar]
- De Ponti F, Azpiroz F, Malagelada JR. Relaxatory responses of canine proximal stomach to esophageal and duodenal distension. Importance of vagal pathways. Dig Dis Sci. 1989;34:873–881. doi: 10.1007/BF01540272. [DOI] [PubMed] [Google Scholar]
- Glise H, Lindahl BO, Abrahamsson H. Reflex adrenergic inhibition of gastric motility by nociceptive intestinal stimulation and peritoneal irritation in the cat. Scand J Gastroenterol. 1980;15:673–681. doi: 10.3109/00365528009181514. [DOI] [PubMed] [Google Scholar]
- Holzer HH, Raybould HE. Vagal and splanchnic sensory pathways mediate inhibition of gastric motility induced by duodenal distension. Am J Physiol. 1992;262:G603–G608. doi: 10.1152/ajpgi.1992.262.4.G603. [DOI] [PubMed] [Google Scholar]
- Holzer P, Painsipp E, Schuligoi R. Differential effects of intragastric acid and capsaicin on gastric emptying and afferent input to the rat spinal cord and brainstem. BMC Neurosci. 2005;6:60. doi: 10.1186/1471-2202-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol. 1984;230:133–141. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- Neuhuber W, Niederle B. Spinal ganglion cells innervating the stomach of the rat as demonstrated by somatopetal transport of horseradish peroxidase (HRP) Anat Embryol. 1979;155:355–362. doi: 10.1007/BF00317648. [DOI] [PubMed] [Google Scholar]
- Nijsen MJ, Ongenae NG, Coulie B, Meulemans AL. Telemetric animal model to evaluate visceral pain in the freely moving rat. Pain. 2003;105:115–123. doi: 10.1016/s0304-3959(03)00170-2. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Sengupta JN, Gebhart GF. Mechanosensitive properties of gastric vagal afferent fibers in the rat. J Neurophysiol. 1999;82:2210–2220. doi: 10.1152/jn.1999.82.5.2210. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Gebhart GF. Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am J Physiol. 2001;281:G1449–G1459. doi: 10.1152/ajpgi.2001.281.6.G1449. [DOI] [PubMed] [Google Scholar]
- Qin C, Chandler MJ, Miller KE, Foreman RD. Responses and afferent pathways of C(1)-C(2) spinal neurons to gastric distension in rats. Auton Neurosci. 2003;104:128–136. doi: 10.1016/S1566-0702(03)00002-X. [DOI] [PubMed] [Google Scholar]
- Qin C, Chen JD, Zhang J, Foreman RD. Duodenal afferent input converges onto T9-T10 spinal neurons responsive to gastric distension in rats. W1592, DDW Abstracts. 2007a doi: 10.1016/j.brainres.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on rat thoracic spinal neurons receiving input from the stomach. Neurosci Res. 2007b;57:29–39. doi: 10.1016/j.neures.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Chen JD, Zhang J, Foreman RD. Characterization of T9-T10 spinal neurons with duodenal input and modulation by gastric electrical stimulation in rats. Brain Res. 2007c;1152:75–86. doi: 10.1016/j.brainres.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinson N, Niel JP, Miolan JP. Nitric oxide released by gastric mechanoreceptors modulates nicotinic activation of coeliac plexus neurons in the rabbit. Eur J Neurosci. 2000;12:1521–1524. doi: 10.1046/j.1460-9568.2000.00056.x. [DOI] [PubMed] [Google Scholar]
- Raybould HE, Glatzle J, Robin C, Meyer JH, Phan T, Wong H, Sternini C. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2003;284:G367–G372. doi: 10.1152/ajpgi.00292.2001. [DOI] [PubMed] [Google Scholar]
- Schicho R, Donnerer J, Liebmann I, Lippe IT. Nociceptive transmitter release in the dorsal spinal cord by capsaicin-sensitive fibers after noxious gastric stimulation. Brain Res. 2005;1039:108–115. doi: 10.1016/j.brainres.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Schloithe AC, Woods CM, Davison JS, Blackshaw LA, Toouli J, Saccone GT. Pancreatobiliary afferent recordings in the anaesthetised Australian possum. Auton Neurosci. 2006;126–127:292–8. doi: 10.1016/j.autneu.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Schuligoi R, Herzeg G, Wachter C, Jocic M, Holzer P. Differential expression of c-fos messenger RNA in the rat spinal cord after mucosal and serosal irritation of the stomach. Neuroscience. 1996;72:535–544. doi: 10.1016/0306-4522(95)00552-8. [DOI] [PubMed] [Google Scholar]
- Stam R, van Laar TJ, Wiegant VM. Physiological and behavioural responses to duodenal pain in freely moving rats. Physiol Behav. 2004;81:163–169. doi: 10.1016/j.physbeh.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Texter EC. Ulcer pain mechanisms. The clinical features of active peptic ulcer disease and implications for therapy. Scand J Gastroenterol Suppl. 1987;134:1–20. doi: 10.3109/00365528709090135. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Sengupta JN, Gebhart GF. Differential c-fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distention in the rat. Neurosci. 1996;74:873–884. doi: 10.1016/0306-4522(96)00173-x. [DOI] [PubMed] [Google Scholar]
- Werdmuller BF, van der Putten AB, Loffeld RJ. The clinical presentation of peptic ulcer disease. Neth J Med. 1997;50:115–119. doi: 10.1016/s0300-2977(96)00075-7. [DOI] [PubMed] [Google Scholar]
- Zhang X, Renehan WE, Fogel R. Neurons in the vagal complex of the rat respond to mechanical and chemical stimulation of the GI tract. Am J Physiol. 1998;274:G331–G341. doi: 10.1152/ajpgi.1998.274.2.G331. [DOI] [PubMed] [Google Scholar]