Abstract
The human AF-6, a scaffold protein between cell membrane-associated proteins and the actin cytoskeleton, plays an important role in special cell–cell junctions and signal transduction. It can be phosphorylated by the protein kinase Bcr, which allows efficient binding of the C terminus of Bcr to the PDZ domain of AF-6 and consequently enhances the binding affinity of AF-6 to Ras. Formation of the AF-6, Bcr, and Ras ternary complex results in down-regulation of the Ras-mediated signal transduction pathway. To better understand the molecular basis for the recognition of the AF-6 PDZ domain and Bcr, we solve the solution structure of the AF-6 PDZ domain complexed with the C-terminal peptide of Bcr and explore the interactions between them in detail. Compared with previously reported structures, the complex exhibits a noncanonical binding mode of PDZ/peptide. Owing to the distinct residues involved in the AF-6 PDZ domain and Bcr peptide interaction, the interaction mode does not adapt to the existing classification rules that have been put forward, based on the ligand or the PDZ domain specificity. Furthermore, the PDZ domain of AF-6 can bind to the C terminus of Bcr efficiently after phosphorylation of AF-6 by the Bcr kinase. The phosphorylation may induce a conformational change of AF-6, which makes the binding surface on the PDZ domain accessible to Bcr for efficient binding. This study not only characterizes the structural details of the AF-6 PDZ/Bcr peptide complex, but also provides a potential target for future drug design and disease therapy.
Keywords: PDZ domain, AF-6, complex, solution structure, backbone dynamics
Cells in multicellular organisms recognize their neighboring cells, adhere to them, and form intercellular junctions that play essential roles in various cellular processes, including morphogenesis, differentiation, proliferation, and migration or differentiation. The human AF-6 protein is a component of both the tight junctions and adhesion junctions and is involved in connecting the junctional complexes with the cortical actin cytoskeleton (Matter and Balda 2003). It belongs to a novel cell–cell adhesion system composed of nectin and afadin (homologous molecule of AF-6 in mouse). This novel adhesion system performs essential functions in assembling the membrane complexes and bringing signaling pathway components into proximity (Takai and Nakanishi 2003). AF-6 is a critical regulator of cell–cell junctions during mouse development. AF-6-deficient mice die because of defects of cell–cell junctions and the reduced cell polarity of neuroepithelial cells (Zhadanov et al. 1999). The Drosophila melanogaster homolog of AF-6, Canoe, is also targeted to junctional complexes in embryonic epithelia. Loss-of-function mutants of Canoe lead to failure in the dorsal closure of embryonic epidermis, demonstrating its essential function in Drosophila morphogenesis (Boettner et al. 2003). AF-6 has been reported to be capable of binding a variety of proteins. It may function as a molecular scaffold, integrating signals related to cell adhesion and cytoskeletal reorganization (Su et al. 2003).
AF-6 is a large multidomain protein, including two Ras-binding domains (Ponting and Benjamin 1996), a Forkhead-associated domain (Hofmann and Bucher 1995), and a class V myosin homology region and DIL motif (Ponting 1995) in the N-terminal part. Located closer to the C terminus is a PDZ domain followed by proline-rich clusters, which may function as docking sites for other molecules.
The PDZ domain is a structurally conserved module with ∼90 residues folded into a compact globular structure comprising six β-strands flanked by two α-helices (Morais Cabral et al. 1996). It is a well-known protein–protein interaction module that plays important roles in assembling membrane proteins, organizing signal transduction complexes, and maintaining cell polarity (Hung and Sheng 2002). One common mode for the interaction of PDZ domains involves association with short peptide fragments at the very C terminus of target proteins, which bind as an antiparallel β-strand in the groove between the βB-strand and the αB-helix of PDZ (Hung and Sheng 2002). Other than this canonical binding mode, some PDZ domains also recognize internal motifs, which are exposed as β-finger structures on their target proteins (Hillier et al. 1999). Additionally, PDZ domains can associate with other PDZ domains to form homo- and hetero-oligomers (Nourry et al. 2003; Chikumi et al. 2004). The PDZ domain of AF-6 can interact with many molecules, such as JAM (Ebnet et al. 2000), Eph receptor (Buchert et al. 1999), SPA-1 (Su et al. 2003), Neurexin (Zhou et al. 2005), and Bcr (Radziwill et al. 2003).
The protein kinase Bcr (breakpoint cluster region protein) is a large soluble oligomeric multidomain protein best known for its involvement in chronic myelogenous leukemia (CML) (Faderl et al. 1999). It is a negative regulator of cell proliferation and oncogenic transformation. It has been revealed that Bcr and AF-6 colocalize in epithelial cells at the plasma membrane. In quiescent cells, the constitutively active Bcr phosphorylates AF-6, which allows efficient binding of the C terminus of Bcr to the PDZ domain of AF-6. This interaction, in turn, increases the affinity of AF-6 for Ras via its Ras-binding domain (RBD). Then the ternary complex of Bcr, AF-6, and Ras at junctional sites of epithelial cell membranes will switch off the downstream Raf/MEK/ERK signal transduction pathway to down-regulate Ras-mediated signaling and cell proliferation (Radziwill et al. 2003). Recently, AF-6 has also been reported to act as a negative regulator of Rap-induced cell adhesion (Zhang et al. 2005).
Although we have previously reported the solution structure of the AF-6 PDZ domain and AF-6 PDZ/Bcr complex model based on chemical shift perturbation data and homology modeling (Zhou et al. 2005), there is no experimental AF-6 PDZ/Bcr complex structure available to date. The complex structure is difficult to determine because of the low affinity between AF-6 PDZ and Bcr. Here, we report the NMR structure of the AF-6 PDZ in complex with the C-terminal peptide of Bcr (amino acid residues 1261–1271). The key residues involved in PDZ–Bcr recognition are identified. Our data reveal that the binding mode of AF-6–PDZ/Bcr is significantly different from that of the canonical class I or class II PDZ domain. The unique Gln70, the first amino acid of αB in the AF-6 PDZ domain, determines the distinct binding mode of the AF-6 PDZ domain/Bcr peptide. Furthermore, with the backbone dynamics study, we demonstrate the flexibility of the AF-6 PDZ domain in free and binding form. The flexibility difference between the two forms is not obviously observed, and the analysis of 15N relaxation data shows a normal pattern of more rigid secondary structures and more flexible loop structures. From the correlation time estimation, we presume that the AF-6 PDZ domain might be in a monomer–dimer equilibrium in solution. However, the concentration-dependent chemical shift changes imply that dimerization neither changes the conformation nor affects the complex structure determination. Our work not only provides a clear view of the interaction between the AF-6 PDZ and the Bcr peptide, but also gives experimental data for further research on the PDZ domain classification and a structural basis for specific ligand screening and drug design.
Results
Structure determination
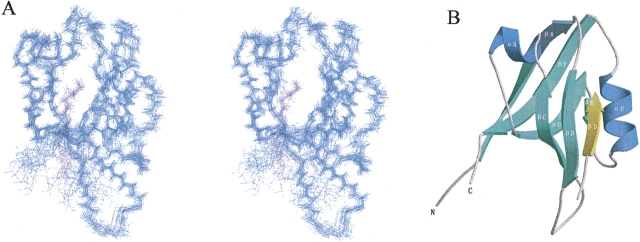
The solution structure of the AF-6 PDZ domain complexed with the C-terminal peptide of Bcr (KRQSILFSTEV) is shown in Figures 1 and 2. The complex structure is solved using a total of 1606 experimental restraints, including 1471 intramolecular NOEs, 61 intermolecular NOEs, and 74 dihedral angle restraints derived from NMR spectroscopy (Table 1). NOEs are observed only between the last five amino acid residues (FSTEV) of the Bcr peptide and the AF-6 PDZ domain. Figure 1A shows a stereoview of the superposition of a family of the 20 lowest-energy NMR structures, selected from 200 accepted structures by requiring no NOE violations >0.5 Å and no dihedral angle violations >5°. The final ensemble of the 20 refined structures has been deposited in the Protein Data Bank under accession code 2AIN.
Figure 1.
Structure of the AF-6 PDZ/Bcr complex. (A) Backbone overlay stereoview of the 20 lowest-energy NMR structures of the PDZ domain from human AF-6 complexed with the C-terminal peptide from Bcr, superimposed using backbone atoms (N, Cα, C′). (Blue) The PDZ domain; (purple) the Bcr peptide. This figure was prepared using MOLMOL (Koradi et al. 1996). (B) Ribbon diagram of a representative NMR structure of the complex generated with MOLSCRIPT (Kraulis 1991) and Raster3D (Merritt and Murphy 1994). The β-strands of the PDZ domain are labeled βA–βF, and the α-helices are labeled αA and αB. The ligand peptide (β0) inserts between the βB-strand and the αB-helix of the PDZ domain, forming an antiparallel β-sheet with βB.
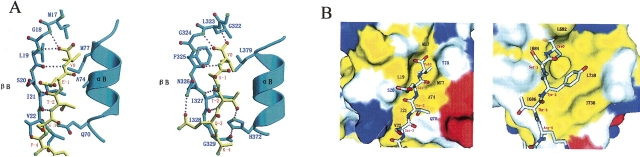
Figure 2.
Detailed interaction between the AF-6 PDZ and Bcr peptide. (A) Structural comparison between (left) AF-6 PDZ/Bcr and (right) the canonical class I complex (PSD-95 PDZ3/peptide, PDB code 1BE9). Hydrogen bonds (dotted pink lines) between residues of the PDZ domain (blue) and the Bcr peptide (yellow) were deduced from the geometry of the structure. (Red) Oxygen atoms; (green) nitrogen atoms. The AF-6 PDZ/Bcr interaction differs significantly from that of the canonical class I PDZ domains for the absence of a hydrogen bond between the −2 position residue of the peptide and the αB:1-position residue His of the PDZ. For clarity, side chains of only selected residues are shown. The programs MOLSCRIPT and Raster3D were used to generate this figure. (B) Structural comparison between (left) AF-6 PDZ/Bcr and (right) the canonical class II complex (Grip1 PDZ6/peptide, PDB code 1N7F). Surface representation of the packing interface is generated with PyMOL (available at www.pymol.org). (Yellow) The hydrophobic residues (Ala, Ile, Leu, Met, Pro, Phe, Tyr, and Val); (red) negatively charged residues (Asp and Glu); (blue) positively charged residues (Arg, His, and Lys); and (white) polar residues (Asn, Gln, Gly, Ser, and Thr). The AF-6 PDZ/Bcr interaction differs obviously from that of the canonical class II PDZ domains for the absence of the second hydrophobic pocket at the −2 position residue of the ligand peptide.
Table 1.
Structural statistics of the PDZ domain/Bcr peptide complex

Similar to other PDZ/peptide complexes, the AF-6 PDZ has a conserved fold consisting of six β-strands (βA–βF) flanked by two α-helices (αA and αB). The NMR data indicate that the C-terminal peptide of Bcr binds directly to the groove formed by αB and βB of the PDZ domains in an antiparallel fashion. Figure 2 shows an enlarged view of the binding site in the PDZ/Bcr complex. Deduced from the geometry of the structure, the C terminus Val0 (ligand residue positions are numbered in reverse direction from the C terminus, which is denoted as 0) of the peptide forms intermolecular hydrogen bonds with the backbone amide groups of residues Gly18 and Leu19, which belong to the conserved GLGF loop (GMGL in the case of the AF-6 PDZ), with the bond lengths of 2.8 Å and 3.3 Å in the average structure, respectively. Turning to the side chains, Val0 is embedded in a deep hydrophobic pocket surrounded by the side chains of residues Met17, Leu19, Ile21, Ala74, Met77, Val84, and Leu86, whereas Thr−2 is in van der Waals contact with αB:1 (Q70). In particular, the long βB/βC-loop seems not to be involved in the recognition between AF-6 and the Bcr peptide.
Molecular basis for peptide recognition
The role of the residue at position −1 for side-chain-dependent affinity differs in various PDZ complexes. In most cases, it makes no contribution to PDZ-specific recognition; in some cases, though, it does contribute directly to the specificity and affinity of the interaction (Karthikeyan et al. 2001). As to the AF-6 PDZ/Bcr system, a strong NOE exists between the Cα and Cβ protons of Glu−1 in the peptide and the Cα proton of Ser20 in the protein, demonstrating the specificity contribution of Glu−1 and the typical antiparallel β-sheet characteristic of the interaction.
Although the side chain of Ser−3 is close to the large amino acid residue Val22 in βB:4, it is directed into the solvent, and thus steric hindrance is avoided. Furthermore, it is also in the proximity of the long side chain of Lys37 in the βC-strand, which is confirmed by several NOEs between them.
In general, PDZ specificity is mainly determined by the last four C-terminal residues of the target protein (Wiedemann et al. 2004). However, our data suggest that five residues should be considered in the case of the AF-6 PDZ/Bcr complex. In contrast to most PDZ domain/peptide bindings, in which the residue at position −3 is to the edge of the peptide-binding groove (Kozlov et al. 2002), the AF-6 PDZ domain/Bcr complex has Phe−4 to the edge of the peptide-binding groove, making direct contact. The side chain of Phe−4 points toward helix αB, and its Cα proton shows multiple NOEs with the side chain of Val22 (Supplemental Fig. S1), indicating subsidiary hydrophobic interaction. As to residues beyond the last five amino acids, 13C/15N-filtered (F1) 13C-edited (F3), 3D NOESY experiments demonstrated that they do not participate in the molecular interaction.
Several studies have reported that besides the groove formed by the βB-strand and the αB-helix, other positions, especially the βB/βC-loop, may also be involved in ligand binding (Songyang et al. 1997; Kozlov et al. 2002; Birrane et al. 2003). However, these atypical interactions are restricted to individual PDZ domains or even individual PDZ domain/ligand pairs. In the case of the AF-6/Bcr complex, no NOEs were found in the long βB/βC-loop, which is in agreement with previously reported chemical shift perturbation results (Zhou et al. 2005). We can draw the conclusion that the βB/βC-loop of the AF-6 PDZ domain is not involved in Bcr binding and has no contribution to the binding affinity.
Structural comparison with the ligand-free form
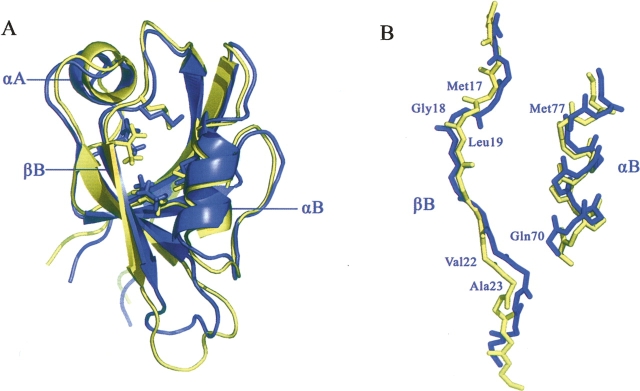
The three-dimensional structure of the ligand-free form of the AF6 PDZ domain has been solved by NMR spectroscopy in our group (Zhou et al. 2005). To better understand the binding mechanism of Bcr, we compared the structures of the ligand-free and ligand-bound AF-6 PDZ domains. Although the spectral analysis shows that the overall structure of the PDZ domain is very similar in the free and complex forms (Fig. 3A), significant deviations with backbone RMSD values above 1.0 are observed in αB, βB, and αA. Upon binding of Bcr, the Cα atom of Gln70 at the beginning of αA is displaced by 2.0 Å, resulting in the widening of the peptide-binding groove (Fig. 3B). In addition, the ɛ protons of Met17 and Met77 project directly down into the peptide-binding hydrophobic pocket in the complex form, whereas in the free form they point toward the interior of the protein instead. The sulfur atoms of the Met17 and Met77 side chains are also rearranged to increase the hydrophobicity of the binding pocket. Closer inspection of the superimposed structures reveals slight side-chain rearrangements of residues Met17, Leu19, and Met77 in the ligand-binding groove to accommodate the bulky methyl group of Val0 from Bcr. The side chains of the other hydrophobic residues lining this pocket (Ile21, Ala74, Val84, and Leu86) are virtually unchanged. Similarly, the backbones of residues Val22 and Ala23 are also rearranged to avoid steric hindrance of the bulky side chain of Phe−4. Notably, residues from αA, which are distant from the peptide-binding sites, also show large backbone deviation with the RMSD value of 1.13 Å between free and complex forms. This is consistent with the results from chemical shift perturbation (Zhou et al. 2005).
Figure 3.
Comparison of the AF-6 PDZ in the (blue) ligand-free and (yellow) ligand-bound states; the peptide of Bcr is not shown (stereoview). Both figures were prepared using the program PyMol. (A) The overall structure of the PDZ domain is only slightly changed upon Bcr binding. (B) Although the overall structure of the PDZ domain is very similar in the free and complex forms, backbone deviations are observed in αB, βB region.
Dynamics of AF-6 PDZ domain/Bcr peptide complex from 15N relaxation measurements
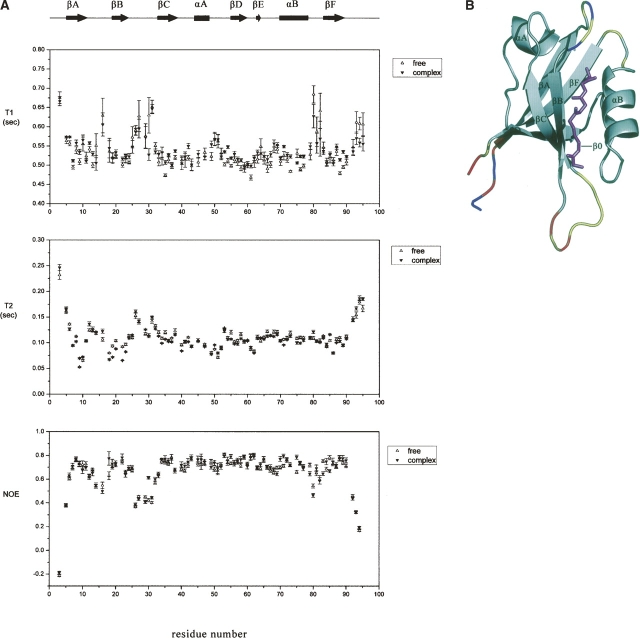
Figure 4 presents the 15N relaxation data of the AF-6 PDZ domain backbone amide nitrogens in both the free and complex forms, including steady-state {1H}–15N NOE intensities and T1 and T2 relaxation times as a function of PDZ sequence. T1, T2, and NOE values were determined for 86 out of 93 residues within the PDZ domain. Of the seven uncharacterized residues, Asn15, Met17, and Asp46 were too weak to obtain reliable experimental data, and the remaining residues were overlapped in the 2D 1H–15N HSQC spectrum.
Figure 4.
Backbone dynamics of the AF-6 PDZ/Bcr peptide complex. (A) The backbone 15N relaxation parameters for (open triangles) free and (filled triangles) peptide-bound states of the AF-6 PDZ domain are plotted versus the residue number. The error bars represent standard deviations. (B) The amides of the AF-6 PDZ domain in the complex form showing enhanced mobility on a sub-nanosecond timescale, as evident by reduced {1H}–15N NOE values, are highlighted in color on the representation of the ensemble of NMR-derived structures. (Red) Residues with {1H}–15N NOE values of <0.2 (highest mobility); (orange) between 0.2 and 0.4; (yellow) between 0.4 and 0.6; and (gray) >0.6. (Blue) Proline residues or residues for which data are not available (e.g., because of spectral overlap). (Pink) The Bcr peptide in ball-and-stick models. This figure was prepared using PyMOL.
As shown in Figure 4A, the backbone 15N relaxation parameters are broadly similar, and localized differences are not obviously observed between the free and complex forms of the AF-6 PDZ. The 15N T1 and T2 relaxation times of the complex form are slightly smaller than those of the free form. The steady-state {1H}–15N NOE, a sensitive indicator of motions on a sub-nanosecond timescale, shows a similar pattern in both free and complex forms. As demonstrated in Figure 4B, residues in the defined secondary structure exhibit a highly restricted mobility, while residues at both termini of the domain, as well as in the βA/βB-loop (residues 13–18), αB/βF-loop (residues 79–82), and to a larger extent, the βB/βC-loop (residues 25–32), exhibit reduced {1H}–15N NOE values indicative of local flexibility. This is in complete agreement with structure calculations. Furthermore, the loop region of βB/βC appears to exhibit little change in the {1H}–15N NOE value upon Bcr binding.
The FAST-Modelfree approach was used to obtain the apparent values of the overall correlation time τm. Interestingly, the overall correlation times of the free and complex forms of the AF-6 PDZ are found to be 7.25 and 6.93 nsec, respectively, at ∼1.0 mM, which are higher than expected for a monomeric 10-kDa protein, yet lower than expected for a dimeric protein (Wagner 1997). Since the overall correlation time of a molecule should be approximately proportional to its molecular weight, the values of these correlation times suggest that the PDZ domain is in a monomer–dimer equilibrium and the equilibrium may shift with the addition of ligand (Farrow et al. 1994). Our result can be partly supported by previous study of the AF-6 PDZ with lower concentration, the global correlation time of which was 6.59 nsec (data not shown).
Because the overall correlation time is close to the theoretic value of a monomer, we presume that the degree of dimerization is not high in our experimental condition. Furthermore, the concentration-dependent chemical shift changes are investigated over a concentration range from 0.275 mM to 2 mM (Supplemental Fig. S2). These spectra are very similar, except for a few cross-peaks exhibiting small chemical shift perturbations <0.04 ppm in the 1H and 0.4 ppm in the 15N dimensions, suggesting a rather fast chemical exchange between the monomer and dimer forms, and thus the structural determination is not affected under the NMR experimental conditions.
Discussion
PDZ classification
Although a diverse family of PDZ domains has been identified, the rules that govern their ligand specificity are not clear. Proper classification may not only help to organize PDZ domains and multi-PDZ domain proteins, but also provide some clues about their functions in cells. According to the classification rule described by Songyang et al. (1997), the binding specificity of PDZ domains is critically determined by the interaction between the first residue in helix αB (position αB:1) and the side chain of the −2 position residue in the C-terminal peptide. In the class I PDZ domain interactions, such as those of PSD-95, a serine or threonine residue occupies the −2 position. The side-chain hydroxyl group of this residue forms a highly conserved hydrogen bond with the N-3 nitrogen of the histidine residue at position αB:1 (Fig. 2A; Doyle et al. 1996; Songyang et al. 1997). In the case of the AF-6/Bcr complex, instead of histidine, there is a hydrophilic residue, Gln, in the position of αB:1 with no hydrogen bonds to the peptide. The AF-6 PDZ domain/Bcr peptide interaction is mainly through van der Waals contacts between the Thr−2 side chains of the peptide and the protein surface consisting of Ile21, Gln70, and Ala74. This type of recognition via van der Waals contacts is obviously in contrast to the hallmark of class I PDZ domains.
Class II PDZ domain interactions are characterized by hydrophobic residues at both the −2 position of the ligand and the αB:1 position of the PDZ domain, presenting two hydrophobic binding pockets at positions 0 and −2 of the ligand (Fig. 2B; Songyang et al. 1997; Im et al. 2003). In addition, a third class of PDZ domains, such as nNOS, prefers negatively charged amino acids at the −2 position. Specificity is determined by the coordination of the hydroxyl group of a tyrosine residue at position αB:1 with the side-chain carboxylate of the −2 residue (Stricker et al. 1997; Tochio et al. 1999). The AF-6/Bcr complex apparently differs from class II and class III PDZ domain interactions for the absence of hydrophobic or negatively charged amino acids at the −2 position as shown in Figure 2B. The unique Gln70 at αB:1 of the AF-6 PDZ domain determines the distinct binding mode of the AF-6 PDZ/Bcr peptide. In conclusion, the AF-6 PDZ/Bcr peptide displays a noncanonical interaction manner and suggests that the classification of PDZ domains solely by the classes of their ligands may not be sufficient to describe the complexity of the PDZ domain family.
With the increasing number of PDZ-mediated interactions that do not conform to the canonical type of recognition, Bezprozvanny and Maximov (2001) have proposed a novel classification of PDZ domains based on the nature of amino acids in the two critical positions in the PDZ domain fold, referred to as “Pos1” and “Pos2.” Using these principles, they classified the binding specificities of PDZ domains into 25 groups, and accordingly, the AF-6 PDZ belongs to the (Sp, p) group with Ala23 in position β:B5 and Gln70 in position αB:1 (“Sp” for small and polar, and “p” for polar). They point out that the specific ligand of the (Sp, p) group is E/D-WC, a ligand of the PTPN13-3 domain (Gross et al. 2001; Bezprozvanny and Maximov 2002). However, it is previously reported that the AF-6 PDZ displays dual ligand specificity in that it not only binds to the Neurexin C tail with an EYYV sequence (canonical class II ligand), but also binds to Bcr with an STEV end (canonical class I ligand) (Zhou et al. 2005). When all the ligands of the AF-6 PDZ that have been confirmed in vivo are considered (Table 2), it is obvious that all these ligands present the classical type II binding mode except Bcr and bear little relationship to the E/D-WC motif. Shedding light on the variety of primary sequences of ligands, we find that the last four C-terminal residues as well as the more upstream sites could accommodate various kinds of residues. This indicates that the PDZ domain of AF-6 is a very flexible interaction domain, with the potential to bind to diverse target sequences. Besides the AF-6 PDZ domain, we also screen PDZ domains from the same (Sp, p) group using DALI (http://www.ebi.ac.uk/dali/) and find that the Syntenin PDZ1 domain has binding specificity for peptides from both class I and class III (Kang et al. 2003). These results, in conjunction with other emerging structural data on PDZ domains, call for further research on classification and the mechanism of the PDZ/ligand interaction.
Table 2.
Binding sites of AF-6 PDZ ligands that have been reported in human
Since no structural data are available for the complexes of the AF-6 PDZ domain with other ligands, the general binding mode of the AF-6 PDZ cannot be fully elucidated. It is the sequence specificity of both the AF-6 PDZ domain and the Bcr peptide that makes their binding a noncanonical mode. More 3D structures and experimental information are needed for better understanding the molecular binding mode between the AF-6 PDZ and its ligands.
Biological implications
It has been reported that in vivo the AF-6 PDZ domain/ligand interaction is regulated by the Bcr kinase, which phosphorylates Thr893 of AF-6 and consequently allows efficient binding of the C terminus of Bcr to the PDZ domain of AF-6. The kinase-defective N-terminal deletion mutant of Bcr shows very weak binding to AF-6 protein, demonstrating that Bcr binds to the full-length AF-6 only if the Bcr kinase is intact. However, the isolated PDZ domain of AF-6 can bind to both wild-type and mutant Bcr that abrogates the kinase activity with almost the same affinity (Radziwill et al. 2003). Our structure also confirms that without phosphorylation, the PDZ domain of AF-6 can still bind to the C terminus of Bcr in vitro. All these results support the notion that the AF-6 PDZ domain exists as a downstream effector. It is hypothesized that the phosphorylation may induce a conformational change of AF-6, which makes the embedded binding surface of the PDZ domain exposed and accessible for the efficient binding of Bcr. The complex structure may provide some clues about the more complicated AF-6/Bcr/Ras ternary complex formation in the Ras-mediated signal transduction pathway in cells. Furthermore, as a protein–protein interaction module, the AF-6 PDZ domain may play an important role in drug discovery and chemical-tool generation. The structure determination of the AF-6 PDZ domain/Bcr peptide complex provides detailed information on the individual residues’ contribution to ligand binding, which is valuable for drug design.
Materials and Methods
Protein expression and purification
The human AF-6 PDZ domain (amino acid residues 987–1078) was amplified from the human brain cDNA library and inserted into the plasmid pET22b (+) (Novagen) as it was described before (Zhou et al. 2005). It was expressed as a His-tag fusion protein in Escherichia coli BL21 (DE3) by induction with 0.1 mM IPTG overnight at 25°C. Uniformly 15N- and 15N/13C-labeled proteins were prepared by growing the bacteria in SV40 medium using 15NH4Cl (0.5 g/L) and 13C6-glucose (2.5 g/L) as stable isotope sources. Recombinant AF-6 PDZ was purified using Hitrap chelating column (Pharmacia) chromatography. The purified protein was confirmed by SDS-PAGE, and the concentrations were determined using the BCA method.
The C-terminal peptide of Bcr (KRQSILFSTEV, amino acid residues 1261–1271) was chemically synthesized using standard FMOC chemistry at Shanghai Zillion Pharmaceuticals Co., Ltd. The synthetic peptide was purified by a reverse-phase HPLC C18 column eluted with an acetonitrile gradient of 15%–30%. The final product was verified by electrospray mass spectrometry and NMR signal assignments.
Sample preparation
The NMR samples typically contained 0.5–1.0 mM 15N, 13C-labeled AF-6 PDZ domain, 50 mM phosphate buffer (pH 5.9), 1 mM EDTA, and 10% (v/v) D2O. A “100% D2O” sample was prepared by lyophilization and resuspension in 10 mM acetate buffer (pH 5.5), 0.5 mM EDTA, and 99.96% D2O. Aliquots of the Bcr peptide (KRQSILFSTEV) were added to the 15N, 13C-labeled AF-6 PDZ domain until no further changes of intensity for the 1H–15N HSQC peaks of the complexed protein were observable. Because the 15N-HSQC spectrum typically indicates fast exchange behavior, to avoid intermediate exchange, we added the unlabeled Bcr peptide in excess during the titration till PDZ was saturated. A freshly prepared 15N-labeled sample in acetate buffer was used for relaxation data measurements.
NMR spectroscopy
All NMR experiments were carried out at 298 K on Bruker DMX500 or DMX600 spectrometers equipped with triple resonances, self-shielded z-axis gradient probes. Data were processed using the programs NMRDraw/NMRPipe (Delaglio et al. 1995). Spectra were analyzed and assigned using the program SPARKY 3 (T.D. Goddard and D.G. Kneller, University of California, San Francisco).
Backbone resonance assignments for AF-6 PDZ were carried out based on a complete series of experiments acquired at 500 MHz in H2O solution: 3D CBCANH, CBCA(CO)NH, HNCO, HN(CA)CO, and 3D HBHA(CBCACO)NH. AF-6 PDZ side-chain assignments were completed from 3D HCCH-TOCSY and HCCH-COSY spectra acquired at 500 MHz in H2O solution. Peptide resonance assignments were obtained from Heteronuclear X-Filter 1H PFG Double-Quantum experiments (Dalvit et al. 1998) at 500 MHz in H2O solution.
Distance restraints of the AF-6 PDZ domain were obtained from 3D 15N-separated NOESY and 13C-separated NOESY spectra acquired at 500 MHz, with mixing times 100 and 130 msec, respectively. Restraints within the unlabeled Bcr peptide were generated with 13C/15N-filtered 2D 1H NOESY spectra using 149 msec of mixing time. Intermolecular interactions were identified unambiguously in a 13C/15N-filtered (F1), 13C-edited (F3) 3D NOESY spectrum with a mixing time of 130 msec at 600 MHz.
Structure calculations
The structures were calculated using the program CNS v1.1 (Accelrys) (Brunger et al. 1998). NOE cross-peak intensities were classified as strong, medium, weak, and very weak, and assigned to restraints of 1.8–3.0 Å, 1.8–4.0 Å, 1.8–5.0 Å, and 1.8–6.0 Å, respectively. ϕ and ψ dihedral angle restraints were obtained based on analysis of 13Cα, 13Cβ, 13CO, and 1Hα chemical shifts using the program CSI (Wishart and Sykes 1994). Two hundred structures were calculated using torsion angle dynamics followed by Cartesian dynamics and minimization. Twenty structures of the lowest restraint violation energy were chosen to represent the solution structure of the AF-6 PDZ domain/Bcr peptide complex. Details of the input restraints and structural statistics are presented in Table 1. The geometrical quality of the resulting model was checked using the programs PROCHECK (Laskowski et al. 1996) and MOLMOL (Koradi et al. 1996).
Backbone 15N NMR relaxation measurements
15N T1, T2, and {1H}–15N steady-state NOE values were determined on the 500-MHz spectrometer at 298 K as described previously (Farrow et al. 1994). 15N T1 values were measured from HSQC spectra recorded with relaxation delays of 11.2, 61.6, 142, 243, 364, 525, 757, and 1150 msec. 15N T2 values were determined with relaxation delays of 0, 17.6, 35.2, 52.8, 70.4, 105.6, and 140.8 msec. {1H}–15N steady-state NOEs were obtained by recording spectra with and without 1H presaturation of duration 3 sec plus a relaxation delay of 5 sec at 500 MHz. All data were processed with Sparky and fitted with the program Fast-Modelfree (v1.1) (Cole and Loria 2003).
Data deposition
The structures of the AF-6 PDZ domain/Bcr peptide complex have been deposited in the Protein Data Bank with accession code 2AIN.
Electronic supplemental material
The Supplemental material includes spectra illustration of the NOEs between Val22 and Phe−4 (Supplemental Fig. S1) and concentration-dependent chemical shift changes of the AF-6 PDZ domain (Supplemental Fig. S2).
Acknowledgments
We thank Dr. Hy Zhou for providing the recombinant plasmid of the human AF-6 PDZ domain, Dr. Yd Yang for computer support, and Dr. Ym Zhou for peptide synthesis. Excellent technical assistance from Jh Zhang is gratefully acknowledged. We thank Dr. F. Delaglio and Prof. A. Bax for providing the software NMRPipe, Profs. T.D. Goddard and D.G. Kneller for providing Sparky, Prof. A.T. Brünger for providing the program CNS, Dr. R. Koradi and Prof. K. Wüthrich for providing MOLMOL, and Dr. L.D. Warren for providing PyMOL. This work was supported by the Chinese National Fundamental Research Project (Grants 2002CB713806, 2006CB806507, and 2006CB910201), the Chinese National Natural Science Foundation (Grants 30121001, 30570361, and 30670426), and the Key Project of the National High Technology Research and Development Program of China (Grant 2002BA711A13).
Footnotes
Supplemental material: see www.proteinscience.org
Reprint requests to: Yunyu Shi or Jihui Wu, School of Life Science, University of Science and Technology of China, Hefei, Anhui 230026, China; e-mail: yyshi@ustc.edu.cn or wujihui@ustc.edu.cn; fax: 86-551-3601443.
Abbreviations: AF-6, ALL-1 fusion partner from Chromosome 6; PDZ, PSD-95/discs large/ZO-1; AJ, adherens junction; PRR/nectin, the poliovirus receptor-related protein; JAM, junctional adhesion molecule; NOE, nuclear Overhauser effect; NOESY, nuclear Overhauser enhancement spectroscopy; COSY, correlated spectroscopy; TOCSY, total correlation spectroscopy; RMSD, root mean square deviation.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.062440607.
References
- Bezprozvanny I. and Maximov, A. 2001. Classification of PDZ domains. FEBS Lett. 509: 457–462. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. and Maximov, A. 2002. PDZ domains: Evolving classification. FEBS Lett. 512: 347–349. [DOI] [PubMed] [Google Scholar]
- Birrane G., Chung, J., and Ladias, J.A. 2003. Novel mode of ligand recognition by the Erbin PDZ domain. J. Biol. Chem. 278: 1399–1402. [DOI] [PubMed] [Google Scholar]
- Boettner B., Harjes, P., Ishimaru, S., Heke, M., Fan, H.Q., Qin, Y., Van Aelst, L., and Gaul, U. 2003. The AF-6 homolog canoe acts as a Rap1 effector during dorsal closure of the Drosophila embryo. Genetics 165: 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunger A.T., Adams, P.D., Clore, G.M., DeLano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., Pannu, N.S., et al. 1998. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54: 905–921. [DOI] [PubMed] [Google Scholar]
- Buchert M., Schneider, S., Meskenaite, V., Adams, M.T., Canaani, E., Baechi, T., Moelling, K., and Hovens, C.M. 1999. The junction-associated protein AF-6 interacts and clusters with specific Eph receptor tyrosine kinases at specialized sites of cell–cell contact in the brain. J. Cell Biol. 144: 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikumi H., Barac, A., Behbahani, B., Gao, Y., Teramoto, H., Zheng, Y., and Gutkind, J.S. 2004. Homo- and hetero-oligomerization of PDZ-RhoGEF, LARG, and p115RhoGEF by their C-terminal region regulates their in vivo Rho GEF activity and transforming potential. Oncogene 23: 233–240. [DOI] [PubMed] [Google Scholar]
- Cole R. and Loria, J.P. 2003. FAST-Modelfree: A program for rapid automated analysis of solution NMR spin-relaxation data. J. Biomol. NMR 26: 203–213. [DOI] [PubMed] [Google Scholar]
- Dalvit C., Ramage, P., and Hommel, U. 1998. Heteronuclear X-filter 1H PFG double-quantum experiments for the proton resonance assignment of a ligand bound to a protein. J. Magn. Reson. 131: 148–153. [DOI] [PubMed] [Google Scholar]
- Delaglio F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J., and Bax, A. 1995. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6: 277–293. [DOI] [PubMed] [Google Scholar]
- Doyle D.A., Lee, A., Lewis, J., Kim, E., Sheng, M., and MacKinnon, R. 1996. Crystal structures of a complexed and peptide-free membrane protein-binding domain: Molecular basis of peptide recognition by PDZ. Cell 85: 1067–1076. [DOI] [PubMed] [Google Scholar]
- Ebnet K., Schulz, C.U., Meyer Zu Brickwedde, M.K., Pendl, G.G., and Vestweber, D. 2000. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J. Biol. Chem. 275: 27979–27988. [DOI] [PubMed] [Google Scholar]
- Faderl S., Talpaz, M., Estrov, Z., O'Brien, S., Kurzrock, R., and Kantarjian, H.M. 1999. The biology of chronic myeloid leukemia. N. Engl. J. Med. 341: 164–172. [DOI] [PubMed] [Google Scholar]
- Farrow N.A., Muhandiram, R., Singer, A.U., Pascal, S.M., Kay, C.M., Gish, G., Shoelson, S.E., Pawson, T., Forman-Kay, J.D., and Kay, L.E. 1994. Backbone dynamics of a free and phosphopeptide-complexed Src homology 2 domain studied by 15N NMR relaxation. Biochemistry 33: 5984–6003. [DOI] [PubMed] [Google Scholar]
- Gross C., Heumann, R., and Erdmann, K.S. 2001. The protein kinase C-related kinase PRK2 interacts with the protein tyrosine phosphatase PTP-BL via a novel PDZ domain binding motif. FEBS Lett. 496: 101–104. [DOI] [PubMed] [Google Scholar]
- Hillier B.J., Christopherson, K.S., Prehoda, K.E., Bredt, D.S., and Lim, W.A. 1999. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS–syntrophin complex. Science 284: 812–815. [PubMed] [Google Scholar]
- Hofmann K. and Bucher, P. 1995. The FHA domain: A putative nuclear signalling domain found in protein kinases and transcription factors. Trends Biochem. Sci. 20: 347–349. [DOI] [PubMed] [Google Scholar]
- Hung A.Y. and Sheng, M. 2002. PDZ domains: Structural modules for protein complex assembly. J. Biol. Chem. 277: 5699–5702. [DOI] [PubMed] [Google Scholar]
- Im Y.J., Park, S.H., Rho, S.H., Lee, J.H., Kang, G.B., Sheng, M., Kim, E., and Eom, S.H. 2003. Crystal structure of GRIP1 PDZ6-peptide complex reveals the structural basis for class II PDZ target recognition and PDZ domain-mediated multimerization. J. Biol. Chem. 278: 8501–8507. [DOI] [PubMed] [Google Scholar]
- Kang B.S., Cooper, D.R., Jelen, F., Devedjiev, Y., Derewenda, U., Dauter, Z., Otlewski, J., and Derewenda, Z.S. 2003. PDZ tandem of human syntenin: Crystal structure and functional properties. Structure 11: 459–468. [DOI] [PubMed] [Google Scholar]
- Karthikeyan S., Leung, T., and Ladias, J.A. 2001. Structural basis of the Na+/H+ exchanger regulatory factor PDZ1 interaction with the carboxyl-terminal region of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 276: 19683–19686. [DOI] [PubMed] [Google Scholar]
- Koradi R., Billeter, M., and Wuthrich, K. 1996. MOLMOL: A program for display and analysis of macromolecular structures. J. Mol. Graph. 14: 29–32. [DOI] [PubMed] [Google Scholar]
- Kozlov G., Banville, D., Gehring, K., and Ekiel, I. 2002. Solution structure of the PDZ2 domain from cytosolic human phosphatase hPTP1E complexed with a peptide reveals contribution of the β2-Sβ3 loop to PDZ domain–ligand interactions. J. Mol. Biol. 320: 813–820. [DOI] [PubMed] [Google Scholar]
- Kraulis P.J. 1991. MOLSCRIPT: A program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24: 946–950. [Google Scholar]
- Laskowski R.A., Rullmannn, J.A., MacArthur, M.W., Kaptein, R., and Thornton, J.M. 1996. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 8: 477–486. [DOI] [PubMed] [Google Scholar]
- Matter K. and Balda, M.S. 2003. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 4: 225–236. [DOI] [PubMed] [Google Scholar]
- Merritt E.A. and Murphy, M.E. 1994. Raster3D Version 2.0: A program for photorealistic molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 50: 869–873. [DOI] [PubMed] [Google Scholar]
- Morais Cabral J.H., Petosa, C., Sutcliffe, M.J., Raza, S., Byron, O., Poy, F., Marfatia, S.M., Chishti, A.H., and Liddington, R.C. 1996. Crystal structure of a PDZ domain. Nature 382: 649–652. [DOI] [PubMed] [Google Scholar]
- Nourry C., Grant, S.G., and Borg, J.P. 2003. PDZ domain proteins: Plug and play! Sci. STKE 2003: RE7. [DOI] [PubMed] [Google Scholar]
- Ponting C.P. 1995. AF-6/cno: Neither a kinesin nor a myosin, but a bit of both. Trends Biochem. Sci. 20: 265–266. [DOI] [PubMed] [Google Scholar]
- Ponting C.P. and Benjamin, D.R. 1996. A novel family of Ras-binding domains. Trends Biochem. Sci. 21: 422–425. [DOI] [PubMed] [Google Scholar]
- Radziwill G., Erdmann, R.A., Margelisch, U., and Moelling, K. 2003. The Bcr kinase downregulates Ras signaling by phosphorylating AF-6 and binding to its PDZ domain. Mol. Cell. Biol. 23: 4663–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z., Fanning, A.S., Fu, C., Xu, J., Marfatia, S.M., Chishti, A.H., Crompton, A., Chan, A.C., Anderson, J.M., and Cantley, L.C. 1997. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Sci ence 275: 73–77. [DOI] [PubMed] [Google Scholar]
- Stricker N.L., Christopherson, K.S., Yi, B.A., Schatz, P.J., Raab, R.W., Dawes, G., Bassett Jr, D.E., Bredt, D.S., and Li, M. 1997. PDZ domain of neuronal nitric oxide synthase recognizes novel C-terminal peptide sequences. Nat. Biotechnol. 15: 336–342. [DOI] [PubMed] [Google Scholar]
- Su L., Hattori, M., Moriyama, M., Murata, N., Harazaki, M., Kaibuchi, K., and Minato, N. 2003. AF-6 controls integrin-mediated cell adhesion by regulating Rap1 activation through the specific recruitment of Rap1GTP and SPA-1. J. Biol. Chem. 278: 15232–15238. [DOI] [PubMed] [Google Scholar]
- Takai Y. and Nakanishi, H. 2003. Nectin and afadin: Novel organizers of intercellular junctions. J. Cell Sci. 116: 17–27. [DOI] [PubMed] [Google Scholar]
- Tochio H., Zhang, Q., Mandal, P., Li, M., and Zhang, M. 1999. Solution structure of the extended neuronal nitric oxide synthase PDZ domain complexed with an associated peptide. Nat. Struct. Biol. 6: 417–421. [DOI] [PubMed] [Google Scholar]
- Wagner G. 1997. An account of NMR in structural biology. Nat. Struct. Biol. 4S: 841–844. [PubMed] [Google Scholar]
- Wiedemann U., Boisguerin, P., Leben, R., Leitner, D., Krause, G., Moelling, K., Volkmer-Engert, R., and Oschkinat, H. 2004. Quantification of PDZ domain specificity, prediction of ligand affinity and rational design of super-binding peptides. J. Mol. Biol. 343: 703–718. [DOI] [PubMed] [Google Scholar]
- Wishart D.S. and Sykes, B.D. 1994. The 13C chemical-shift index: A simple method for the identification of protein secondary structure using 13C chemical-shift data. J. Biomol. NMR 4: 171–180. [DOI] [PubMed] [Google Scholar]
- Zhadanov A.B., Provance Jr, D.W., Speer, C.A., Coffin, J.D., Goss, D., Blixt, J.A., Reichert, C.M., and Mercer, J.A. 1999. Absence of the tight junctional protein AF-6 disrupts epithelial cell–cell junctions and cell polarity during mouse development. Curr. Biol. 9: 880–888. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Rehmann, H., Price, L.S., Riedl, J., and Bos, J.L. 2005. AF6 negatively regulates Rap1-induced cell adhesion. J. Biol. Chem. 280: 33200–33205. [DOI] [PubMed] [Google Scholar]
- Zhou H., Xu, Y., Yang, Y., Huang, A., Wu, J., and Shi, Y. 2005. Solution structure of AF-6 PDZ domain and its interaction with the C-terminal peptides from Neurexin and Bcr. J. Biol. Chem. 280: 13841–13847. [DOI] [PubMed] [Google Scholar]