Abstract
Traditionally, the adrenal gland has been considered an important endocrine component of the pathway to inhibit acute inflammation via hypothalamic corticotropin-releasing hormone (CRH)-mediated secretion of glucocorticoid. Immunoreactive CRH found in inflamed tissues is a potent proinflammatory factor. Using genetic and pharmacological models of CRH deficiency, we now show that CRH deficiency unmasks a major proinflammatory effect of epinephrine secreted from the adrenal medulla. Together, epinephrine and peripheral CRH stimulate inflammation, and glucocorticoid acts as a counterbalancing force in this regard. Our findings suggest that stimulation of the acute inflammatory response should be included with the other “fight-or-flight” actions of epinephrine.
Immune responses, both acquired and innate (1), are balanced between proinflammatory and antiinflammatory actions. Some immune regulators such as IL-1β mediate both roles, depending on their site of action. Corticotropin-releasing hormone (CRH; ref. 2) also has opposing, site-specific effects; although, traditionally, hypothalamic CRH within the hypothalamic–pituitary–adrenal axis has been considered to be on the antiinflammatory side of this equilibrium, immunoreactive CRH present in peripheral tissues has proinflammatory effects (3, 4).
To define the relative balance that peripheral CRH and adrenal glucocorticoids exert on the innate immune response, we quantitated the degree of inflammation in normal and CRH-deficient mice (5) after the subcutaneous administration of the seaweed polysaccharide carrageenin (3), a potent activator of the innate immune response (6). Mice were studied both before and after adrenalectomy, as well as after supplementation with exogenous glucocorticoids (7, 8). Although adrenalectomy is often employed experimentally to remove the source of endogenous glucocorticoids, this procedure also removes the adrenal medulla. During the course of these studies, we discovered that CRH deficiency unmasks a major proinflammatory effect of epinephrine, the principal catecholamine secreted by the adrenal medulla.
MATERIALS AND METHODS
Materials.
The CRH-receptor-1 antagonist, antalarmin (9), was a kind gift from G. P. Chrousos (National Institute of Child Health and Human Development, Bethesda, MD). The compound was dissolved in 100% EtOH (100 mg/500 μl EtOH) at 37°C for 5 min and then an equal volume of emulphor (BASF Bioresearch, Mt. Olive, NJ) was added. The stock solution was adjusted with sterile water to a final concentration of 15 mg/ml. Before carrageenin treatment, mice were administered i.p. either 1 ml of antalarmin solution (30 mg/kg) or 1 ml of vehicle (controls). Corticosterone plasma levels measured 7 h after the antagonist administration, the peak of the inflammatory response, were similar in antagonist and vehicle-treated mice (30.2 ± 5.7 μg/dl vs. 29.3 ± 7.4 μg/dl, respectively). Corticosterone (Aldrich), cholesterol (Sigma), and propranolol (Sigma) were commercially available.
Animals and Experimental Procedures.
All animal experiments were approved by the Animal Care and Use Committee of Children’s Hospital. Male, wild-type (WT) or CRH deficient (knockout, KO; ref. 5), 8- to 12-week-old, ≈30-g mice were used in all experiments. Mice were kept in a 12 h light/dark cycle (lights on at 7 am) and fed ad libitum. Mice were housed individually for 2 days before induction of inflammation to obtain basal corticosterone levels. Carrageenin-induced acute inflammation was performed as described (3). Briefly, 5 ml of air was injected subcutaneously between the scapulae on day 1. On day 2, 3 ml of 2% (wt/vol) carrageenin-λ (Sigma) in 0.9% NaCl was injected in the preformed pouch. Mice were killed 7 h later. The inflammatory exudates were aspirated. Their cellularity was evaluated as an index of the inflammatory reaction. Blood corticosterone was measured by using a commercial radioimmunoassay kit (Incstar, Stillwater, MN).
Adrenalectomy and sham adrenalectomy were performed by the retroperitoneal route under ketamine anesthesia as described (7), 6 days before the induction of inflammation. Corticosterone/cholesterol or vehicle (cholesterol alone) pellets weighing 35 mg were made as described (8) and implanted subcutaneously to provide constant circulating levels of corticosterone. A 35-mg 10% corticosterone/90% cholesterol (wt/wt) pellet implanted in adrenalectomized mice weighing 30–35 g restores circulating corticosterone levels to those normally found in the morning (≈3 μg/dl; ref. 8). To provide circulating corticosterone levels similar to those achieved by WT mice during inflammation (20–25 μg/dl; Fig. 1B), we implanted two 35-mg 50% corticosterone/50% cholesterol (wt/wt) at the time of adrenalectomy.
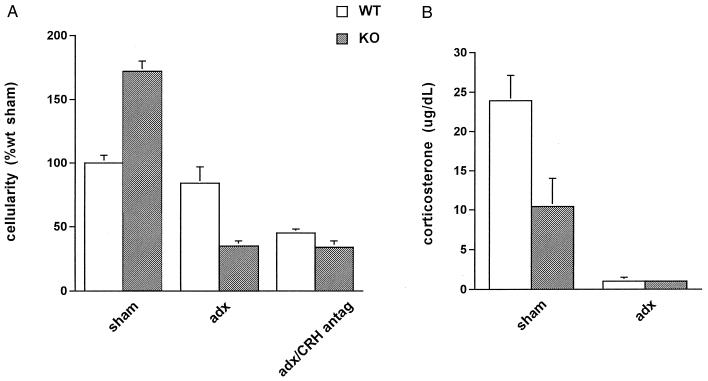
Figure 1.
Acute inflammatory response in WT (white bars) and CRH-deficient (KO; gray bars) mice. (A) Cellularity of the inflammatory exudate during carrageenin-induced acute inflammation (3), expressed as percentage of the cellularity of sham-adrenalectomized (sham) WT mice. CRH KO sham had higher cellularity than did WT sham (P < 0.05). Adrenalectomy (adx) had no significant effect on the cellularity of WT mice, whereas adrenalectomy significantly decreased the cellularity of CRH KO mice to 30% of WT sham (P < 0.05). Adrenalectomy combined with administration of CRH antagonist (adx/CRH antag) caused a significant reduction in exudate cellularity (P < 0.05) in WT mice, similar to that seen in the adx CRH KO group. CRH antagonist had no effect on the exudate cellularity of CRH KO mice. (B) Plasma corticosterone levels at the peak of the inflammatory response. Corticosterone levels of CRH KO sham were significantly lower than those of WT sham mice (P < 0.05), whereas levels in adrenalectomized mice were equally low in both genotypes.
Statistical Analysis.
In all experiments, each experimental group included 3–6 mice, and each individual experiment was performed three or more times. Statistical analysis was performed by ANOVA, with significance accepted at P < 0.05. In all figures, the cellularities shown represent mean values ± SEM from one representative experiment (expressed as percentage of the cellularity of WT/sham mice in that experiment, which averaged 65 ± 8 × 105 cells per ml in all experiments). Where standard error bars are not visible, they are less than the resolution of the figure.
RESULTS
Acute Inflammation in CRH Deficiency.
To examine the acute inflammatory response in the absence of CRH, we first administered carrageenin to WT and CRH KO mice. Although we expected the absence of peripheral CRH to be associated with less inflammation, CRH KO mice had a cellular inflammatory response 50% greater than that of WT mice (Fig. 1A, sham). We reasoned that this finding could be due to the much lower plasma corticosterone concentration in CRH KO mice after the inflammatory stimulus (Fig. 1B, sham) and therefore equalized corticosterone levels between genotypes by adrenalectomy of both groups (Figs. 1B, adx). As shown, adrenalectomy led to a 7-fold fall in the inflammatory response of CRH KO mice, whereas it had no effect in WT mice (Fig. 1A, adx), despite the dramatic fall in their corticosterone levels (Fig. 1B, adx). Thus, with intact adrenal glands, inflammation in CRH KO mice was more than 50% greater than in WT mice, whereas, after adrenalectomy, inflammation in CRH KO mice was one-third that of WT mice. This result suggested the presence of a proinflammatory factor within the adrenal gland whose effect is unmasked by CRH deficiency.
In the genetic model described above, the antiinflammatory effect of CRH deficiency was apparent only in the absence of the adrenal glands. We extended this observation in WT mice by using a pharmacological model of CRH deficiency (Fig. 1A, adx/CRH antag). When a CRH antagonist (9) was given to adrenalectomized WT mice, inflammation fell to levels observed in adrenalectomized CRH KO mice. As expected, the antagonist had no effect in CRH KO mice. Thus, pharmacological CRH deficiency, like genetic CRH deficiency, when coupled with adrenalectomy, results in suppression of inflammation. These findings suggested that both WT and CRH KO mice possess a proinflammatory adrenal factor that is unmasked by CRH deficiency.
Proinflammatory and Antiinflammatory Adrenal Factors.
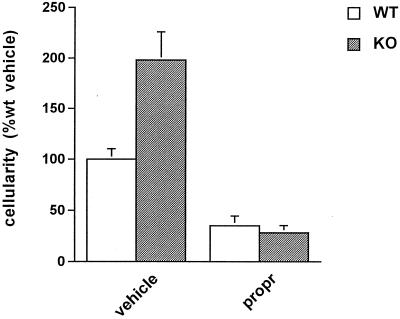
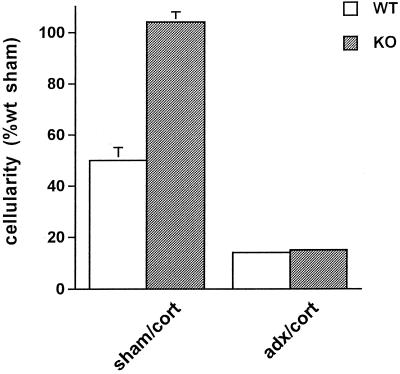
We reasoned that the proinflammatory factor present within the adrenal gland was unlikely to be a glucocorticoid because of the well known antiinflammatory activity of this class of hormones. Another major hormone of the adrenal gland is epinephrine, the absence of which, we considered, might be responsible for the decreased inflammatory responses observed after adrenalectomy in both CRH-KO and CRH-antagonist-treated WT mice. To test this hypothesis, we administered the specific β-adrenergic antagonist, propranolol, to both WT and CRH KO mice before they received carrageenin. We found that in both genotypes, propranolol significantly decreased the acute inflammatory response by more than 50% (Fig. 2). Thus, in WT mice, epinephrine deficiency, via β-adrenergic receptor blockade, coupled with an inflammation-induced rise in plasma corticosterone, was associated with a marked decrease in the acute inflammatory response (Fig. 2), whereas combined epinephrine and corticosterone deficiency (via adrenalectomy) was not (Fig. 1A, adx). To determine whether the level of corticosterone could explain this difference, we measured the acute inflammatory response in WT mice whose corticosterone levels (19.6 ± 2.3 μg/dl) were maintained at the same high level achieved after carrageenin administration to intact WT mice (23 ± 2 μg/dl). Corticosterone treatment reduced inflammation by ≈50% in WT mice with intact adrenal glands (Fig. 3, sham/cort). As before, adrenalectomy alone in WT mice had minimal effect on the inflammatory response (data not shown). When corticosterone treatment was combined with adrenalectomy, inflammation was reduced to ≈15% that of control WT mice (Fig. 3, adx/cort). These data, together with the results obtained after administration of propranolol, indicate that the inflammatory response is regulated by two counteracting adrenal factors: epinephrine, which stimulates the response, and corticosterone, which inhibits it. CRH KO mice with intact adrenal glands treated with the same dose of corticosterone also responded to glucocorticoid administration with a reduction in their inflammatory responses (Fig. 3, sham/cort). Interestingly, the degree of inflammation of corticosterone-treated, sham-adrenalectomized CRH KO mice was similar to that of WT sham-adrenalectomized mice not given supplemental corticosterone but higher than that of corticosterone-treated WT mice; however, after combined adrenalectomy and corticosterone administration, the inflammatory response was indistinguishable between the two genotypes (Figs. 1A and 3). The markedly lower inflammatory response of adrenalectomized CRH KO compared with adrenalectomized WT mice (Fig. 1A, adx) and the equalization of this response after adrenalectomy coupled with corticosterone supplementation (Fig. 3, adx/cort) support a proinflammatory role for peripheral CRH in the WT, a role that is apparent with adrenalectomy and masked by concomitant high-level corticosterone replacement.
Figure 2.
Effect of epinephrine on the acute inflammatory response. Cellularity of the inflammatory exudate after administration of either vehicle or the β-adrenergic antagonist, propranolol (propr), in WT (white bars) and CRH KO (gray bars) mice. Propranolol was administered i.p. at a dose of 10 mg/kg at the time of carrageenin injection. Propranolol reduced (P < 0.05) the cellularity of the exudate of both WT and CRH KO mice compared with untreated mice of the same genotype.
Figure 3.
Effect of glucocorticoid on the acute inflammatory response. The cellularity of the inflammatory exudate was measured in WT (white bars) or CRH KO (gray bars) mice treated either with sham adrenalectomy/corticosterone pellet supplementation (sham/cort) or with adrenalectomy/corticosterone pellet supplementation (adx/cort). Corticosterone administration to both WT and CRH KO mice, with or without adrenalectomy, significantly reduced (P < 0.05) the inflammatory response, compared with sham-adrenalectomized WT mice.
Synergistic Proinflammatory Effects of CRH and Epinephrine.
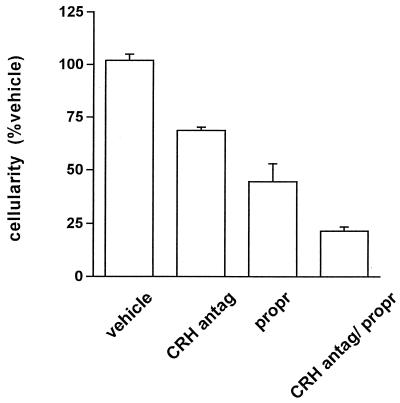
To evaluate the relative stimulatory contributions of CRH and epinephrine to acute inflammation, we performed an additional experiment in which CRH antagonist, β-adrenergic antagonist, or both were given to WT mice (Fig. 4). The antiinflammatory effects of these two antagonists seem additive, with inflammation inhibited most when both antagonists are present and impaired only partly in the absence of one or the other. Because CRH KO mice have epinephrine-dependent inflammation (Fig. 2), CRH is not required for the proinflammatory effect of epinephrine. Because adrenalectomized WT mice have CRH-dependent inflammation in the absence of epinephrine (Fig. 1A, adx/CRH antag), neither is epinephrine required for the proinflammatory effect of CRH.
Figure 4.
Relative contribution of CRH and epinephrine to the acute inflammatory response in WT mice. Cellularity of inflammatory exudates after administration of vehicle, CRH antagonist (CRH antag), propranolol (propr), or combination of both (CRH antag/propr). Although each agent alone inhibited the inflammatory response significantly, maximal inhibition was obtained by the combination of the two antagonists (P < 0.05, between all pairs).
DISCUSSION
Immunoreactive CRH is expressed in peripheral tissues in several models of inflammation in humans and rodents (3, 10–13). The mechanisms involved in the proinflammatory actions of CRH are not known but may include the stimulation of mast cell degranulation (14), nitric oxide-dependent vasodilatation (15), enhanced vascular permeability (16), leukocyte proliferation, and cytokine release by activated leukocytes (17). Our results and those of others (9) indicate that these actions of CRH are mediated to a significant extent by a CRH-receptor-1-dependent mechanism (18–19), which can also mediate the effects of urocortin, a recently identified CRH-like molecule (20).
Proinflammatory properties of epinephrine, reversible by β-receptor blockade or adrenal medullectomy, have been suggested by other in vivo models of inflammation (21–24). However, both immunosuppressive and immunostimulatory effects of catecholamines have been described (25), with several recent in vitro studies suggesting that catecholamines suppress the release of proinflammatory cytokines (26–27). Our data clearly establish the ability of epinephrine to augment the acute inflammatory response in vivo. Moreover, our models of CRH deficiency indicate that CRH largely masks this action of epinephrine. Possible mechanisms for epinephrine’s effects include stimulation of neutrophil infiltration (24), increased capillary permeability by stimulation of kinin release (28), stimulation of cytokine release (29), adhesion of leukocytes to endothelial cells (30), and stimulation of peripheral nerve-mediated inflammatory signals (21).
Our studies raise the question of whether variation in the endogenous secretion of epinephrine from the adrenal medulla modulates the inflammatory response. CRH-deficient mice have a low basal epinephrine level associated with decreased expression of phenylethanolamine-N-methyl transferase in the adrenal medulla.§,¶ Despite the low basal level, epinephrine secretion after inflammation increases to levels greater than those of WT mice.§ Perhaps this increased epinephrine secretion is responsible for the greater than normal level of carrageenin-induced inflammation found in CRH KO mice even after glucocorticoid supplementation (Fig. 3), because this inflammation is eliminated following the removal of epinephrine by adrenalectomy.
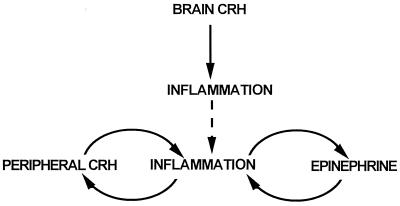
These data indicate that epinephrine and CRH can act independently to stimulate the acute inflammatory response. That blockade of both epinephrine and CRH action is necessary to maximally decrease inflammation suggests that both factors may ultimately act through a common pathway (Fig. 5). We suggest that CRH and inflammation interact in a positive-feedback, or feed-forward manner, because CRH increases inflammation (3), which leads to the greater production of CRH. Likewise, the relationship between epinephrine and inflammation may be similar, because epinephrine stimulates inflammation, and inflammatory mediators most likely stimulate the further release of epinephrine from the adrenal medulla. Such positive-feedback systems, once activated, would cause a rapid escalation in inflammation, which is one of the characteristics of the innate immune response (31). Glucocorticoid, stimulated by inflammatory mediators, down-regulates the proinflammatory response (32), either by inhibiting the expression of peripheral immunoreactive CRH (33) or through other pathways (34–36).
Figure 5.
Model of interaction of CRH, epinephrine, and glucocorticoid in regulating acute inflammation. Solid and dashed lines represent stimulatory and inhibitory effects, respectively.
Epinephrine, identified as the hormone of “fight-or-flight” in the early part of this century (37), prepares an organism under duress for emergency action by enhancing cardiorespiratory function and metabolic fuel availability. Because physical trauma is often sustained under these circumstances, anticipatory measures that would enhance wound healing, such as the stimulation of the acute inflammatory response (38), would be advantageous. Our findings suggest that stimulation of the acute inflammatory response should be included with the other fight-or-flight actions of epinephrine.
We have shown that epinephrine and CRH have additive roles in stimulating the innate immune response to acute inflammation, with the presence of either masking a deficiency in the other. More generally, our findings suggest that directed experimental manipulations in transgenic animals can identify aspects of physiology otherwise masked by complex systems, such as the inflammatory process, even in the presence of compensatory changes resulting from the transgenic state.
Acknowledgments
We thank Drs. George Chrousos and Elizabeth Webster for providing the CRH R1 antagonist antalarmin and its solubilizing agent, emulphor. This work was supported by National Institutes of Health Grants RO1 DK47977 (to K.K.) and RO1 DK50511 (to J.M.).
ABBREVIATIONS
- CRH
corticotropin-releasing hormone
- WT
wild type
- KO
knockout
Footnotes
Karalis, K. P., Kontopoulos, E. V., Pacak, K. & Majzoub, J. A., The Endocrine Society 80th Annual Meeting, June 24–27, 1998, New Orleans, abstr. P2-565.
Jeong, K.-H., Pacak, K., Jacobson, L., Widmaier, E. & Majzoub, J. A., The Endocrine Society 79th Annual Meeting, June 11–14, 1997, Minneapolis, abstr. OR24-2.
References
- 1.Fearon D T, Locksley R M. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 2.Vale W W, Spiess J, Rivier C, Rivier J. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 3.Karalis K, Sano H, Redwine J, Listwak S, Wilder R L, Chrousos G P. Science. 1991;254:421–424. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- 4.Turnbull A V, Rivier C. Endocrinology. 1996;137:455–463. doi: 10.1210/endo.137.2.8593789. [DOI] [PubMed] [Google Scholar]
- 5.Muglia L, Jacobson L, Dikkes P, Majzoub J A. Nature (London) 1995;373:427–431. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa H, Komorita N. Biochem Biophys Res Commun. 1993;194:1181–1187. doi: 10.1006/bbrc.1993.1947. [DOI] [PubMed] [Google Scholar]
- 7.Muglia L J, Jacobson L, Weninger S C, Luedke C E, Bae D S, Jeong K-H, Majzoub J A. J Clin Invest. 1997;99:2923–2929. doi: 10.1172/JCI119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson L. Endocrinology. 1999;140:310–317. doi: 10.1210/endo.140.1.6416. [DOI] [PubMed] [Google Scholar]
- 9.Webster E L, Lewis D B, Torpy D J, Zachman E K, Rice K C, Chrousos G P. Endocrinology. 1996;137:5747–5750. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- 10.Crofford L, Sano H, Karalis K, Webster E L, Goldmuntz E A, Chrousos G P, Wilder R L. J Clin Invest. 1992;90:2555–2564. doi: 10.1172/JCI116150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crofford L, Sano H, Karalis K, Friedman T C, Epps H R, Remmers E F, Mathern P, Chrousos G P. J Immunol. 1993;151:1587–1596. [PubMed] [Google Scholar]
- 12.Skopa C D, Mastorakos G, Melachrinou M, Merino M J, Chrousos G P. Am J Pathol. 1994;145:1159–1167. [PMC free article] [PubMed] [Google Scholar]
- 13.van Tol E A, Petrusz P, Lund P K, Yamanchi M, Sartor R B. Gut. 1996;39:385–392. doi: 10.1136/gut.39.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theoharides T C, Singh L K, Boucher W, Pang X, Letourneau R, Webster E, Chrousos G P. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 15.Clifton V L, Read M A, Leitch I M, Giles W B, Boura A L, Robinson P J, Smith R. J Clin Endocrinol Metab. 1995;80:2888–2893. doi: 10.1210/jcem.80.10.7559870. [DOI] [PubMed] [Google Scholar]
- 16.Jain V, Vedernikov Y P, Saade G R, Chwalisz K, Garfield R E. Am J Obstet Gynecol. 1997;176:234–248. doi: 10.1016/s0002-9378(97)80042-7. [DOI] [PubMed] [Google Scholar]
- 17.Karalis K, Muglia L J, Bae D, Hilderbrand H, Majzoub J A. J Neuroimmunol. 1997;72:131–136. doi: 10.1016/s0165-5728(96)00178-6. [DOI] [PubMed] [Google Scholar]
- 18.Perrin M H, Haas Y, Rivier J E, Vale W W. Endocrinology. 1986;118:1171–1176. doi: 10.1210/endo-118-3-1171. [DOI] [PubMed] [Google Scholar]
- 19.Chen R, Lewis K, Perrin M H, Vale W W. Proc Natl Acad Sci USA. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughan J, Donaldson C, Bittencourt J, Perrin M H, Lewis K, Sutton S, Chan R, Turnbull A V, Lovejoy D, Rivier C, et al. Nature (London) 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 21.Levine J D, Coderre J, Helms C, Bausbaum A I. Proc Natl Acad Sci USA. 1988;85:4553–4558. doi: 10.1073/pnas.85.12.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordling L, Lundberg T, Liedberg H, Theordsson E, Ekman P. Neurosci Lett. 1992;139:169–172. doi: 10.1016/0304-3940(92)90544-h. [DOI] [PubMed] [Google Scholar]
- 23.Bhalla T N, Sinha J N, Tangri K K, Bhargav K P. Eur J Pharmacol. 1970;13:90–96. doi: 10.1016/0014-2999(70)90187-1. [DOI] [PubMed] [Google Scholar]
- 24.Kaan S K, Mei Q B, Cho C H. Eur J Pharmacol. 1996;317:115–122. doi: 10.1016/s0014-2999(96)00705-4. [DOI] [PubMed] [Google Scholar]
- 25.Felten D L, Felten S Y, Bellinger D L, Carlson S L, Ackerman K D, Madden K S, Olschowski J A, Livnat S. Immunol Rev. 1987;100:225–260. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 26.Panina-Bordignon P, Mazzeo D, DiLucia P, D’Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F. J Clin Invest. 1997;100:1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramer-Quinn D S, Baker R A, Sanders V M. J Immunol. 1997;159:4857–4867. [PubMed] [Google Scholar]
- 28.Burns A M, Keogan M, Donaldson M, Brown D L, Park G R. Br J Anaesth. 1997;78:530–535. doi: 10.1093/bja/78.5.530. [DOI] [PubMed] [Google Scholar]
- 29.Severn A, Rapson N T, Hunter C A, Liew F Y. J Immunol. 1992;148:3441–3445. [PubMed] [Google Scholar]
- 30.Sung C P, Arleth A J, Storer B, Feuerstein G Z. Life Sci. 1992;49:375–382. doi: 10.1016/0024-3205(91)90445-h. [DOI] [PubMed] [Google Scholar]
- 31.Abbas A K, Lichtman A H, Pober J S. Cellular and Molecular Immunology. 2nd Ed. Philadelphia: Saunders; 1994. pp. 299–301. [Google Scholar]
- 32.Boumpas D T, Chrousos G P, Wilder R L, Cupps T R, Balow J E. Ann Intern Med. 1993;119:1198–1208. doi: 10.7326/0003-4819-119-12-199312150-00007. [DOI] [PubMed] [Google Scholar]
- 33.Karalis K, Mastorakos G, Sano H, Wilder R L, Chrousos G P. Endocrinology. 1995;136:4133–4138. doi: 10.1210/endo.136.9.7544277. [DOI] [PubMed] [Google Scholar]
- 34.Kunz D, Walker G, Eberhardt W, Pfeilschifter J. Proc Natl Acad Sci USA. 1996;93:255–259. doi: 10.1073/pnas.93.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Auphan N, DiDonato J A, Rosette C, Helmberg A, Korin M. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 36.Scheinman R I, Cogswell P C, Lolquist A, Badwin J A., Jr Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 37.Cannon W B. Am J Physiol. 1929;89:84–107. [Google Scholar]
- 38.Clark R A F. In: The Molecular and Cellular Biology of Wound Repair. 2nd Ed. Clark R A F, editor. New York: Plenum; 1995. pp. 3–50. [Google Scholar]