Abstract
This review addresses genes differentially expressed in the mammary gland transcriptome during the progression of mammary carcinogenesis in BALB/c mice that are transgenic for the rat neu (ERBB2, or HER-2/neu) oncogene (BALB-neuT664V-E mice). The Ingenuity knowledge database was used to characterize four functional association networks whose hub genes are directly linked to inflammation (specifically, the genes encoding IL-1β, tumour necrosis factor, interferon-γ, and monocyte chemoattractant protein-1/CC chemokine ligand-2) and are increasingly expressed during such progression. In silico meta-analysis in a human breast cancer dataset suggests that proinflammatory activation in the mammary glands of these mice reflects a general pattern of human breast cancer.
Introduction
Inflammation, the archetypal response to invasion, has evolved as a local protective response to life-threatening invasion; it is required to be rapid and devastating, sometimes regardless of the cost to the host. When viewed in the light of evolutionary pressure, the frequently disastrous long-term consequences of inflammation are a small price to pay for the protection against the immediate danger avoided.
It is now clear, however, that various distinct reactions are encompassed within the term 'inflammation'. Development of the immune system has led to sophisticated decoding of danger signals elicited by incursion of foreign bodies, and retaliatory responses are adjusted accordingly. The type of cells recruited, their state of activation, the reactive substances that they release, their guidance by cytokines and soluble factors, and the time frame over which the reaction occurs will all depend on both the type of signals and the ability of the invader to resist responses. Therefore, it is hardly surprising that some of the molecular mechanisms that sustain flares of inflammation may have opposing effects on the progression of early neoplastic lesions [1].
Acute inflammation cures lethal tumours
Acute inflammation can certainly destroy both initial and established neoplastic lesions. Indeed, much of our current understanding of tumour immunology can be traced back to Cooley's observations of the ability of the acute inflammation elicited by bacterial infections to cure human carcinomas [2]. Similar observations have also been reported in countless studies of experimental tumours. However, it has also been shown that tumours can be destroyed by a more focused assault triggered by double-strand polynucleotides [3] and unmethylated-CpG oligodesoxynucleotides [4]. Moreover, because cytokines guide inflammatory reactions, most can initiate and guide inflammatory patterns that have a distinct ability to inhibit tumour growth. The powerful anti-tumour activity of tumour necrosis factor (TNF)-α driven inflammation, for instance, is accompanied by dramatic adverse effects that confine its therapeutic use to isolated tissue perfusion [5]. IL-2 also recruits natural immunity mechanisms that exhibit marked anti-tumour activity in patients with advanced cancer [6]. Administration of minute amounts of IL-2 at the tumour site induces neutrophil infiltration, which produces dramatic, albeit temporary, regression [7].
Several lines of experimental data have shown that tumour cells engineered to release a cytokine trigger a strong inflammatory reaction, which results in acute tumour destruction and induction of tumour-specific immune memory [8]. Systematic investigation of the reaction elicited by a mouse mammary adenocarcinoma cell line (TSA) transfected with several cytokine genes has shown that the cytokines produced induce distinct local inflammatory reactions. Neutrophils are the dominant infiltrating cells recruited by TSA-IL-2 cells (TSA cells transfected with the gene encoding IL-2) [9], eosinophils by TSA-IL-4 and TSA-IL-5 cells [10,11], natural killer cells by TSA-IL-12 cells [12], and macrophages by TSA-IFN-γ cells [10]. Other cytokines such as IL-7 [13], TNF-α [13] and IFN-α [14], or chemokines such as CC chemokine ligand (CCL)16 (liver expressed chemokine [LEC]) [15] activate acute inflammatory reactions and recruit mixed leucocyte populations. Although reactions elicited by cytokines are markedly different histologically, those elicited by TSA tumour cells engineered to release IL-2, IL-4, IL-7, IL-12, TNF-α, IFN-γ and LEC/CCL16 rapidly neutralize a TSA challenge that would otherwise be lethal. Other cytokines only delay tumour growth (IFN-γ) or have no inhibitory effect (IL-5 and IL-6) [10,11,13]. Similarly, protective inflammatory reactions are triggered through repeated local injection of recombinant cytokines [8,16,17].
Inflammation modulates neu (ERBB2, or HER-2/neu) driven mammary carcinogenesis
The time frame obviously differentiates acute inflammation from the chronic reactions that set the stage for initiation of carcinogenesis and its progression. Because chronic inflammation is a long-lasting response to an enduring invasion, it can persist for a significant period of time in the life of the individual. Prolonged release of a mixture of highly reactive oxidants secreted by infiltrating leucocytes may damage the genomes of nearby cells and increase their rate of mutation [18]. In addition to this direct oncogenic effect, a chronically inflamed microenvironment possesses many mechanisms with which to promote progression of a preneoplastic lesion, as described elsewhere in this series of review articles.
Cancer-prone transgenic mice provide models of autochthonous tumour formation. We conducted a microarray gene expression analysis to determine how proinflammatory genes are switched on during the stepwise progression of neu oncogene driven mammary carcinogenesis in BALB/c mice.
BALB/c mice transgenic for the transforming rat neu664V-E oncogene under the transcriptional control of the mouse mammary tumour virus promoter (BALB-neuT664V-E mice) are genetically predestined to develop one of the most aggressive forms of mammary carcinogenesis, with penetrance of all of their mammary glands. Starting from the week 3 or 4 of age, the protein product of the neu oncogene (p185neu) is diffusely over-expressed in the terminal buds of mammary glands of virgin mice. The over-expressing cells form foci of atypical hyperplasia. At around 8 weeks of age, these enlarge to in situ carcinomas that progress to invasive cancer between weeks 17 and 22. At around weeks 17 to 18 one or more tumours are palpable in the mammary pad of all mice, and at around week 33 a tumour is palpable in each of the 10 mammary glands [19]. The p185neu over-expression in the thymus and mammary gland has a marked effect on the T-cell repertoire of these mice, and CD8+ T-cell clones reacting with dominant p185neu epitopes are deleted [20]. Moreover, during progression of the mammary lesions, both suppressor CD4+ CD25+ FOXP3+ GITR+ Treg cells [21] and CD11b+ Gr1+ immature myeloid cells [22] expand. This mammary carcinogenesis appears to be driven in a straightforward manner by the transgenic neu oncogene, which provides abnormal growth signals and inhibits cell death pathways [23].
Because the genetic alteration is the driving force of tumor development in the BALB-neuT664V-E model, and because the mammary gland is not commonly susceptible to chronic inflammation, interactions between the incipient tumour and its inflammatory microenvironment presumably do not play a major role. In contrast, progression of this devastating form of carcinogenesis is markedly modulated by the inflammatory microenvironment. The inflammation-related reaction elicited by repeated injections of recombinant IL-12 during the early stages of carcinogenesis results in restricted and delayed tumour formation [24], mostly resulting from the ability of IL-12 to trigger innate immunity reaction mechanisms and induce downstream factors that inhibit the angiogenic switch, which promotes progression from in situ to invasive cancer [25]. Conversely, a marked modulation of the progression of neu carcinogenesis is evident in mice in which the gene encoding IFN-γ [26] or that encoding monocyte chemo-attractant protein (MCP)-1/CCL2 [27] has been knocked out.
Inflammatory gene expression during neu carcinogenesis
We have used DNA microarray technology to compare patterns of transcription in 2-week pregnant wild-type BALB/c mice with those in the mammary glands of BALB-neuT664V-E mice during progression of neu carcinogenesis [28,29]. We also characterized the transcription profile in the gland when carcinogenesis is halted by immune mechanisms triggered by cell [30] and DNA-based anti-neu vaccines [28,31]. Meta-analysis of these transcription profiles [32] has provided suggestions regarding possible new onco-antigens for use in anti-tumour vaccines.
A recent transcription profiling study using full, genome-wide mouse arrays was run to identify changes in transcription in BALB-neuT664V-E mammary glands progressing from atypical hyperplasia and in situ carcinomas (10- to 15-week-old mice) to invasive cancer (19- to 22-week-old mice) (unpublished data; the microarray dataset has been released, GSE7395, on the GEO database, http://www.ncbi.nlm.nih.gov/geo). The total RNAs extracted from mammary glands were analyzed using Mouse Genome Survey Microarrays (Applied Biosystems, Foster City, CA, USA). Identification of differentially expressed genes associated with the transition from the preneoplastic state to neoplasia was done by means of a linear modelling approach [33]. An empirical Bayesian method [34] was used to assess differential expression, along with false discovery rate correction of the P value [35] to moderate the standard errors of the estimated log-fold changes. We identified 2,758 differentially expressed probes (2,651 transcripts) by applying a false discovery rate of 0.05, associated with an absolute log2 (fold change) threshold of 1. These differentially expressed genes represent the element of the mammary gland transcriptome whose expression changes as the tumour microenvironment evolves and the tumour mass increases. Therefore, these transcripts are defined as genes associated with mammary tumour microenvironment (GATMs).
The Ingenuity Pathways Knowledge Base [36] is currently the world's largest database of knowledge on biological networks, with annotation curated by experts. We exploited this database to define the presence of functional associations within the GATMs and to identify differences between the ontological gene classes [37] that were enriched among the upregulated and downregulated genes (Figure 1). This ontological gene classification provides the controlled vocabulary to describe gene and gene product attributes. The principal Ingenuity ontological classes found to be enriched in BALB-neuT664V-E GATM sets are those related to cellular movement and cellular growth and proliferation. However, among the other significantly enriched groups were genes belonging to the classes inflammatory diseases, haematological system development and function, and immune and lymphatic system development and function. This highlights the collaboration between transformed cells and body defence systems during initial tumour development.
Figure 1.
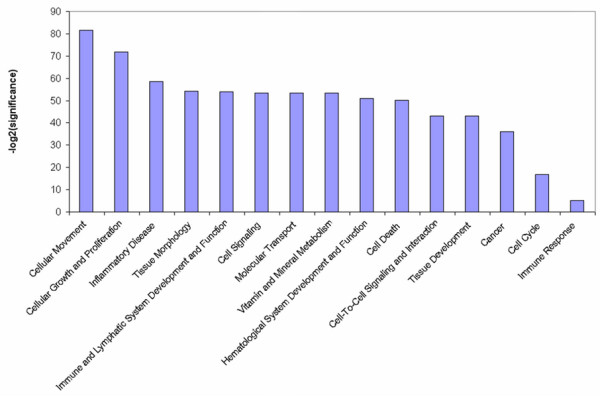
Most significant Ingenuity functional classes found to be enriched in GATMs. GATM, gene associated with mammary tumour microenvironment.
Furthermore, we generated significant functional association networks [37] encompassing GATMs (Table 1). These are graphical descriptions of literature associations identified by the Ingenuity knowledge database, in which gene products are linked if some kind of experimentally measured association has been reported. The 14 most significant gene networks, each composed of 35 GATMs (nodes), are characterized by the presence of one hub gene (the highest connected node), which is the main player in the biological events that connect the GATMs. Remarkably, four of these 14 networks are highly associated with the inflammatory response: TNF (Figure 2), IFN-γ (Figure 3), IL-1β (Figure 4) and MCP-1/CCL2 (Figure 5).
Table 1.
Functional network generated by Ingenuity knowledge database analysis
| Hub gene | Score | GATMs | Top functions |
| TNF↑ | 34 | 35 | Lipid metabolism, small molecule biochemistry, cell death |
| IFNG↑ | 34 | 35 | Lipid metabolism, molecular transport, small molecule biochemistry |
| IL1B↑ | 34 | 35 | Immune response, cellular movement, haematological system development and function |
| MYC↑ | 34 | 35 | Cell cycle, cellular movement, cancer |
| CCL2↑, KDR↓, VEGF↓ | 34 | 35 | Cellular movement, cancer, tumour morphology |
| SRC↑ | 34 | 35 | Haematological system development and function, immune and lymphatic system development and function, tissue morphology |
| BCL2↑ | 34 | 35 | Cancer, cell death, cell morphology |
| AGT↓ | 34 | 35 | Cell-to-cell signalling and interaction, haematological system development and function, immune and lymphatic system development and function |
| TIMP1↑, EDN1↓ | 34 | 35 | Cancer, cellular movement, cellular development |
| LEP↓ | 34 | 35 | Lipid metabolism, molecular transport, small molecule biochemistry |
| AR↓ | 34 | 35 | Cellular growth and proliferation, gene expression, cellular development |
| IGF2↓, ERBB3↑ | 34 | 35 | DNA replication, recombination, and repair, cancer, cell cycle |
| HOXA9↓, CDH1↑ | 34 | 35 | Cellular growth and proliferation, cell death, cancer |
| ARNT2↑, ZAP70↑ | 34 | 35 | Cellular growth and proliferation, haematological system development and function, immune response |
| PPARA↓, IL6, SERBF1 | 15 | 24 | Lipid metabolism, small molecule biochemistry, molecular transport |
| CDC42, RAC1 | 13 | 22 | Cell signalling, free radical scavenging, nucleic acid metabolism |
| ERBB2↑ | 12 | 17 | Cancer, tumour morphology, cell cycle |
| FN1 | 12 | 21 | Cell-to-cell signalling and interaction, cellular movement, reproductive system development and function |
| FOS | 12 | 21 | Gene expression, cell cycle, haematological system development and function |
| IGF1, CCND1, BMP2 | 12 | 21 | Cellular growth and proliferation, connective tissue development and function, skeletal and muscular system development and function |
| IL4 | 12 | 21 | Cellular assembly and organization, cell cycle, DNA replication, recombination, and repair |
| NFYB | 11 | 16 | Cancer, cell cycle, cellular assembly and organization |
| IL13, MAPK8, NFKB1 | 11 | 20 | Haematological system development and function, immune and lymphatic system development and function, tissue morphology |
| STAT3, INSR, PTPN | 11 | 20 | Cellular growth and proliferation, haematological system development and function, immune response |
| NFKBIA, CD40LG, HGF | 11 | 20 | Cell death, cellular movement, haematological system development and function |
| KRAS, CDKN1A | 10 | 19 | Cellular growth and proliferation, cell signalling, cancer |
| TNF↑ | 10 | 19 | Carbohydrate metabolism, molecular transport, small molecule biochemistry |
Out of the 55 available networks, only those containing at least 10 genes associated with mammary tumour microenvironment (GATMs) are shown. ↑ and ↓ indicate gene expression increasing and decreasing on passing from the pre-neoplastic condition to neoplasia, respectively.
Figure 2.
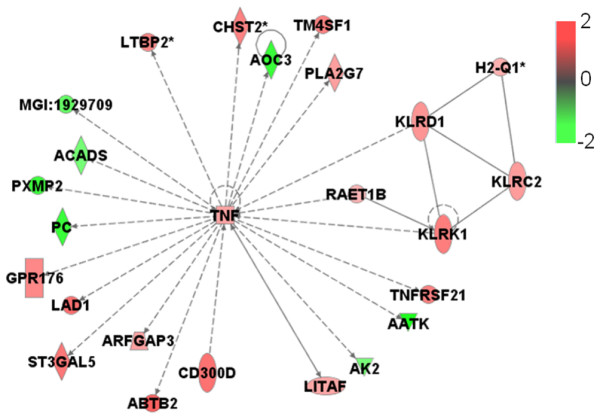
Network of functional association between TNF gene and other GATMs generated by Ingenuity database analysis. RAET1B, KLRD1 and KLRK1 are genes associated with cytotoxicity; TNFRSF21 and AATK are genes involved in apoptosis. LITAF, AK2 and KLRC2 are genes associated with proliferation regulation. PLA2G7 is a gene associated with inflammatory response. All of the other genes have unknown cell functions. Genes are shown by their symbols [44]. The nodes represent the genes and the edges reflect direct links or connections between them. GATM, gene associated with mammary tumour microenvironment; TNF, tumour necrosis factor.
Figure 3.
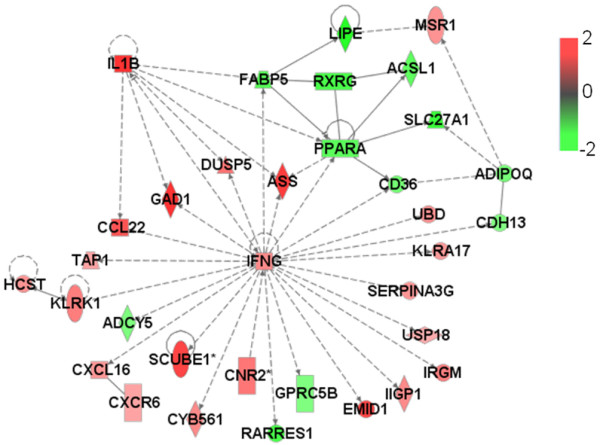
Network of functional association between IFN-γ gene and other GATMs generated by Ingenuity database analysis. ADIPOQ, CCL22, IFNG, IL1B, PPARA, RXRG, MSR1, GAD1 and TAP1 are genes associated with proliferation, whereas USP18 and CDH13 are genes linked to growth. ASS, DUSP5, ADCY5 and UBD are genes associated with apoptosis/survival. CXCR6, CXCL16 and CNR2 are genes associated with chemotaxis/trafficking; KLRK1 and HCST are associated with cytolysis/cytotoxicity; and RARRES1 and CD36 are linked to migration. All of the other genes have unknown cell functions. Genes are shown by their symbols. GATM, gene associated with mammary tumour microenvironment; IFN, interferon.
Figure 4.
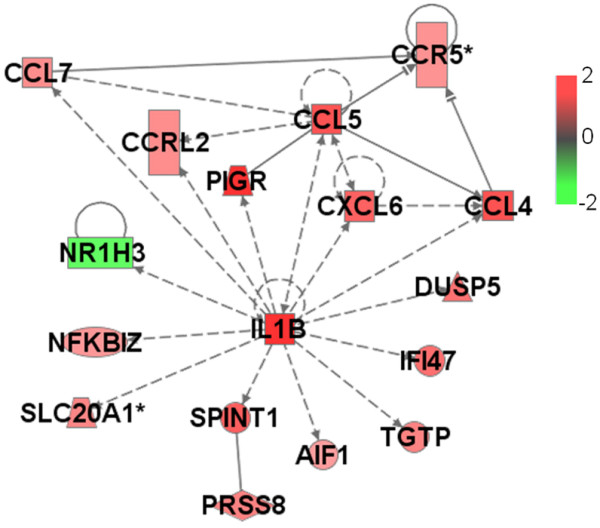
Network of functional relationships between IL-1β gene and other GATMs generated by Ingenuity database analysis. AIF1, CCL4, CCL5, CCL7, CXCL6, IL1B, NFKBIZ and PIGR are associated with the inflammatory response. All of the other genes have unknown cell functions. Genes are shown by their symbols. GATM, gene associated with mammary tumour microenvironment; IL, interleukin.
Figure 5.
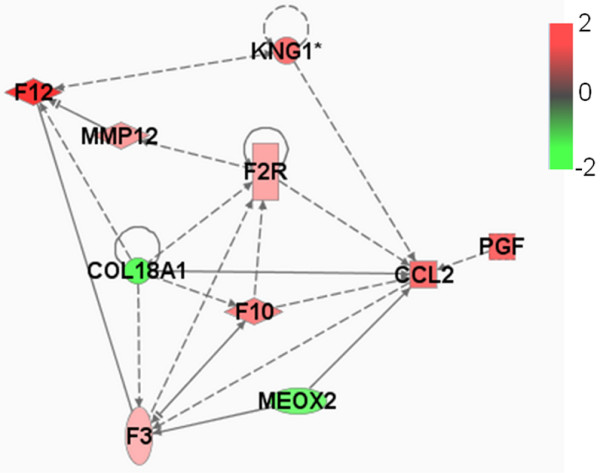
Network of functional association between CCL2 gene and other GATMs generated by Ingenuity database analysis. F3, F2R, F12, F10 and KNG1 are part of the complement and coagulation cascades. MMP12 encodes a matrix metalloproteinase involved in tissue remodelling. HBEGF and PGF encode growth factors. Associations with VEGF and KDR have been omitted for the sake of legibility. Genes are shown by their symbols. CCL, CC chemokine ligand; GATM, gene associated with mammary tumour microenvironment.
During neu carcinogenesis, these four functional association networks, centred on genes that encode proinflammatory molecules, can be defined. To ensure that these networks were not an artefact of the experimental model, we assessed their expression in breast cancer specimens in the light of recently published data exploring associations between recurrent copy number abnormalities, gene expression, and clinical outcomes in a set of aggressively treated early-stage breast tumours [38].
The presence of GATMs associated with TNF, IL-1β, IFN-γ, and MCP-1/CCL2 hub genes, and characterized by human orthologs (a total of 77 genes), was sought in the dataset reported by Chin and coworkers [38]. This is one of the largest collections of breast cancer samples (n = 118) analyzed by microarray and for which the clinical outcome is known. The study showed that the accuracy with which early-stage breast cancer patients can be stratified according to outcome can be improved by combining analyses of gene expression and genome copy number.
We identified 65 out of 77 GATMs. Hierarchical clustering of their expression profile (a method for dividing a dataset into subsets whose individual data ideally share some common traits [37]) was used to group the sample reported by Chin and coworkers into three subsets (Figure 6). This clustering was based on a proinflammatory gene signature, and it revealed different types or degrees of leucocyte infiltration. This suggests that proinflammatory activation in the mammary glands of BALB-neuT664V-E mice reflects a general pattern of human breast cancer. Only a limited overlap between the A group and a subset of samples characterized by negative oestrogen receptor status was observed (data not shown). This is not surprising, because our study addressed BALB-neuT664V-E carcinogenesis sequentially, whereas only full-blown cancers were considered in the report by Chin and coworkers [38]. Moreover, sampling bias in the human setting may mask any correlation with clinical outcome.
Figure 6.
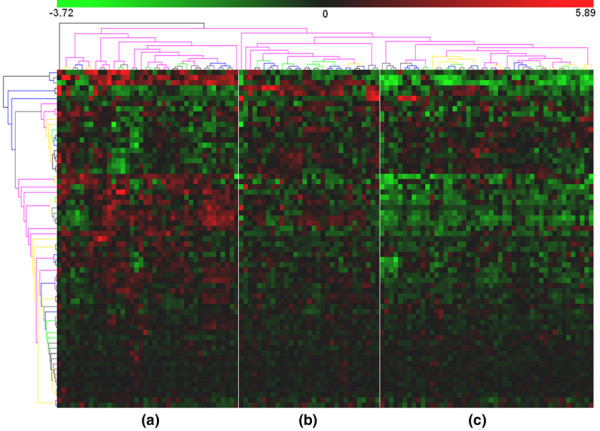
Clustering of expression of genes associated to inflammation. Shown is hierarchical clustering of gene-centred expression of the 65 genes present in the IFN-γ, TNF, IL-1β and MCP-1/CCL2 gene functional association networks. Samples from the dataset, presented by Chin and coworkers [38], cluster in three groups (A, B and C) if the expression levels of the proinflammatory genes are used. CCL, CC chemokine ligand; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; TNF, tumour necrosis factor.
A complementary dataset that could corroborate our observations is that report by Neve and coworkers [39]. Those investigators found that the recurrent genomic and transcriptional characteristics of a large set of breast cancer cell lines mirrored those of primary breast tumours, although some significant differences were documented.
The work reported by Chin [38] and Neve [39] and their colleagues offers a unique opportunity to discriminate between transcripts that belong to the tumour microenvironment and those associated with tumour cells. Taking advantage of these transcriptional profiles [38,39] and specific data mining techniques [40], one can determine whether a set of genes shares little expression similarity between tumour specimens and cell lines. This would suggest that their expression is linked to cell infiltrates and does not belong to tumour cells. Such a scenario was actually observed for the set of proinflammatory genes that we identified in BALB-neuT664V-E mice because of the absence of correlation [40] between gene expression observed in tumour specimens [38] and in tumour cell lines [39] (Figure 7).
Figure 7.
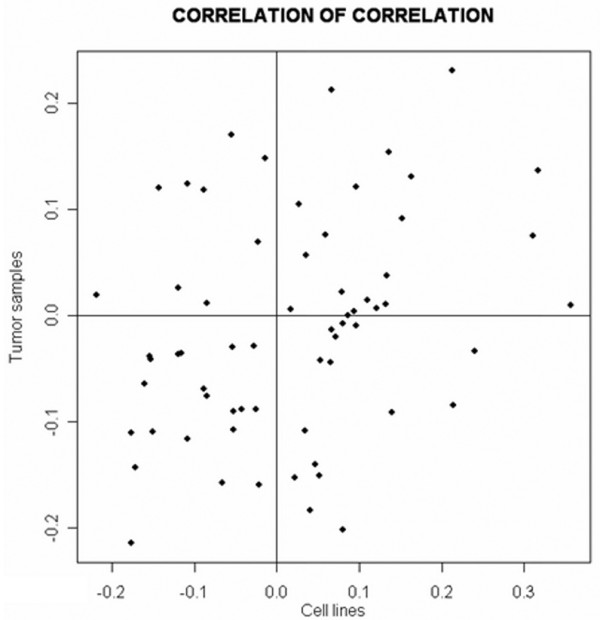
Scatterplot of pair-wise correlation comparison within the probe sets in the two datasets. Integrative correlation coefficient [40] was used to quantify the extent of the similarity between the tumour specimens and breast cancer cell line transcription profiles. All pair-wise correlations (Pearson correlation coefficient) of gene expression across samples within individual projects were calculated, and the reproducibility of the results was defined without relying on direct comparison of expression across platforms.
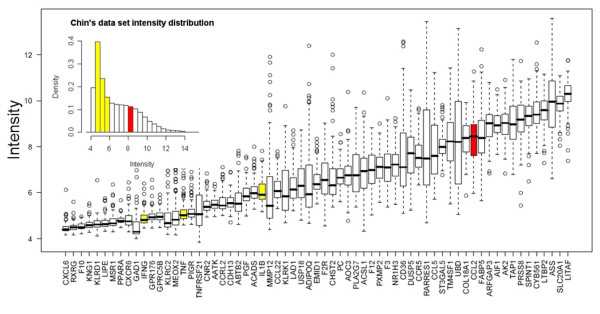
It should nonetheless be noted that the gene encoding MCP-1/CCL2 is the only one of the four hub genes (those encoding IFN-γ, TNF, IL-1β and MCP-1/CCL2) that constantly exhibit relatively high levels of expression (Figure 8).
Figure 8.
Box-plot of the expression level distributions of the 65 GATMs presented by Chin and coworkers. The IFN-γ, TNF, IL-1β and MCP-1/CCL2 hub genes are shown in grey. The inset figure shows their expression levels within the intensity distribution of tumour dataset presented by Chin and coworkers [38]. CCL, CC chemokine ligand; GATM, gene associated with mammary tumour microenvironment; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; TNF, tumour necrosis factor.
Conclusion
The neu oncogene is the central driving force of the mammary cancer that inexorably kills all female BALB-neuT664V-E mice. Even so, the timeframe within which neu carcinomas appear and progress is modulated by the proinflammatory components of the reactive stroma that surrounds the cancer lesions.
Our transcription profiling search for modulation of GATMs during the progression of neu mammary lesions in BALB-neuT664V-E mice generated a large body of data. The Ingenuity knowledge database was used to identify four functional association networks whose hub genes are directly linked to inflammation (namely, the genes that encode IFN-γ, TNF, IL-1β and MCP-1/CCL2). Data from such analyses clearly show that the progression of neu-driven autochthonous carcinogenesis is directly associated with increased expression of these four hub GATMs. These data were from a representative but artificial experimental model. However, an in silico meta-analysis in a human breast cancer dataset suggests that proinflammatory activation in the mammary glands of BALB-neuT664V-E mice reflects the general pattern of human breast cancer.
However, the way in which the proinflammatory cytokines encoded by these genes affect the progression of neu carcinogenesis is neither simple nor unequivocal. Different amounts of the same cytokine in the tumour microenvironment may have different influence, whereas the significance of this influence can fluctuate during the distinct stages of cancer progression. For instance, increased expression of IFN-γ in the tumour microenvironment during neu tumour progression does not appear to favour tumour expansion but plays a major inhibitory role. In BALB-neuT664V-E IFN-γ knockout mice, absence of IFN-γ leads to accelerated progression of neu carcinogenesis [26]. Work in progress suggests that this more rapid progression is associated with a major increase in tumoural neoangiogenesis (Iezzi M, unpublished data). Indeed, it is well known that IFN-γ, as well as the downstream factors that it induces, have marked anti-angiogenic activity that naturally restricts tumour neoangiogenesis and hampers tumour growth [41].
Moreover, our work on administration of recombinant mouse IL-12 shows that IL-12 mediated inhibition of neu carcinogenesis relies on production of IFN-γ in the tumour microenvironment [41]. In BALB-neuT664V-E IFN-γ knockout mice, IL-12 no longer impairs tumour growth [25]. We have also found that the ability of IL-12 induced IFN-γ to inhibit the progression of neu carcinogenesis is related to tumour stage. It is very marked during the tumour-induced angiogenic switch that accompanies the passage from an in situ lesion to invasive cancer. The newly formed capillary sprouts that characterize the early events in the angiogenic switch are sensitive to the inhibitory activity of IFN-γ, whereas this activity is nearly absent in the stages that precede and follow the switch [24].
Our data show that the expression of the gene that encodes the MCP-1/CCL2 chemokine increases as neu carcinogenesis progresses. The presence of this chemokine in the reactive stroma around the tumour appears to be directly associated with enhanced progression, because in BALB-neuT664V-E MCP-1/CCL2 knockout mice progression is slower and mice survive longer [27].
The influence of different levels of TNF and IL-1β has not been assessed in BALB-neuT664V-E mice. However, data from other experimental systems suggest that local increases in these factors may favour cancer progression. Skin carcinogenesis is dramatically impaired in TNF-α knockout mice [42]. In various experimental models, IL-1β appears to increase tumour invasiveness and metastasis [43].
In conclusion, our Ingenuity analysis identifies four genes that encode inflammatory cytokines whose increased expression in the tumour microenvironment is naturally associated with mammary cancer progression. Much greater concentrations of the very same cytokines, artificially reached in the tumour microenvironment either through their local injection or from cells engineered to release them, lead to both tumour rejection and induction of long-lasting tumour-specific immune memory [10].
Abbreviations
CCL = CC chemokine ligand; GATM = gene associated with mammary tumour microenvironment; IFN = interferon; LEC = liver expressed chemokine; MCP = monocyte chemoattractant protein; TNF = tumour necrosis factor.
Competing interests
The authors declare that they have no competing interests.
Note
This article is part of a review series on Inflammation and breast cancer, edited by Mina J Bissell and Jeffrey W Pollard.
Other articles in the series can be found online at http://breast-cancer-research.com/articles/review-series.asp?series=bcr_Inflammation
Acknowledgments
Acknowledgements
This work was supported by grants from Italian Association for Cancer Research; the Italian Ministero dell'Università e della Ricerca; the University of Torino; the Compagnia di San Paolo, Torino; the Fondazione Denegri, Torino; and the Regione Piemonte: bando regionale sulla ricerca scientifica applicata 2004.
The Authors wish to thank Ms Irene F Merighi for her skilled technical assistance.
References
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Nauts HC, McLaren JR. Coley toxins: the first century. Adv Exp Med Biol. 1990;267:483–500. doi: 10.1007/978-1-4684-5766-7_52. [DOI] [PubMed] [Google Scholar]
- Weinstein AJ, Gazdar AF, Sims HL, Levy HB. Lack of correlation between interferon induction and antitumour effect of poly I-poly C. Nat New Biol. 1971;231:53–54. doi: 10.1038/231053a0. [DOI] [PubMed] [Google Scholar]
- Krieg AM. CpG motifs: the active ingredient in bacterial extracts? Nat Med. 2003;9:831–835. doi: 10.1038/nm0703-831. [DOI] [PubMed] [Google Scholar]
- Lejeune FJ, Lienard D, Matter M, Ruegg C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 2006;6:6. [PubMed] [Google Scholar]
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- Cortesina G, De Stefani A, Galeazzi E, Cavallo GP, Badellino F, Margarino G, Jemma C, Forni G. Temporary regression of recurrent squamous cell carcinoma of the head and neck is achieved with a low but not with a high dose of recombinant interleukin 2 injected perilymphatically. Br J Cancer. 1994;69:572–576. doi: 10.1038/bjc.1994.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni G, Santoni A, Frati L. Cytokine-Induced Tumor Immunogenicity. London, UK: Academic Press; 1994. [Google Scholar]
- Cavallo F, Giovarelli M, Gulino A, Vacca A, Stoppacciaro A, Modesti A, Forni G. Role of neutrophils and CD4+T lymphocytes in the primary and memory response to nonimmunogenic murine mammary adenocarcinoma made immunogenic by IL-2 gene. J Immunol. 1992;149:3627–3635. [PubMed] [Google Scholar]
- Musiani P, Modesti A, Giovarelli M, Cavallo F, Colombo MP, Lollini PL, Forni G. Cytokines, tumour-cell death and immunogenicity: a question of choice. Immunol Today. 1997;18:32–36. doi: 10.1016/S0167-5699(97)80012-6. [DOI] [PubMed] [Google Scholar]
- Di Carlo E, Modesti A, Coletti A, Colombo MP, Giovarelli M, Forni G, Diodoro MG, Musiani P. Interaction between endothelial cells and the secreted cytokine drives the fate of an IL4- or an IL5-transduced tumour. J Pathol. 1998;186:390–397. doi: 10.1002/(SICI)1096-9896(199812)186:4<390::AID-PATH194>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Cavallo F, Signorelli P, Giovarelli M, Musiani P, Modesti A, Brunda MJ, Colombo MP, Forni G. Antitumor efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12. J Natl Cancer Inst. 1997;89:1049–1058. doi: 10.1093/jnci/89.14.1049. [DOI] [PubMed] [Google Scholar]
- Allione A, Consalvo M, Nanni P, Lollini PL, Cavallo F, Giovarelli M, Forni M, Gulino A, Colombo MP, Dellabona P. Immunizing and curative potential of replicating and nonreplicating murine mammary adenocarcinoma cells engineered with interleukin (IL)-2, IL-4, IL-6, IL-7, IL-10, tumor necrosis factor alpha, granulocyte-macrophage colony-stimulating factor, and gamma-interferon gene or admixed with conventional adjuvants. Cancer Res. 1994;54:6022–6026. [PubMed] [Google Scholar]
- Ferrantini M, Giovarelli M, Modesti A, Musiani P, Modica A, Venditti M, Peretti E, Lollini PL, Nanni P, Forni G. IFN-alpha 1 gene expression into a metastatic murine adenocarcinoma (TS/A) results in CD8+T cell-mediated tumor rejection and development of antitumor immunity. Comparative studies with IFN-gamma-producing TS/A cells. J Immunol. 1994;153:4604–4615. [PubMed] [Google Scholar]
- Giovarelli M, Cappello P, Forni G, Salcedo T, Moore PA, LeFleur DW, Nardelli B, Carlo ED, Lollini PL, Ruben S, et al. Tumor rejection and immune memory elicited by locally released LEC chemokine are associated with an impressive recruitment of APCs, lymphocytes, and granulocytes. J Immunol. 2000;164:3200–3206. doi: 10.4049/jimmunol.164.6.3200. [DOI] [PubMed] [Google Scholar]
- Forni G, Giovarelli M, Santoni A. Lymphokine-activated tumor inhibition in vivo. I. The local administration of interleukin 2 triggers nonreactive lymphocytes from tumor-bearing mice to inhibit tumor growth. J Immunol. 1985;134:1305–1311. [PubMed] [Google Scholar]
- Bosco M, Giovarelli M, Forni M, Modesti A, Scarpa S, Masuelli L, Forni G. Low doses of IL-4 injected perilymphatically in tumor-bearing mice inhibit the growth of poorly and apparently non-immunogenic tumors and induce a tumor-specific immune memory. J Immunol. 1990;145:3136–3143. [PubMed] [Google Scholar]
- Weinberg RA. The Biology of Cancer. Oxford, UK: Garland Science; 2006. [Google Scholar]
- Pannellini T, Forni G, Musiani P. Immunobiology of her-2/neu transgenic mice. Breast Disease. 2004;20:33–42. doi: 10.3233/bd-2004-20105. [DOI] [PubMed] [Google Scholar]
- Rolla S, Nicolo C, Malinarich S, Orsini M, Forni G, Cavallo F, Ria F. Distinct and non-overlapping T cell receptor repertoires expanded by DNA vaccination in wild-type and HER-2 transgenic BALB/c mice. J Immunol. 2006;177:7626–7633. doi: 10.4049/jimmunol.177.11.7626. [DOI] [PubMed] [Google Scholar]
- Ambrosino E, Spadaro M, Iezzi M, Curcio C, Forni G, Musiani P, Wei WZ, Cavallo F. Immunosurveillance of Erbb2 carcinogenesis in transgenic mice is concealed by a dominant regulatory T-cell self-tolerance. Cancer Res. 2006;66:7734–7740. doi: 10.1158/0008-5472.CAN-06-1432. [DOI] [PubMed] [Google Scholar]
- Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102:2138–2145. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Boggio K, Nicoletti G, Di Carlo E, Cavallo F, Landuzzi L, Melani C, Giovarelli M, Rossi I, Nanni P, De Giovanni C, et al. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J Exp Med. 1998;188:589–596. doi: 10.1084/jem.188.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifaldi L, Quaglino E, Di Carlo E, Musiani P, Spadaro M, Lollini PL, Wolf S, Boggio K, Forni G, Cavallo F. A light, nontoxic inter-leukin 12 protocol inhibits HER-2/neu mammary carcinogenesis in BALB/c transgenic mice with established hyperplasia. Cancer Res. 2001;61:2809–2812. [PubMed] [Google Scholar]
- Spadaro M, Ambrosino E, Iezzi M, Di Carlo E, Sacchetti P, Curcio C, Amici A, Wei WZ, Musiani P, Lollini PL, et al. Cure of mammary carcinomas in Her-2 transgenic mice through sequential stimulation of innate (neoadjuvant interleukin-12) and adaptive (DNA vaccine electroporation) immunity. Clin Cancer Res. 2005;11:1941–1952. doi: 10.1158/1078-0432.CCR-04-1873. [DOI] [PubMed] [Google Scholar]
- Conti I, Dube C, Rollins BJ. Chemokine-based pathogenetic mechanisms in cancer. Novartis Found Symp. 2004;256:29–41. discussion 41–52. [PubMed] [Google Scholar]
- Quaglino E, Rolla S, Iezzi M, Spadaro M, Musiani P, De Giovanni C, Lollini PL, Lanzardo S, Forni G, Sanges R, et al. Concordant morphologic and gene expression data show that a vaccine halts HER-2/neu preneoplastic lesions. J Clin Invest. 2004;113:709–717. doi: 10.1172/JCI200419850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astolfi A, Landuzzi L, Nicoletti G, De Giovanni C, Croci S, Palladini A, Ferrini S, Iezzi M, Musiani P, Cavallo F, et al. Gene expression analysis of immune-mediated arrest of tumorigenesis in a transgenic mouse model of HER-2/neu-positive basal-like mammary carcinoma. Am J Pathol. 2005;166:1205–1216. doi: 10.1016/S0002-9440(10)62339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astolfi A, Rolla S, Nanni P, Quaglino E, De Giovanni C, Iezzi M, Musiani P, Forni G, Lollini PL, Cavallo F, et al. Immune prevention of mammary carcinogenesis in HER-2/neu transgenic mice: a microarray scenario. Cancer Immunol Immunother. 2005;54:599–610. doi: 10.1007/s00262-004-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglino E, Iezzi M, Mastini C, Amici A, Pericle F, Di Carlo E, Pupa SM, De Giovanni C, Spadaro M, Curcio C, et al. Electroporated DNA vaccine clears away multifocal mammary carcinomas in her-2/neu transgenic mice. Cancer Res. 2004;64:2858–2864. doi: 10.1158/0008-5472.CAN-03-2962. [DOI] [PubMed] [Google Scholar]
- Cavallo F, Astolfi A, Iezzi M, Cordero F, Lollini PL, Forni G, Calogero R. An integrated approach of immunogenomics and bioinformatics to identify new tumor associated antigens (TAA) for mammary cancer immunological prevention. BMC Bioinformatics. 2005;6(Suppl 4):S7. doi: 10.1186/1471-2105-6-S4-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettenhall JM, Smyth GK. limmaGUI: a graphical user interface for linear modeling of microarray data. Bioinformatics. 2004;20:3705–3706. doi: 10.1093/bioinformatics/bth449. [DOI] [PubMed] [Google Scholar]
- Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article 3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- Westfall PH, Krishen A, Young SS. Using prior information to allocate significance levels for multiple endpoints. Stat Med. 1998;17:2107–2119. doi: 10.1002/(SICI)1097-0258(19980930)17:18<2107::AID-SIM910>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Ingenuity Systems http://www.ingenuity.com
- Hu P, Bader G, Wigle DA, Emili A. Computational prediction of cancer-gene function. Nat Rev Cancer. 2007;7:23–34. doi: 10.1038/nrc2036. [DOI] [PubMed] [Google Scholar]
- Chin K, DeVries S, Fridlyand J, Spellman PT, Roydasgupta R, Kuo WL, Lapuk A, Neve RM, Qian Z, Ryder T, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10:529–541. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmigiani G, Garrett-Mayer ES, Anbazhagan R, Gabrielson E. A cross-study comparison of gene expression studies for the molecular classification of lung cancer. Clin Cancer Res. 2004;10:2922–2927. doi: 10.1158/1078-0432.CCR-03-0490. [DOI] [PubMed] [Google Scholar]
- Cavallo F, Quaglino E, Cifaldi L, Di Carlo E, Andre A, Bernabei P, Musiani P, Forni G, Calogero RA. Interleukin 12-activated lymphocytes influence tumor genetic programs. Cancer Res. 2001;61:3518–3523. [PubMed] [Google Scholar]
- Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglott D, Ostell J, Pruitt KD, Tatusova T. Entrez Gene: gene-centered information at NCBI. Nucleic Acids Res. 2007;(35 Database):D26–D31. doi: 10.1093/nar/gkl993. [DOI] [PMC free article] [PubMed] [Google Scholar]