Abstract
In patients with melanoma, surgery is pivotal not only for the primary tumor but also for regional and often distant metastases. The minimally invasive technique of sentinel node biopsy has become standard for detection of occult regional node metastasis in patients with intermediate-thickness primary melanoma; in these patients it has a central role in determining prognosis and a significant impact on survival when biopsy results are positive. Its role in thin melanoma remains under evaluation. The regional tumor-draining sentinel node is also a useful model for studies of melanoma-induced immunosuppression. Although completion lymphadenectomy remains the standard of care for patients with sentinel node metastasis, results of ongoing phase III trials will indicate whether sentinel node biopsy without further lymph node surgery is adequate therapy for certain patients with minimal regional node disease.
Although this issue of Seminars in Oncology outlines many exciting nonsurgical developments against melanoma, surgery remains the mainstay of treatment – particularly now that surgical techniques have become less morbid for all stages of disease. At the primary site, margins of excision have been narrowed without sacrificing survival or local control. At the regional lymph nodes, sentinel lymph node biopsy can identify occult metastasis without the complications of elective standard lymphadenectomy. At distant sites, metastasectomy can significantly prolong the survival of carefully selected patients. This review considers the surgical treatment of melanoma, with a focus on sentinel node technology.
Wide Excision of Primary Cutaneous Melanoma
As early as the mid-19th century, British surgeon William Norris recognized the importance of treatment margins in primary melanoma. He recommended surgery to “not only remove the disease, but cut away some of the healthy parts. I would, after excising the part, touch the wound with caustic so as not to leave an atom of the disease, if possible, and occasionally apply the same remedy to the skin in the vicinity.” 1 Although pathologic studies have identified atypical melanocytes at a distance of 5 cm from the primary tumor,2–4 margins of 3 to 5 cm were often accompanied by fairly significant morbidity. As described below, subsequent clinical trials have generally supported the efficacy of narrower excisions (Table 1).5–9
Table 1.
Randomized excision margin trials
| Recurrence | Hazard Ratio for Survival (95% confidence interval) | ||||||
|---|---|---|---|---|---|---|---|
| Study | N | Primary Thickness | Arms (cm) | Local | Nodal | Disease-Free | Overall |
| Veronesi et al5 | 612 | ≤ 2 mm | 1 vs. 3 | NS | NS | NS | |
| Cohn-Cedermark et al8 | 989 | ≤ 2 mm | 2 vs. 5 | NS | NS | 1.02 (0.80–1.30) | 0.96 (0.75–1.24) |
| Khayat et al9 | 337 | ≤ 2.1 mm | 2 vs. 5 | NS | NS | NS | NS |
| Balch et al6 | 486 | 1–4 mm | 2 vs. 4 | NS | NS | ||
| Thomas et al7 | 900 | >2 mm | 1 vs. 3 | NS | NS | 1.26 (1.00–1.59)* | 1.21 (0.99–1.46) |
locoregional recurrence
A prospective randomized study organized by the World Health Organization Melanoma Program examined 1-cm versus 3-cm margins for 612 patients with primary melanomas ≤ 2 mm in thickness.5 There was no advantage with the wider margin. Similarly, trials from the French Group for Research on Malignant Melanoma and the Swedish Melanoma Trial Group found no significant difference in survival or local recurrence between 2-cm and 5-cm margins for melanomas ≤ 2 mm thick.8,9 The Intergroup Melanoma Surgical Trial compared 2-cm and 4-cm margins in 468 patients with intermediate thickness (1–4 mm) melanomas of the proximal extremity or trunk.6,10 Again, there was no increase in local recurrence or decrease in survival with the narrower margin.
In a more recent randomized trial, 900 patients in the United Kingdom underwent excision of ≥ 2-mm melanomas, with margins of 1 cm or 3 cm.7 Narrower margins were associated with an increase in locoregional recurrences (primarily nodal disease) and a trend toward decreased survival. However, there was no difference in local or intransit recurrence, and the higher rate of nodal disease might have reflected this trial’s exclusion of sentinel node biopsy. Another randomized study of patients with > 2-mm primary melanomas did not demonstrate any outcome difference with 2-cm versus 4-cm margins of excision.11
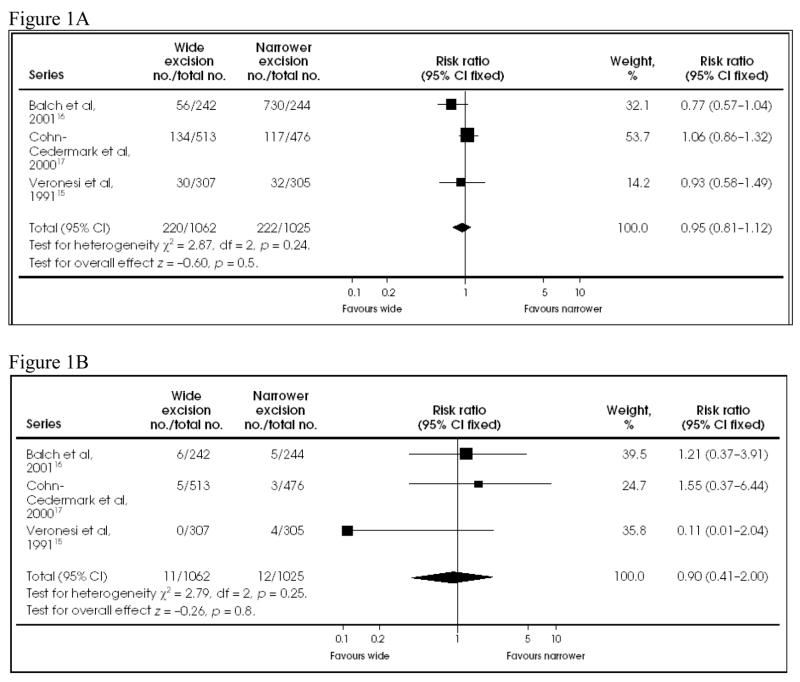
As confirmed in a meta-analysis of several randomized trials (Figure 1),12 a 2-cm margin of excision is safe and effective for intermediate-thickness (1–4 mm) melanoma, although a 1-cm margin is acceptable in anatomically restricted areas such as the face. There is no evidence-based optimal margin for primary melanomas > 4 mm. A 2-cm margin is recommended; wider margins may be considered if easily obtained. A 1-cm margin is appropriate for primary melanomas < 1 mm. Two clinical situations require special attention to margins: lentigo maligna melanoma and desmoplastic melanoma. The former is relatively common in elderly patients with severely sun-damaged skin. Because its histologic extension may exceed its clinically evident borders, a series of punch biopsies may be warranted to evaluate the adequacy of proposed margins, especially before complex reconstructive procedures. Desmoplastic melanoma may also extend for several centimeters beyond the usual 2-cm margin; its increased risk of local recurrence may merit wider margins.13 However, desmoplastic lesions are often on the head or neck, where very wide margins are not practical. The North Central Cancer Treatment Group is conducting a randomized controlled trial of adjuvant radiation for some patients with desmoplastic melanoma.
Figure 1.
A: Comparison of mortality at 8 to 11 years between wide and narrower excision in the 3 follow-up trials of melanoma of the extremities. B: Comparison of local recurrence at 8 to 10 years between wide and narrower excision in the 3 follow-up trials of patients with melanoma of the extremities. Reprinted with permission from Haigh et al.12
Management of the Regional Lymph Nodes
Evolution of Sentinel Node Biopsy
In the late 1800s, Herbert Snow initiated a surgical controversy that would continue for over a century, by recommending elective removal of clinically normal regional lymph nodes in patients with cutaneous melanoma.1 He believed that early removal of “infected” lymph nodes would prevent subsequent metastasis to distant sites and therefore improve patient outcomes. However, most patients with clinically localized primary melanoma do not have nodal involvement and therefore cannot benefit from nodal resection. Since complete lymph node dissection is a substantial operation and entails the risk of significant morbidity, its universal application for all melanoma patients was quite controversial. Eventually the Intergroup Melanoma Surgical Trial demonstrated evidence of a survival benefit in several subgroups, including patients with extremity melanoma, those with non-ulcerated melanoma, and those 60 years old or younger.14 However, the survival difference for the overall group comparison did not reach statistical significance (p=0.12).
Selective biopsy of high-risk regional nodes can eliminate blind use of complete lymphadenectomy – if the biopsy technique accurately identifies the most likely nodal targets of metastasis, the so-called sentinel nodes. The idea of tracking lymphatic drainage from a primary anatomic site had been percolating since its introduction by Virchow in the 1800s.15 In cancer, Gould et al16 in 1960 and Cabanas17 in 1977 described “sentinel” lymph nodes that drained the testicle and penis, respectively, and were the most likely to harbor metastatic disease. However, these nodes were defined by fixed anatomic standards that did not accommodate dynamic variations in lymphatic drainage. The sentinel node concept based on functional assessment of lymphatic pathways began to realize its clinical potential about 20 years ago, with the development of cutaneous lymphoscintigraphy to identify the lymphatic basins receiving drainage from primary melanomas.
In 1977, Holmes et al18 proposed preoperative lymphoscintigraphy to guide the rational treatment of regional lymph nodes. They subsequently reported successful use of lymphoscintigraphy to identify nodal basins draining primary melanomas in 32 patients.19 As the technology improved, lymphoscintigrams often revealed one or more initial nodal targets within a drainage basin. These targets were functionally defined as sentinel nodes (SNs). If the SN is the first regional node to receive lymph from a primary tumor, then it would also be the first site of any tumor cells metastasizing via lymphatic routes. If so, then biopsy and analysis of the SN might be used to determine the tumor status of the entire nodal drainage basin. Intraoperative lymphatic mapping of the SN was initially tested by using vital blue dyes to map lymphatic channels in a feline model.20 In 1985, the first clinical trials of intraoperative lymphatic mapping and sentinel node biopsy (SNB) were begun at the John Wayne Cancer Clinic. At the time of surgery, blue dye was injected at the primary site, and an incision made over the draining basin. Skin flaps were then raised toward the primary site until a blue channel was identified, which was then traced to the sentinel node. The completeness of the procedure was determined by exploration several centimeters around the initial blue node. In 1990, the first report of SNB was presented by Morton’s group21 at the Society of Surgical Oncology. In this study, 223 patients with cutaneous melanoma underwent 237 dye-directed mapping procedures; at least one SN was identified in 194 procedures (82%). All patients underwent complete lymphadenectomy after SNB: of the 40 (21%) specimens containing tumor cells, all but two were associated with tumor-positive SNs. Although an initially skeptical response to this report delayed its publication until 1992,22 SNB was subsequently adopted by many investigators, who confirmed its accuracy and low morbidity.
Initial investigations of SNB used preoperative lymphoscintigraphy to identify the nodal drainage basin, but radioactive tracers were not used for intraoperative identification of SNs within the basin. This changed with the advent of handheld intraoperative gamma probes. Use of a probe for intraoperative mapping of the SN was described in an animal model by Alex et al,23 and its clinical use was reported by Essner et al24 at the Society of Surgical Oncology in 1994. Several radiotracers have been evaluated and are now used clinically,25,26 alone or in conjunction with a blue dye. In fact, dual-agent mapping (dye plus radiotracer) seems to produce the best results. The SN identification rate is approximately 82–87% for dye-directed mapping (although this increases with experience27), as compared with 99% for dual-agent mapping. Use of the probe not only improves the identification of additional SNs within the drainage basin but also eliminates the need for dissection of subcutaneous flaps in the direction of the primary site.
SNB Selection Criteria
Most patients with clinically localized cutaneous melanoma are candidates for SNB. The value of SNB depends on the likelihood of nodal metastasis, which in turn can be estimated by a nomogram that considers the primary tumor’s Breslow thickness, Clark level and ulceration, and the patient’s age (Figure 2).28 Reported rates of SN metastasis are 12–19.7% for 1–2 mm primary melanomas, 28–33.2% for 2–4 mm melanoma,29,30 and 28–44% for melanomas > 4 mm. As indicated by the multivariate analysis in Table 2,29 almost all patients with lesions at least 1 mm in thickness have a sufficiently high risk of nodal metastasis to justify SNB. Gershenwald and colleagues31 noted that patients with thick (>4 mm) melanoma also appear to be good candidates for SNB. In their study of 126 patients with thick primary melanomas, SN status and ulceration were the only independent prognostic factors in multivariate survival analysis. Patients with tumor-negative SNs had an 86.7% 3-year survival (Figure 3).
Figure 2.
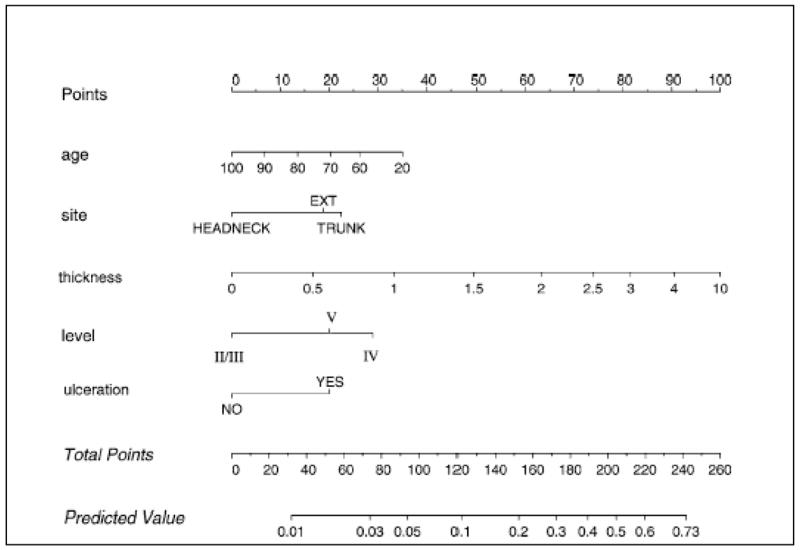
Nomogram to predict positive sentinel lymph nodes. How to use the nomogram: The patient’s values on each axis are located, and a line is drawn upward to determine how many points the patient receives for each variable value. The points are summed; that number is located on the ‘‘total points’’ axis. A line is drawn downward to then determine the predicted probability of sentinel node positivity. Reprinted with permission from Wong et al.28
Table 2.
Multivariate analysis of prognostic factors for predicting sentinel node metastases (N = 1351). Reprinted with permission from Rousseau et al. 29
| Prognostic factor | OR | 95% CI | P value |
|---|---|---|---|
| Tumor thickness | 3.42 | 2.54–4.61 | <.0001 |
| Ulceration | 2.21 | 1.57–3.13 | <.0001 |
| Age ≤ 50 y | 1.81 | 1.31–2.51 | .0003 |
| Axial location | 1.45 | 1.05–2.02 | .026 |
| Clark level IV/V | 1.34 | .94–1.92 | .107 |
| Male sex | 1.09 | .78–1.52 | .629 |
OR, odds ratio; CI, confidence interval
Figure 3.
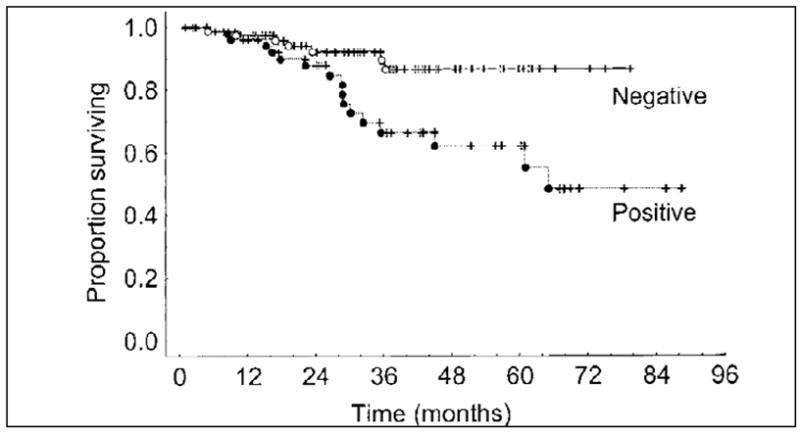
Kaplan-Meier survival for patients with thick (>4 mm) melanoma by SN status. Disease-free and overall survivals were significantly better for patients with a negative SN biopsy (P<.03 and P=.006, respectively). Reprinted with permission from Gershenwald et al.31
SNB for thin melanoma is somewhat more controversial. Ranieri32 demonstrated prognostic value for determination of SN status in patients with thin melanoma, but routine use of SNB for all patients with thin melanoma would not be cost-effective.33 Clinicopathologic factors that might increase the risk of nodal metastasis from a thin primary melanoma include Breslow thickness > 0.75 mm, Clark’s level IV, ulceration, young patient age, mitotic rate greater than zero, absence of tumor-infiltrating lymphocytes, male sex and primary tumor regression (Table 3).30,34–55 Interestingly, however, the rate of clinical nodal recurrence may be higher than the rate of positive SNs among patients with melanomas < 0.76 mm (2.3% versus 0.94%, respectively, based on mean of reported studies), and among those with primary tumors between 0.76 and 1.00 mm (8.6% versus 5.5%, respectively).30, 47–50 This unexpected finding suggests that the selection criteria for SNB need refinement and/or current pathologic techniques are not adequately sensitive. In either case, a better means of risk determination is needed to identify patients who should undergo SNB for a thin melanoma.
Table 3.
Previous studies of SN mapping or clinical recurrence in thin (<1.00 mm) melanoma
| Study | <0.76 | 0.76–1.00 | ≤ 1.00 | Factors | |
|---|---|---|---|---|---|
| Sentinel node | Bedrosian et al35* | 1/40 (2.5%) | 3/31 (9.7%) | 4/71 (5.6%) | VGP, no other |
| Bleicher et al34 | 2/118 (1.7%) | 6/154 (3.9%) | 8/272 (2.9%) | Age, incomplete biopsy | |
| Jacobs et al44 | 1/NR | 1/NR | 2/63 (3.2%) | ||
| Kesmodel et al43 | 1/91 (1.1%) | 8/90 (8.9%) | 9/181 (5.0%) | MR, Br, gender | |
| Lowe et al45 | 2/NR | 1/NR | 3/46 (6.5%) | Clark ≥ III | |
| Nahabedian et al51 | 0/NR | 2/NR | 2/24 (8.3%) | ||
| Oliveira Filho et al46 | NR | NR | 6/77 (7.8%) | ulceration, MR, VGP | |
| Puleoet al41 | NR | 20/409 (4.9%) | NR | None predictive | |
| Wong et al39 | 0/109 | 8/114 (7.0%) | 8/223 (3.6%) | None predictive | |
| Stitzenberg et al36 | 3/NR | 3/NR | 6/146 (4.1%) | None predictive | |
| Hershko et al37 | 2/NR | 3/NR | 5/64 (7.8%) | Age | |
| Morton et al30 | NR | NR | 34/465 (7.3%) | ||
|
| |||||
| Subtotal | 3/325 (0.94%) | 42/767 (5.5%) | 83/1561 (5.3%) | ||
|
| |||||
| Clinical recurrence | Morton et al30 | NR | NR | 238/1979 (12.0%) | |
| Kalady et al50 | NR | NR | 38/1082 (3.5%) | ||
| Massi et al52 ** | 8/174 (4.6%) | 17/113 (15%) | 25/287 (8.7%) | TIL | |
| Karakousis et al49 | 21/684 (3.1%) | 17/198 (8.6%) | 38/882 (4.3%) | male,<60,axial | |
| Corsetti et al48 | 0/NR | 5/NR | 5/68 (7.4%) | Median TTR 52mo | |
| Schmid-Wendtner et al53 | 37/2301 (1.6%) | NR | NR | male, ALM or LMM | |
| McKinnon et al54 | NR | NR | NR | TTR 49.8mo | |
| Woods et al47 | 5/400 (1.3%) | NR | NR | 2.8% total recur | |
| Naruns et al55 | 28/649 (4.3%) | NR | NR | male, regression | |
| Subtotal | 99/4208 (2.35) | 17/198 (8.6) | 319/4011 (8.0%) | ||
|
| |||||
| TOTAL | 102/4533(2.3%) | 59/965 (6.1%) | 402/5572 (7.2%) | ||
VGP, vertical growth phase; MR, mitotic rate; Br, Breslow; NR, not reported; TTR, time to recurrence; TIL, tumor-infiltrating lymphocytes; ALM, acral lentigenous melanoma; LMM, lentigo maligna melanoma
These patients also in Kesmodel et al,43 not duplicated in total figures.
up to 1.5 mm included, not included in total figures.
In addition to thickness, age is an important determinant of the risk of nodal metastasis. Paradoxically, although increasing age is associated with worse overall survival, it is also associated with less nodal metastasis. This relationship appears to be a continuous variable with different studies setting cut points at 45, 50 or 60 years. 29,50,56 There is a relationship between age and lymphatic function as measured by radiotracer uptake in SNs; patients older than 50 years have significantly lower counts in the hottest SN.57 It is possible that an age-related decrease in lymphatic function decreases the potential for nodal disease but increases the risk of systemic metastasis.
Finally, histologic subtype may play a role in determining the risk of nodal metastasis, particularly with regard to desmoplastic melanoma (DM). Studies of SN positivity in desmoplastic melanoma have shown conflicting rates of nodal metastasis. Some have demonstrated rates equivalent to those of standard melanoma58; others reported almost no nodal involvement in DM.59 These conflicting findings may reflect pathologic classification of DM. Recently “pure” and “mixed” types of DM have been described.60,61 Pure DM is characterized by prominent desmoplasia throughout the lesion and the absence of another histologic subtype. The rate of SN involvement in pure DM appears to be quite low.
SNB Technique
SNB for clinically localized melanoma is an outpatient procedure. It should be preceded by preoperative lymphoscintigraphy to identify the regional drainage basin(s) and the approximate site of SNs within that basin.62 Dynamic lymphoscintigraphy can distinguish true SNs, which receive direct drainage from the primary tumor site, from second-echelon non-SNs, which may be near SNs but do not receive direct drainage from the primary tumor. Preoperative lymphoscintigraphy can also identify ectopic nodes in popliteal and epitrochlear basins of patients with distal extremity lesions, in the triangular intermuscular space of patients with lesions on the back and flank, or in intercostal locations of patients with lesions on the abdomen and chest.26,63,64 These sites contain SNs in approximately 5–10% of cases.
After SN sites have been marked on the skin by the nuclear medicine physician, the patient is taken to the operating room where blue dye is injected intradermally at the primary site. Patent V and isosulfan blue are both effective20; methylene blue is less effective in highlighting lymph channels and SNs and has been associated with significant soft tissue necrosis in some cases.20,65
As mentioned earlier, dual-agent mapping with dye and radiotracer is optimal: the probe facilitates identification of blue-stained SNs and it may identify further SNs that do not stain blue. However, there is no firm consensus on the level of radioactivity that corresponds to a sentinel versus nonsentinel node. Some investigators define probe-identified SNs by a radioactivity level of greater than twice background radioactivity or as more than 10% of the hottest identified node. McMasters and colleagues66 reviewed data derived from the Sunbelt Melanoma Trial: resection of all blue-stained nodes and all nodes with >10% of the hottest node’s radioactivity was associated with a estimated false-negative rate of 0.4% (Table 4). However, it is often very difficult to determine a node’s true level of radioactivity until it has been removed; ex-vivo counts may be 2 or 3 times higher than in-vivo counts. A surgeon’s experience with probe-directed mapping becomes a critical determinant of the number of resected nodes. In addition, nodes which are replaced by tumor may fail to take up mapping agents (dye or radiotracer). As a result, any clinically suspicious nodes found during lymphatic mapping should be removed. Complete node dissection may be recommended when an SN cannot be identified.
Table 4.
Impact of sentinel node definition on false-negative results. Reprinted with permission from McMasters et al.66
| Criteria for removal of SN | No. of false negatives/no. of basins with positive nodes | False-negative ratea |
|---|---|---|
| A. Only hottest node removed | 40/288b | 13.9% |
| B. Hottest node and all obviously blue nodes removedc,d | 19/285e | 6.7% |
| C. Hottest node and all blue nodes removed | 6/285 | 2.1% |
| D. 1st or 2nd SN identified | 5/288 | 1.7% |
| E. All blue nodes and all nodes >10% of hottest node | 1/285 | 0.4% |
The actual false-negative rate can be determined only by long-term follow-up for recurrent nodal metastases in basins with negative SNs
All results are statistically different from category A and reduce the risk of false-negative results (P<.02).
This assumes that the faintly blue or obviously blue nodes would have been identified without the gamma probe, or that blue dye staining could be established prior to removing the node.
Cases in which blue dye was not used have been excluded
Category B is statistically different from categories C, D, and E (P<.01)
Excised SNs are processed for analysis as permanent rather than frozen sections. Analysis of frozen sections has a high rate of false-negative results; in addition, some diagnostic material is lost during frozen section processing. In breast cancer, touch prep analysis of SN specimens appears to be fairly accurate in selected settings.67–69 Data are limited in melanoma,70 but a small study by Shidham et al71 reported good sensitivity and specificity for imprint cytology and rapid immunohistochemical staining. However, the issue may be moot because complete lymphadenectomy is usually undertaken as a separate procedure, after results of fixed-section analysis are available and after the patient has been informed. As discussed below, most patients with tumor-positive SNs have no further regional node involvement, and completion dissection may not prove to be necessary in all cases.
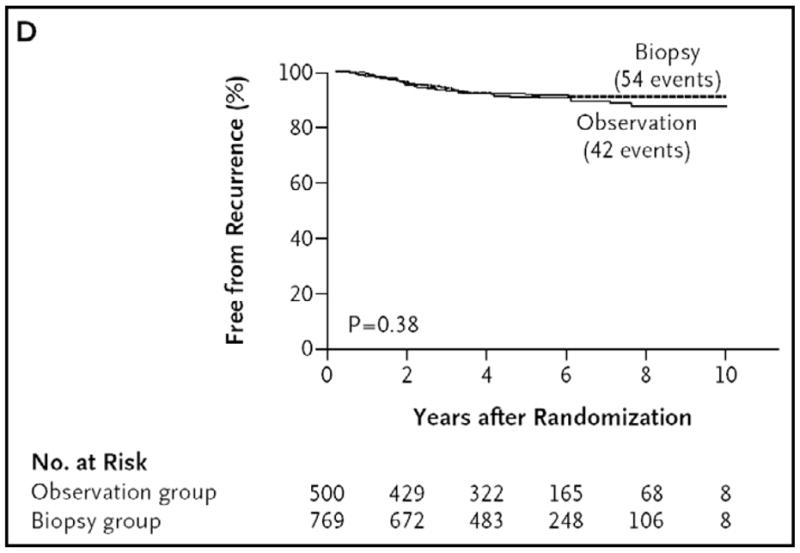
The SN procedure generally entails very little morbidity. Risks include wound separation (0.2–1.2%), seroma/hematoma (2.3–5.5%), wound infection (1.1–4.6%), and lymphedema (0.6–0.7%).72,73 Severe allergic reactions to blue dye have been reported, but these appear to be more common for lymphatic mapping of primary breast cancers, and have not been seen in large multicenter studies of melanoma. Reports of an SNB-related increase in intransit metastasis74,75 are not supported by results of larger retrospective studies.76–80 Recently published data from a prospective randomized trial confirm no increase in intransit metastasis with SN mapping, putting the question to rest (Figure 4).81
Figure 4.
Time to local or intransit metastasis according to the type of treatment (wide excision alone or wide excision plus SNB) shows no significant difference.Reprinted with permission from Morton et al.81
Multicenter Selective Lymphadenectomy Trial of SNB
The first Multicenter Selective Lymphadenectomy Trial (MSLT-I)27,72,81 included patients with clinically localized melanoma >1 mm or > Clark IV who were less than 10 weeks from their initial melanoma biopsy. Patients were randomized to either wide local excision alone (WEO) alone (40%) or to WEO with SNB (LM/SNB) (60%) and complete nodal dissection if the SN was involved. The trial enrolled 2001 patients between January 1994 and March 2002 at centers in North America, Australia, and Europe. There was good compliance with the protocol treatment assignments with small and balanced numbers of patients receiving unassigned treatments. Of those assigned to WEO, 5.0% had LM/SNB and of those assigned to LM/SNB 4.8% had WEO.
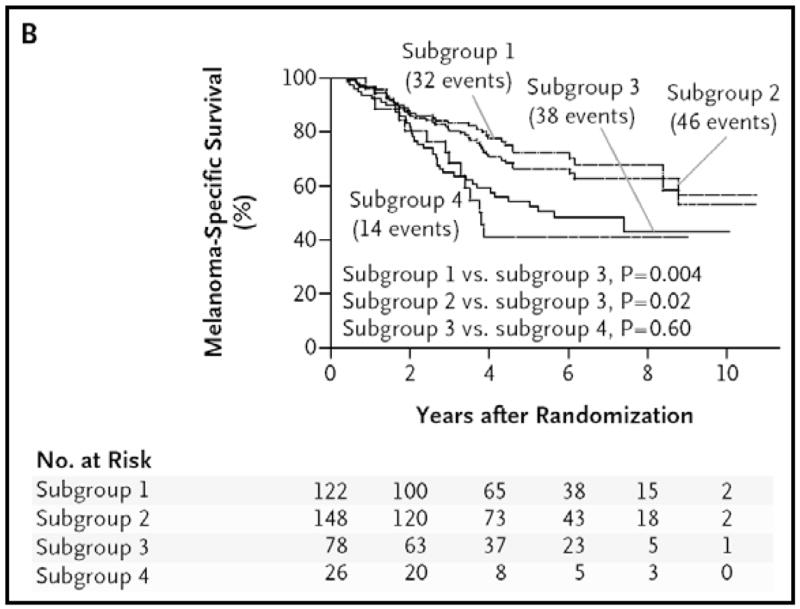
After a planned interim analysis, the Data Safety Monitoring Board recommended release of the study results, even though the two treatment arms showed no difference in the primary endpoint (overall survival). This recommendation was based on highly significant differences in disease-free survival (78.3% in the LM/SNB arm versus 73.1% in the WEO arm; hazard ratio 0.74, 95% CI 0.59–0.93). In addition, although the unexpectedly low number of metastases indicated insufficient power to detect a significant difference in overall survival, data showed a significant melanoma-specific survival benefit for SNB among the subgroup of patients with nodal metastases (Figure 5; hazard ratio 0.51, 95%CI 0.32–0.81).81 Obviously patients with nodal metastases could not be identified at the time of randomization, but this subgroup comparison was validated by the comparable distribution of prognostic variables and similar rates of nodal positivity between those with SN metastasis and those who developed clinical evidence of nodal recurrence during observation.
Figure 5.
Melanoma-specific survival among patients with nodal metastases. Subgroup 1 comprised patients with a tumor-positive sentinel node; subgroup 2, the patients in subgroup 1 plus those in subgroup 4 with a nodal recurrence after a negative result on biopsy; subgroup 3, those with nodal recurrence during observation; and subgroup 4, those with nodal recurrence after a negative result on biopsy. Reprinted with permission from Morton et al.81
Although MSLT-I data confirm SNB as standard of care for intermediate-thickness melanoma, they do not indicate whether completion lymphadenectomy is always necessary when the SNB specimen contains evidence of tumor. The current standard of care is removal of the remaining nodes from the basin containing an involved SN, but only about one in five such basins will yield additional involved nodes. Studies suggest that the risk of nonsentinel metastasis in a patient with a tumor-positive SN depends on the thickness of the primary melanoma, the amount of melanoma in the SN (measured by a number of methods), and the immune status of the SN.82–86 Another uncertainty is the clinical impact of removing tumor-involved nonsentinel nodes in a patient population that has a high risk of occult distant metastasis. The second MSLT (MSLT-II) addresses these issues by randomizing patients with tumor-positive SNs to immediate completion lymphadenectomy or to observation. Patients in the observation arm undergo periodic nodal ultrasonography. The trial is ongoing at sites in North America, Australia and Europe.
Ongoing SNB Research
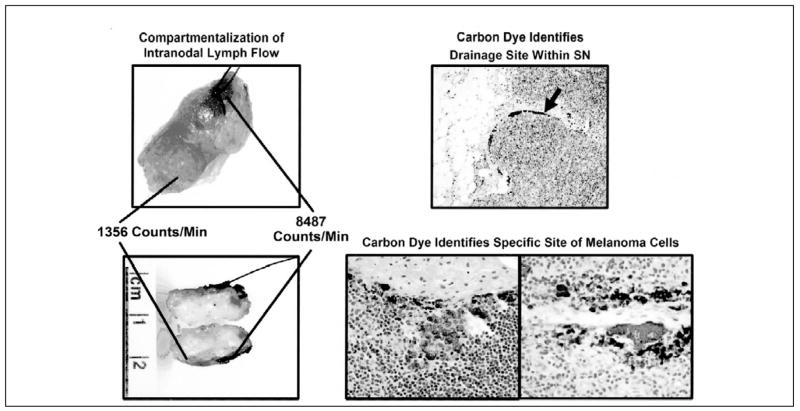
Researchers are developing new radiocolloid agents for lymphatic mapping. One of these, [99mTc]diethylenetriamine pentaacetic acid (DTPA)-mannosyldextran (Lymphoseek, Neoprobe Corp., Dublin, OH), is a radiopharmaceutical which binds to mannose receptors on antigen-presenting cells of lymph nodes.87 Preclinical studies suggest specific binding to nodes and relatively rapid clearance from the primary injection site. It is currently being evaluated in clinical trials. Another mapping agent under investigation is carbon dye. Unlike blue dyes and radiotracers, which eventually move through the SN to nodes further along the lymphatic chain, carbon dye is relatively inert; it tends to remain within the SN – and it tends to localize within a particular portion of this node (Figure 6).30 These characteristics suggest that carbon might not only confirm a node as sentinel but also indicate which areas within the SN are most likely to harbor tumor cells. Preclinical and clinical studies of lymphatic mapping with carbon dye have confirmed the presence of carbon within SNs and its co-localization with tumor foci in SNs.30 Further studies will determine if the use of carbon dye as an adjunctive mapping agent can help decrease false-negative results of SNB by facilitating pathological evaluation.
Figure 6.
Compartmentalization of intranodal lymph flow as detected in the operating room by radiopharaceutical, blue dye, and carbon dye (left) and confirmed in the pathology department by carbon dye (right). Reprinted with permission from Morton et al.30
Another potential refinement to the pathologic evaluation of SN is the incorporation of RT-PCR and molecular staging to detect submicroscopic evidence of melanoma. In the multicenter Sunbelt Melanoma Trial, a portion of each SN was analyzed by PCR assay for the presence of tyrosinase, MART-1, MAGE-3 and gp100 mRNA.88 Samples that were positive for tyrosinase and at least one other marker were considered positive. Although PCR analysis significantly increased the number of SNs with evidence of tumor, it did not impact disease-free survival or overall survival. However, melanogenesis antigens such as tyrosinase are not specific for melanoma; they can also be expressed in benign nodal nevus cells. Subsequent studies have evaluated marker panels that are more specific for melanoma. Also, this study used frozen portions of the SN, sacrificing tissue from pathologic analysis; subsequent introduction of technology for RT-PCR analysis of paraffin-embedded nodes89 has allowed complete standard pathologic evaluation prior to performing molecular assays.
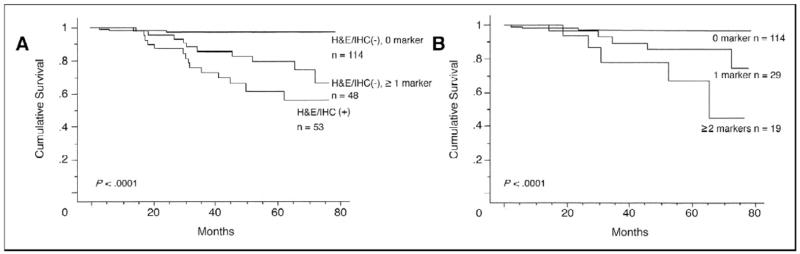
Takeuchi et al89 used multimarker RT-PCR to examine SNs from 215 patients whose SN analyses were negative for tumor by standard pathology. The markers examined were MART-1 (antigen recognized by T cells-1), MAGE-A3 (melanoma antigen gene-A3 family), GalNAc-T (β1–4-N-acetylgalactosaminyl-transferase), and Pax3 (paired-box homeotic gene transcription factor 3). These markers are from non-overlapping biologic pathways and are not present in normal melanocytes. As shown in Figure 7,89 marker expression had a marked impact on disease-free and overall survival; in addition, prognosis was inversely correlated with the number of positive markers. This multimarker RT-PCR assay is being prospectively evaluated as a clinical decision-making tool in MSLT-II.
Figure 7.
(A) Kaplan-Meier curve analysis of overall survival (OS) according to multiple marker quantitative realtime reverse transcriptase polymerase chain reaction and histopathology status in 215 patients; (B) Kaplan-Meier curve analysis of OS according to number of molecular markers in 162 SN histopathology-negative patients. H&E, hematoxylin and eosin; IHC, immunohistochemistry. Reprinted with permission from Takeuchi et al.89
Perhaps the most challenging area of SNB research is the immunology of the SN and its microenvironment. The SN is emerging as a potential site to study melanoma-induced immunosuppression.90 In theory, interleukin-10, cyclooxygenase (COX-2), TGF-β and other immunosuppressive factors produced by a primary melanoma91–94 will have their most profound effects in the closest immune organ, in this case the regional lymph node. Early studies suggested that lymph nodes closest to the primary tumor site were downregulated as evidenced by lower density, frequency and dendrite formation of antigen-presenting cells.94,95 However, after lymphatic mapping showed that the closest regional lymph node is not always the direct target of lymphatic drainage from a primary tumor, investigators started to compare immune parameters of sentinel and nonsentinel nodes. They found that SNs have fewer high-endothelial venules (HEV) as demonstrated by CD105 staining.96 These vessels are important for trafficking of lymphocytes to the node for interaction with antigen-presenting cells. In addition, decreases in dendritic cell frequency, density and dendrite formation seen in earlier anatomic studies were confirmed in comparisons of SN and non-SN.97 These decreases in paracortical dendritic cells have been confirmed by staining for S100, CD1a, MHC class II, CD40, CD80, CD86, CD207 and DC-LAMP. In addition, DC meshworks (visualized by fascin staining), which are potentially important for effector cell-antigen-presenting cell interactions, are markedly diminished in SNs relative to non-SNs. These immune changes appear to affect the likelihood of non-SN metastases.97 RT-PCR analysis of SNs has also demonstrated relative increases in IL-10 and IFN-γ, and these changes correlated with changes in the immunosuppressive enzyme indoamine 2,3-deoxygenase (IDO).93 Taken together, these findings suggest not only the mechanisms by which melanoma evades immune recognition, but also potential methods to counteract these effects for clinical benefit.
Resection of Distant Metastatic Melanoma
Surgery is not often considered when a solid tumor metastasizes to distant sites. However, the immunogenic/immunosuppressive nature of melanoma, its pattern of metastasis, and its poor response to nonsurgical interventions make surgery a reasonable consideration in properly selected patients in stage IV melanoma.98–100 Retrospective series have demonstrated long-term survival in patients with melanoma metastatic to the lung,101,102 liver,103 adrenal glands,104 gastrointestinal tract,105 or soft tissue sites. A recent international phase III trial evaluated adjuvant vaccine immunotherapy in patients with resected stage IV disease.106 Although the trial did not demonstrate significant survival differences between treatment arms, overall 5-year survival rate reached about 40%. This impressive result, which probably reflects patient selection and possibly nonspecific immune stimulation in the placebo arm, compares very favorably with the reported long-term survival rates of 5–15% for systemic chemotherapy in stage IV disease.107,108
Conclusions
Surgical treatment at the site of primary tumors has become less morbid without loss of efficacy through the use of narrower excision margins. Treatment of regional nodal disease has also become less morbid through the use of SNB. This technique is generally considered the standard treatment for clinically localized, intermediate thickness melanoma. It has become a standard procedure for patients with breast cancer, eliminating unnecessary axillary lymph node dissections.109 Groups around the world have also demonstrated the effectiveness of the concept in colorectal, thyroid, lung, stomach, vulvar, prostate, penile, uterine, squamous cell and Merkel cell carcinomas.110–112 The International Sentinel Node Society recently held its 5th Biennial Congress where over 180 abstracts were presented regarding sentinel node research in a wide array of tumor types.
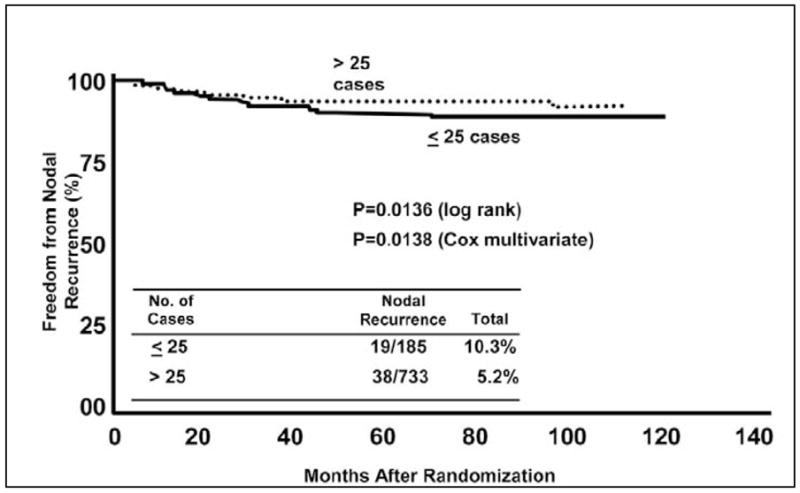
However, although the SN concept is simple, its implementation requires expertise in multiple disciplines. Both the SN identification rate and false-negative rate are significantly influenced by the surgeon’s experience.22,27 In MSLT-I, the rate of in-basin nodal recurrence (false-negative) at each center was 10.3% for the first 25 patients versus 5.2% for subsequent patients (Figure 8).72 Since all centers were required to accurately perform 30 SN procedures prior to enrolling patients in the trial, this suggests a fairly long learning curve of about 50 cases.
Figure 8.
Relationship between nodal recurrence after a tumor-negative lymphatic mapping and sentinel node biopsy procedure and volume of cases at 10 MSLT-1 centers. Minimum duration of follow-up was 36 months. Reprinted with permission from Morton et al.72
As SNB becomes standard practice for clinically localized melanoma, long-term studies will further evaluate the clinical impact of SN micrometastases, particularly those identified by molecular techniques. These studies will be facilitated by the use of paraffin-embedded specimens; archived specimens can be used to examine the prognostic importance of a positive molecular test, without the loss of diagnostic material. In addition, the SN will continue to serve as a model for melanoma-induced immunosuppression.
Surgical resection of distant metastasis has been associated with very favorable outcomes in both single-institution and multicenter studies; 5-year survival rate is about 40%. The use of surgery to treat distant metastases appears to have merit in properly selected patients.
Acknowledgments
Supported by grant CA29605 from the National Cancer Institute and by funding from the Amyx Foundation, Inc. (Boise, ID), the Wayne and Gladys Valley Foundation (Oakland, CA), and from Mr. and Mrs. Louis Johnson, (Stanfield, AZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McLeod G, Davis N, Sober A. A history of melanoma from Hunter to Clark. In: Balch C, Houghton A, Sober A, Soong S, editors. Cutaneous Melanoma. 4. St. Louis: Quality Medical Publishing; 2003. pp. 1–11. [Google Scholar]
- 2.Olsen G. The malignant melanoma of the skin. New theories based on a study of 500 cases Acta Chir Scand Suppl. 1966;365:1–222. [PubMed] [Google Scholar]
- 3.Wong CK. A study of melanocytes in the normal skin surrounding malignant melanomata. Dermatologica. 1970;141(3):215–25. doi: 10.1159/000252469. [DOI] [PubMed] [Google Scholar]
- 4.Cochran AJ. Studies of the melanocytes of the epidermis adjacent to tumors. J Invest Dermatol. 1971;57(1):38–43. doi: 10.1111/1523-1747.ep12292060. [DOI] [PubMed] [Google Scholar]
- 5.Veronesi U, Cascinelli N. Narrow excision (1-cm margin). A safe procedure for thin cutaneous melanoma. Arch Surg. 1991;126(4):438–41. doi: 10.1001/archsurg.1991.01410280036004. [DOI] [PubMed] [Google Scholar]
- 6.Balch CM, Soong SJ, Smith T, Ross MI, Urist MM, Karakousis CP, Temple WJ, Mihm MC, Barnhill RL, Jewell WR, et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1–4 mm melanomas. Ann Surg Oncol. 2001;8(2):101–8. doi: 10.1007/s10434-001-0101-x. [DOI] [PubMed] [Google Scholar]
- 7.Thomas JM, Newton-Bishop J, A’Hern R, Coombes G, Timmons M, Evans J, Cook M, Theaker J, Fallowfield M, O’Neill T, et al. Excision margins in high-risk malignant melanoma. N Engl J Med. 2004;350(8):757–66. doi: 10.1056/NEJMoa030681. [DOI] [PubMed] [Google Scholar]
- 8.Cohn-Cedermark G, Rutqvist LE, Andersson R, Breivald M, Ingvar C, Johansson H, Jonsson PE, Krysander L, Lindholm C, Ringborg U. Long term results of a randomized study by the Swedish Melanoma Study Group on 2-cm versus 5-cm resection margins for patients with cutaneous melanoma with a tumor thickness of 0.8–2.0 mm. Cancer. 2000;89(7):1495–501. [PubMed] [Google Scholar]
- 9.Khayat D, Rixe O, Martin G, Soubrane C, Banzet M, Bazex JA, Lauret P, Verola O, Auclerc G, Harper P, et al. Surgical margins in cutaneous melanoma (2 cm versus 5 cm for lesions measuring less than 2.1-mm thick) Cancer. 2003;97(8):1941–6. doi: 10.1002/cncr.11272. [DOI] [PubMed] [Google Scholar]
- 10.Balch CM, Urist MM, Karakousis CP, Smith TJ, Temple WJ, Drzewiecki K, Jewell WR, Bartolucci AA, Mihm MC, Jr, Barnhill R, et al. Efficacy of 2-cm surgical margins for intermediate-thickness melanomas (1 to 4 mm). Results of a multi-institutional randomized surgical trial. Ann Surg. 1993;218(3):262–7. doi: 10.1097/00000658-199309000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ringborg U, Brahme EM, Drewiecki K. Ranomized trial of a resection margin of 2 cm versus 4 cm for cutaneous malignant melanoma with a tumor thickness of more than 2 mm. World Congress on Melanoma; September 6–10, 2005; Vancouver, British Columbia. [Google Scholar]
- 12.Haigh PI, DiFronzo LA, McCready DR. Optimal excision margins for primary cutaneous melanoma: a systematic review and meta-analysis. Can J Surg. 2003;46(6):419–26. [PMC free article] [PubMed] [Google Scholar]
- 13.Posther KE, Selim MA, Mosca PJ, Stanley WE, Johnson JL, Tyler DS, Seigler HF. Histopathologic characteristics, recurrence patterns, and survival of 129 patients with desmoplastic melanoma. Ann Surg Oncol. 2006;13(5):728–39. doi: 10.1245/ASO.2006.03.091. [DOI] [PubMed] [Google Scholar]
- 14.Balch C, Soong S, Ross M, Urist M, Karakousis C, Temple W, MC Mihm J, Barnhill R, Jewell W, Wanebo H, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0–4.0 mm) Ann Surg Oncol. 2000;7(2):87–97. doi: 10.1007/s10434-000-0087-9. [DOI] [PubMed] [Google Scholar]
- 15.Thompson JF, Scolyer RA, Uren RF. Surgical management of primary cutaneous melanoma: excision margins and the role of sentinel lymph node examination. Surg Oncol Clin N Am. 2006;15(2):301–18. doi: 10.1016/j.soc.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Gould EA, Winship T, Philbin PH, Kerr HH. Observations on a “sentinel node” in cancer of the parotid. Cancer. 1960;13:77–8. doi: 10.1002/1097-0142(196001/02)13:1<77::aid-cncr2820130114>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 17.Cabanas R. An approach for the treatment of penile carcinoma. Cancer. 1977;39(2):456–66. doi: 10.1002/1097-0142(197702)39:2<456::aid-cncr2820390214>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 18.Holmes E, Moseley H, Morton D, Clark W, Robinson D, Urist M. A rational approach to the surgical management of melanoma. Ann Surg. 1977;186(4):481–90. doi: 10.1097/00000658-197710000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fee HJ, Robinson DS, Sample WF, Graham LS, Holmes EC, Morton DL. The determination of lymph shed by colloidal gold scanning in patients with malignant melanoma: a preliminary study. Surgery. 1978;84(5):626–32. [PubMed] [Google Scholar]
- 20.Wong J, Cagle L, Morton D. Lymphatic drainage of skin to a sentinel lymph node in a feline model. Ann Surg. 1991;214(5):637–41. doi: 10.1097/00000658-199111000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morton D, Cagle L, Wong J. Intraoperative lymphatic mapping and selective lymphadenectomy: Technical details of a new procedure for clinical stage I melanoma. Washington, DC: 1990. [Google Scholar]
- 22.Morton D, Wen D, Wong J, Economou J, Cagle L, Storm F, Foshag L, Cochran A. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392–9. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 23.Alex JC, Krag DN. Gamma-probe guided localization of lymph nodes. Surg Oncol. 1993;2(3):137–43. doi: 10.1016/0960-7404(93)90001-f. [DOI] [PubMed] [Google Scholar]
- 24.Essner R, Foshag L, Morton D. Intraoperative radiolymphoscintigraphy: A useful adjunct to intraoperative lymphatic mapping and selective lymphadenectomy in patients with clinical stage I melanoma. Society of Surgical Oncology Annual Meeting; March 17–20, 1994; Houston, TX. [Google Scholar]
- 25.Glass EC, Essner R, Morton DL. Kinetics of three lymphoscintigraphic agents in patients with cutaneous melanoma. J Nucl Med. 1998;39(7):1185–90. [PubMed] [Google Scholar]
- 26.Uren RF, Thompson JF, Howman-Giles R, Chung DK. The role of lymphoscintigraphy in the detection of lymph node drainage in melanoma. Surg Oncol Clin N Am. 2006;15(2):285–300. doi: 10.1016/j.soc.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Morton DL, Thompson JF, Essner R, Elashoff R, Stern SL, Nieweg OE, Roses DF, Karakousis CP, Mozzillo N, Reintgen D, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg. 1999;230(4):453–63. doi: 10.1097/00000658-199910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong SL, Kattan MW, McMasters KM, Coit DG. A nomogram that predicts the presence of sentinel node metastasis in melanoma with better discrimination than the American Joint Committee on Cancer staging system. Ann Surg Oncol. 2005;12(4):282–8. doi: 10.1245/ASO.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Rousseau DL, Jr, Ross MI, Johnson MM, Prieto VG, Lee JE, Mansfield PF, Gershenwald JE. Revised American Joint Committee on Cancer staging criteria accurately predict sentinel lymph node positivity in clinically node-negative melanoma patients. Ann Surg Oncol. 2003;10(5):569–74. doi: 10.1245/aso.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Morton D, Hoon D, Cochran A, Turner R, Essner R, Takeuchi H, Wanek L, Glass E, Foshag L, Hsueh E, et al. Lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: therapeutic utility and implications of nodal microanatomy and molecular staging for improving the accuracy of detection of nodal micrometastases. Ann Surg. 2003;238(4):538–49. doi: 10.1097/01.sla.0000086543.45557.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gershenwald JE, Mansfield PF, Lee JE, Ross MI. Role for lymphatic mapping and sentinel lymph node biopsy in patients with thick (> or = 4 mm) primary melanoma. Ann Surg Oncol. 2000;7(2):160–5. doi: 10.1007/s10434-000-0160-4. [DOI] [PubMed] [Google Scholar]
- 32.Ranieri JM, Wagner JD, Wenck S, Johnson CS, Coleman JJ., 3rd The prognostic importance of sentinel lymph node biopsy in thin melanoma. Ann Surg Oncol. 2006;13(7):927–32. doi: 10.1245/ASO.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 33.Agnese DM, Abdessalam SF, Burak WE, Jr, Magro CM, Pozderac RV, Walker MJ. Cost-effectiveness of sentinel lymph node biopsy in thin melanomas. Surgery. 2003;134(4):542–7. doi: 10.1016/s0039-6060(03)00275-7. [DOI] [PubMed] [Google Scholar]
- 34.Bleicher RJ, Essner R, Foshag LJ, Wanek LA, Morton DL. Role of sentinel lymphadenectomy in thin invasive cutaneous melanomas. J Clin Oncol. 2003;21(7):1326–31. doi: 10.1200/JCO.2003.06.123. [DOI] [PubMed] [Google Scholar]
- 35.Bedrosian I, Faries MB, Guerry Dt, Elenitsas R, Schuchter L, Mick R, Spitz FR, Bucky LP, Alavi A, Elder DE, et al. Incidence of sentinel node metastasis in patients with thin primary melanoma (< or = 1 mm) with vertical growth phase. Ann Surg Oncol. 2000;7(4):262–7. doi: 10.1007/s10434-000-0262-z. [DOI] [PubMed] [Google Scholar]
- 36.Stitzenberg KB, Groben PA, Stern SL, Thomas NE, Hensing TA, Sansbury LB, Ollila DW. Indications for lymphatic mapping and sentinel lymphadenectomy in patients with thin melanoma (Breslow thickness < or =1.0 mm) Ann Surg Oncol. 2004;11(10):900–6. doi: 10.1245/ASO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Hershko DD, Robb BW, Lowy AM, Ahmad SA, Ramadas GH, Soldano DA, Sussman JJ. Sentinel lymph node biopsy in thin melanoma patients. J Surg Oncol. 2006;93(4):279–85. doi: 10.1002/jso.20415. [DOI] [PubMed] [Google Scholar]
- 38.Olah J, Gyulai R, Korom I, Varga E, Dobozy A. Tumour regression predicts higher risk of sentinel node involvement in thin cutaneous melanomas. Br J Dermatol. 2003;149(3):662–3. doi: 10.1046/j.1365-2133.2003.05502.x. [DOI] [PubMed] [Google Scholar]
- 39.Wong SL, Brady MS, Busam KJ, Coit DG. Results of sentinel lymph node biopsy in patients with thin melanoma. Ann Surg Oncol. 2006;13(3):302–9. doi: 10.1245/ASO.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Vaquerano J, Kraybill WG, Driscoll DL, Cheney R, Kane JM., 3rd American Joint Committee on Cancer clinical stage as a selection criterion for sentinel lymph node biopsy in thin melanoma. Ann Surg Oncol. 2006;13(2):198–204. doi: 10.1245/ASO.2006.03.092. [DOI] [PubMed] [Google Scholar]
- 41.Puleo CA, Messina JL, Riker AI, Glass LF, Nelson C, Cruse CW, Johnson TM, Sondak VK. Sentinel node biopsy for thin melanomas: which patients should be considered? Cancer Control. 2005;12(4):230–5. doi: 10.1177/107327480501200404. [DOI] [PubMed] [Google Scholar]
- 42.Koskivuo I, Suominen E, Niinikoski J, Talve L. Sentinel node metastasectomy in thin <or=1-mm melanoma. Langenbecks Arch Surg. 2005;390(5):403–7. doi: 10.1007/s00423-005-0572-5. [DOI] [PubMed] [Google Scholar]
- 43.Kesmodel SB, Karakousis GC, Botbyl JD, Canter RJ, Lewis RT, Wahl PM, Terhune KP, Alavi A, Elder DE, Ming ME, et al. Mitotic rate as a predictor of sentinel lymph node positivity in patients with thin melanomas. Ann Surg Oncol. 2005;12(6):449–58. doi: 10.1245/ASO.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs IA, Chang CK, DasGupta TK, Salti GI. Role of sentinel lymph node biopsy in patients with thin (<1 mm) primary melanoma. Ann Surg Oncol. 2003;10(5):558–61. doi: 10.1245/aso.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 45.Lowe JB, Hurst E, Moley JF, Cornelius LA. Sentinel lymph node biopsy in patients with thin melanoma. Arch Dermatol. 2003;139(5):617–21. doi: 10.1001/archderm.139.5.617. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira Filho RS, Ferreira LM, Biasi LJ, Enokihara MM, Paiva GR, Wagner J. Vertical growth phase and positive sentinel node in thin melanoma. Braz J Med Biol Res. 2003;36(3):347–50. doi: 10.1590/s0100-879x2003000300009. [DOI] [PubMed] [Google Scholar]
- 47.Woods JE, Soule EH, Creagan ET. Metastasis and death in patients with thin melanomas (less than 0.76 mm) Ann Surg. 1983;198(1):63–4. doi: 10.1097/00000658-198307000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corsetti RL, Allen HM, Wanebo HJ. Thin < or = 1 mm level III and IV melanomas are higher risk lesions for regional failure and warrant sentinel lymph node biopsy. Ann Surg Oncol. 2000;7(6):456–60. doi: 10.1007/s10434-000-0456-4. [DOI] [PubMed] [Google Scholar]
- 49.Karakousis GC, Gimotty PA, Botbyl JD, Kesmodel SB, Elder DE, Elenitsas R, Ming ME, Guerry D, Fraker DL, Czerniecki BJ, et al. Predictors of regional nodal disease in patients with thin melanomas. Ann Surg Oncol. 2006;13(4):533–41. doi: 10.1245/ASO.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 50.Kalady MF, White RR, Johnson JL, Tyler DS, Seigler HF. Thin melanomas: predictive lethal characteristics from a 30-year clinical experience. Ann Surg. 2003;238(4):528–35. doi: 10.1097/01.sla.0000090446.63327.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nahabedian MY, Tufaro AP, Manson PN. Sentinel lymph node biopsy for the T1 (thin) melanoma: is it necessary? Ann Plast Surg. 2003;50(6):601–6. doi: 10.1097/01.SAP.0000069065.00486.1E. [DOI] [PubMed] [Google Scholar]
- 52.Massi D, Franchi A, Borgognoni L, et al. Thin cutaneous malignant melanomas (< or =1.5 mm): identification of risk factors indicative of progression. Cancer. 1999;85(5):1067–76. doi: 10.1002/(sici)1097-0142(19990301)85:5<1067::aid-cncr9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 53.Schmid-Wendtner MH, Baumert J, Eberle J, et al. Disease progression in patients with thin cutaneous melanomas (tumour thickness < or = 0.75 mm): clinical and epidemiological data from the Tumour Center Munich 1977–98. Br J Dermatol. 2003;149(4):788–93. doi: 10.1046/j.1365-2133.2003.05599.x. [DOI] [PubMed] [Google Scholar]
- 54.McKinnon JG, Yu XQ, McCarthy WH, Thompson JF. Prognosis for patients with thin cutaneous melanoma: long-term survival data from New South Wales Central Cancer Registry and the Sydney Melanoma Unit. Cancer. 2003;98(6):1223–31. doi: 10.1002/cncr.11624. [DOI] [PubMed] [Google Scholar]
- 55.Naruns PL, Nizze JA, Cochran AJ, et al. Recurrence potential of thin primary melanomas. Cancer. 1986;57(3):545–8. doi: 10.1002/1097-0142(19860201)57:3<545::aid-cncr2820570323>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 56.Balch C, Buzaid A, Soong S, Atkins M, Cascinelli N, Coit D, Fleming I, Gershenwald J, Houghton A, Kirkwood J, et al. Final version of the American Joint Committee on Cancer Staging System for Cutaneous Melanoma. J Clin Oncol. 2001;19(16):3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 57.Faries MB, Essner R, Foshag L, Bilchik A, Zogakis T, Morton D. Lymph flow decreases with age and correlates with nonsentinel node metastasis and poor survival in stage III melanoma. Proc Am Assoc Cancer Res. 2006:47. Abstract #4766. [Google Scholar]
- 58.Su LD, Fullen DR, Lowe L, Wang TS, Schwartz JL, Cimmino VM, Sondak VK, Johnson TM. Desmoplastic and neurotropic melanoma. Cancer. 2004;100(3):598–604. doi: 10.1002/cncr.11947. [DOI] [PubMed] [Google Scholar]
- 59.Gyorki DE, Busam K, Panageas K, Brady MS, Coit DG. Sentinel lymph node biopsy for patients with cutaneous desmoplastic melanoma. Ann Surg Oncol. 2003;10(4):403–7. doi: 10.1245/aso.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Pawlik TM, Ross MI, Prieto VG, Ballo MT, Johnson MM, Mansfield PF, Lee JE, Cormier JN, Gershenwald JE. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer. 2006;106(4):900–6. doi: 10.1002/cncr.21635. [DOI] [PubMed] [Google Scholar]
- 61.Hawkins WG, Busam KJ, Ben-Porat L, Panageas KS, Coit DG, Gyorki DE, Linehan DC, Brady MS. Desmoplastic melanoma: a pathologically and clinically distinct form of cutaneous melanoma. Ann Surg Oncol. 2005;12(3):207–13. doi: 10.1245/ASO.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 62.Thompson JF, Uren RF. Teaching points on lymphatic mapping for melanoma from the Sydney Melanoma Unit. Semin Oncol. 2004;31(3):349–56. doi: 10.1053/j.seminoncol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Essner R. The role of lymphoscintigraphy and sentinel node mapping in assessing patient risk in melanoma. Semin Oncol. 1997;24(I Suppl 4):S8–S10. [PubMed] [Google Scholar]
- 64.McMasters KM, Chao C, Wong SL, Wrightson WR, Ross MI, Reintgen DS, Noyes RD, Cerrito PB, Edwards MJ. Interval sentinel lymph nodes in melanoma. Arch Surg. 2002;137(5):543–7. doi: 10.1001/archsurg.137.5.543. [DOI] [PubMed] [Google Scholar]
- 65.Stradling B, Aranha G, Gabram S. Adverse skin lesions after methylene blue injections for sentinel lymph node localization. Am J Surg. 2002;184(4):350–2. doi: 10.1016/s0002-9610(02)00945-5. [DOI] [PubMed] [Google Scholar]
- 66.McMasters K, Reintgen D, Ross M, Wong S, Gershenwald J, Krag D, Noyes D, Viar V, Cerrito P, Edwards M. Sentinel lymph node biopsy for melanoma: How many radioactive nodes should be removed? Ann Surg Oncol. 2001;8(3):192–7. doi: 10.1007/s10434-001-0192-4. [DOI] [PubMed] [Google Scholar]
- 67.Forbes RC, Pitchford C, Simpson JF, Balch GC, Kelley MC. Selective use of intraoperative touch prep analysis of sentinel nodes in breast cancer. Am Surg. 2005;71(11):955–60. [PubMed] [Google Scholar]
- 68.Kane JM, 3rd, Edge SB, Winston JS, Watroba N, Hurd TC. Intraoperative pathologic evaluation of a breast cancer sentinel lymph node biopsy as a determinant for synchronous axillary lymph node dissection. Ann Surg Oncol. 2001;8(4):361–7. doi: 10.1007/s10434-001-0361-5. [DOI] [PubMed] [Google Scholar]
- 69.Rubio IT, Korourian S, Cowan C, Krag DN, Colvert M, Klimberg VS. Use of touch preps for intraoperative diagnosis of sentinel lymph node metastases in breast cancer. Ann Surg Oncol. 1998;5(8):689–94. doi: 10.1007/BF02303478. [DOI] [PubMed] [Google Scholar]
- 70.Creager AJ, Shiver SA, Shen P, Geisinger KR, Levine EA. Intraoperative evaluation of sentinel lymph nodes for metastatic melanoma by imprint cytology. Cancer. 2002;94(11):3016–22. doi: 10.1002/cncr.10512. [DOI] [PubMed] [Google Scholar]
- 71.Shidham VB, Chang CC, Komorowski R. MCW melanoma cocktail for the evaluation of micrometastases in sentinel lymph nodes of cutaneous melanoma. Expert Rev Mol Diagn. 2005;5(3):281–90. doi: 10.1586/14737159.5.3.281. [DOI] [PubMed] [Google Scholar]
- 72.Morton D, Cochran A, Thompson J, Essner R, Glass E, Mozzillo N, Nieweg O, Roses D, Hoekstra H, Karakousis C, et al. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-1, an international multicenter trial. Ann Surg. 2005;242(3):302–11. doi: 10.1097/01.sla.0000181092.50141.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wrightson WR, Wong SL, Edwards MJ, Chao C, Reintgen DS, Ross MI, Noyes RD, Viar V, Cerrito PB, McMasters KM. Complications associated with sentinel lymph node biopsy for melanoma. Ann Surg Oncol. 2003;10(6):676–80. doi: 10.1245/aso.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Estourgie SH, Nieweg OE, Kroon BB. High incidence of intransit metastases after sentinel node biopsy in patients with melanoma. Br J Surg. 2004;91(10):1370–1. doi: 10.1002/bjs.4692. [DOI] [PubMed] [Google Scholar]
- 75.Thomas JM, Clark MA. Selective lymphadenectomy in sentinel node-positive patients may increase the risk of local/intransit recurrence in malignant melanoma. Eur J Surg Oncol. 2004;30(6):686–91. doi: 10.1016/j.ejso.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 76.Pawlik TM, Ross MI, Thompson JF, Eggermont AM, Gershenwald JE. The risk of intransit melanoma metastasis depends on tumor biology and not the surgical approach to regional lymph nodes. J Clin Oncol. 2005;23(21):4588–90. doi: 10.1200/JCO.2005.12.245. [DOI] [PubMed] [Google Scholar]
- 77.van Poll D, Thompson JF, Colman MH, McKinnon JG, Saw RP, Stretch JR, Scolyer RA, Uren RF. A sentinel node biopsy does not increase the incidence of intransit metastasis in patients with primary cutaneous melanoma. Ann Surg Oncol. 2005;12(8):597–608. doi: 10.1245/ASO.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 78.Kang JC, Wanek LA, Essner R, Faries MB, Foshag LJ, Morton DL. Sentinel lymphadenectomy does not increase the incidence of intransit metastases in primary melanoma. J Clin Oncol. 2005;23(21):4764–70. doi: 10.1200/JCO.2005.20.537. [DOI] [PubMed] [Google Scholar]
- 79.Essner R, Conforti A, Kelley MC, Wanek L, Stern S, Glass E, Morton DL. Efficacy of lymphatic mapping, sentinel lymphadenectomy, and selective complete lymph node dissection as a therapeutic procedure for early-stage melanoma. Ann Surg Oncol. 1999;6(5):442–9. doi: 10.1007/s10434-999-0442-4. [DOI] [PubMed] [Google Scholar]
- 80.Gershenwald JE, Colome MI, Lee JE, Mansfield PF, Tseng C, Lee JJ, Balch CM, Ross MI. Patterns of recurrence following a negative sentinel lymph node biopsy in 243 patients with stage I or II melanoma. J Clin Oncol. 1998;16(6):2253–60. doi: 10.1200/JCO.1998.16.6.2253. [DOI] [PubMed] [Google Scholar]
- 81.Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, Nieweg OE, Roses DF, Hoekstra HJ, Karakousis CP, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355(13):1307–17. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 82.Lee JH, Essner R, Torisu-Itakura H, Wanek L, Wang H, Morton DL. Factors predictive of tumor-positive nonsentinel lymph nodes after tumor-positive sentinel lymph node dissection for melanoma. J Clin Oncol. 2004;22(18):3677–84. doi: 10.1200/JCO.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 83.Sabel MS, Griffith K, Sondak VK, Lowe L, Schwartz JL, Cimmino VM, Chang AE, Rees RS, Bradford CR, Johnson TM. Predictors of nonsentinel lymph node positivity in patients with a positive sentinel node for melanoma. J Am Coll Surg. 2005;201(1):37–47. doi: 10.1016/j.jamcollsurg.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 84.Reeves ME, Delgado R, Busam KJ, Brady MS, Coit DG. Prediction of nonsentinel lymph node status in melanoma. Ann Surg Oncol. 2003;10(1):27–31. doi: 10.1245/aso.2003.03.020. [DOI] [PubMed] [Google Scholar]
- 85.Wagner JD, Davidson D, Coleman JJ, 3rd, Hutchins G, Schauwecker D, Park HM, Havlik RJ. Lymph node tumor volumes in patients undergoing sentinel lymph node biopsy for cutaneous melanoma. Ann Surg Oncol. 1999;6(4):398–404. doi: 10.1007/s10434-999-0398-4. [DOI] [PubMed] [Google Scholar]
- 86.Cochran AJ, Wen DR, Huang RR, Wang HJ, Elashoff R, Morton DL. Prediction of metastatic melanoma in nonsentinel nodes and clinical outcome based on the primary melanoma and the sentinel node. Mod Pathol. 2004;17(7):747–55. doi: 10.1038/modpathol.3800117. [DOI] [PubMed] [Google Scholar]
- 87.Wallace AM, Hoh CK, Vera DR, Darrah DD, Schulteis G. Lymphoseek: a molecular radiopharmaceutical for sentinel node detection. Ann Surg Oncol. 2003;10(5):531–8. doi: 10.1245/aso.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 88.Scoggins CR, Ross MI, Reintgen DS, Noyes RD, Goydos JS, Beitsch PD, Urist MM, Ariyan S, Davidson BS, Sussman JJ, et al. Prospective multi-institutional study of reverse transcriptase polymerase chain reaction for molecular staging of melanoma. J Clin Oncol. 2006;24(18):2849–57. doi: 10.1200/JCO.2005.03.2342. [DOI] [PubMed] [Google Scholar]
- 89.Takeuchi H, Morton DL, Kuo C, Turner RR, Elashoff D, Elashoff R, Taback B, Fujimoto A, Hoon DS. Prognostic significance of molecular upstaging of paraffin-embedded sentinel lymph nodes in melanoma patients. J Clin Oncol. 2004;22(13):2671–80. doi: 10.1200/JCO.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cochran AJ, Huang RR, Lee J, Itakura E, Leong SP, Essner R. Tumour-induced immune modulation of sentinel lymph nodes. Nat Rev Immunol. 2006;6(9):659–70. doi: 10.1038/nri1919. [DOI] [PubMed] [Google Scholar]
- 91.Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother. 2006;29(3):233–40. doi: 10.1097/01.cji.0000199193.29048.56. [DOI] [PubMed] [Google Scholar]
- 92.Eisengart CA, Mestre JR, Naama HA, Mackrell PJ, Rivadeneira DE, Murphy EM, Stapleton PP, Daly JM. Prostaglandins regulate melanoma-induced cytokine production in macrophages. Cell Immunol. 2000;204(2):143–9. doi: 10.1006/cimm.2000.1686. [DOI] [PubMed] [Google Scholar]
- 93.Lee JH, Torisu-Itakara H, Cochran AJ, Kadison A, Huynh Y, Morton DL, Essner R. Quantitative analysis of melanoma-induced cytokine-mediated immunosuppression in melanoma sentinel nodes. Clin Cancer Res. 2005;11(1):107–12. [PubMed] [Google Scholar]
- 94.Wen DR, Hoon DS, Chang C, Cochran AJ. Variations in lymphokine generation by individual lymph nodes draining human malignant tumors. Cancer Immunol Immunother. 1989;30(5):277–82. doi: 10.1007/BF01744894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cochran AJ, Wen DR, Farzad Z, Stene MA, McBride W, Lana AM, Hoon DS, Morton DL. Immunosuppression by melanoma cells as a factor in the generation of metastatic disease. Anticancer Res. 1989;9(4):859–64. [PubMed] [Google Scholar]
- 96.Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol. 2004;4(5):360–70. doi: 10.1038/nri1354. [DOI] [PubMed] [Google Scholar]
- 97.Cochran AJ, Morton DL, Stern S, Lana AM, Essner R, Wen DR. Sentinel lymph nodes show profound downregulation of antigen-presenting cells of the paracortex: implications for tumor biology and treatment. Mod Pathol. 2001;14(6):604–8. doi: 10.1038/modpathol.3880358. [DOI] [PubMed] [Google Scholar]
- 98.Morton D, Ollila D, Hsueh E, Essner R, Gupta R. Cytoreductive surgery and adjuvant immunotherapy: a new management paradigm for metastatic melanoma. CA Cancer J Clin. 1999;49(2):101–16. doi: 10.3322/canjclin.49.2.101. [DOI] [PubMed] [Google Scholar]
- 99.Ollila DW. Complete metastasectomy in patients with stage IV metastatic melanoma. Lancet Oncol. 2006;7(11):919–24. doi: 10.1016/S1470-2045(06)70938-X. [DOI] [PubMed] [Google Scholar]
- 100.Allen PJ, Coit DG. The surgical management of metastatic melanoma. Ann Surg Oncol. 2002;9(8):762–70. doi: 10.1007/BF02574498. [DOI] [PubMed] [Google Scholar]
- 101.Ollila D, Morton D. Surgical resection as the treatment of choice for melanoma metastatic to the lung. Chest Surg Clin N Am. 1998;8(1):183–196. [PubMed] [Google Scholar]
- 102.Faries MB, Bleicher RJ, Ye X, Essner R, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for primary and metastatic pulmonary malignant neoplasms. Arch Surg. 2004;139(8):870–6. doi: 10.1001/archsurg.139.8.870. [DOI] [PubMed] [Google Scholar]
- 103.Pawlik T, Zorzi D, Abdalla E, Clary B, Gershenwald J, Ross M, Aloia T, Curley S, Camacho L, Capussotti L, et al. Hepatic resection for metastatic melanoma: distinct patterns of recurrence and prognosis for ocular versus cutaneous disease. Ann Surg Oncol. 2006 doi: 10.1245/ASO.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 104.Wood T, DiFronzo A, Rose M, Haigh P, Stern S, Wanek L, Essner R, Morton D. Does complete resection of melanoma metastatic to solid intraabdominal organs improve survival? Ann Surg Oncol. 2001;8(8):658–662. doi: 10.1007/s10434-001-0658-4. [DOI] [PubMed] [Google Scholar]
- 105.Ollila D, Essner R, Wanek L, Morton D. Surgical resection for melanoma metastatic to the gastrointestinal tract. Arch Surg. 1996;131:975–9. doi: 10.1001/archsurg.1996.01430210073013. [DOI] [PubMed] [Google Scholar]
- 106.Morton D, Mozzillo N, Thompson J, Kashani-Sabet M, Kelley M, Gammon G. An international, randomized, double-blind, phase 3 study of the specific active immunotherapy agent, Onamelatucel-L (Canvaxin), compared to placebo as post-surgical adjuvant in AJCC stage IV melanoma. Ann Surg Oncol. 2006;13(2 Suppl):5s. [Google Scholar]
- 107.Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, Pavlick AC, DeConti R, Hersh EM, Hersey P, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24(29):4738–45. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 108.Eton O, Legha SS, Bedikian AY, Lee JJ, Buzaid AC, Hodges C, Ring SE, Papadopoulos NE, Plager C, East MJ, et al. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: results from a phase III randomized trial. J Clin Oncol. 2002;20(8):2045–52. doi: 10.1200/JCO.2002.07.044. [DOI] [PubMed] [Google Scholar]
- 109.Giuliano A, Kirgan D, Guenther J, Morton D. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220(3):391–8. doi: 10.1097/00000658-199409000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liptay M, Grondin S, Fry W, Pozdol C, Carson D, Knop C, Masters G, Perlman R, Watkin W. Intraoperative sentinel lymph node mapping in non-small-cell lung cancer improves detection of micrometastases. J Clin Oncol. 2002;20(8):1984–1988. doi: 10.1200/JCO.2002.08.041. [DOI] [PubMed] [Google Scholar]
- 111.Bilchik A, Nora D, Tollenaar R, Velde Cvd, Wood T, Turner R, Morton D, Hoon D. Ultrastaging of early colon cancer using lymphatic mapping and molecular analysis. Eur J Cancer. 2002;38(7):977–85. doi: 10.1016/s0959-8049(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 112.Bilchik AJ, Giuliano A, Essner R, Bostick P, Kelemen P, Foshag LJ, Sostrin S, Turner RR, Morton DL. Universal application of intraoperative lymphatic mapping and sentinel lymphadenectomy in solid neoplasms. Cancer J Sci Am. 1998;4(6):351–8. [PubMed] [Google Scholar]