Abstract
The nitrophorins from Rhodnius prolixus, the kissing bug, are heme-containing proteins used for the transport of nitric oxide to aide the insect in obtaining a blood meal. The Rhodnius nitrophorins display an eight-stranded antiparallel beta-barrel motif, typical of lipocalins, with a histidine-linked heme in the open end of the barrel. Heme is stabilized in the ferric state and highly distorted, displaying a ruffled conformation that may be of importance in the setting of the reduction potential. To help in understanding the means by which the protein matrix, an inherently soft material, is able to distort the heme from its low-energy planar conformation, we have determined the crystal structure of apo-nitrophorin 4–1.1 Å resolution. Removal of the heme from nitrophorin 4 has very little effect on its structure: The heme binding cavity remains open and the loops near the cavity entrance respond to lower pH in the same manner as the intact protein. We conclude that the general stability of the lipocalin fold and apparent rigidity of the beta-barrel provide the means for distorting the heme cofactor.
Keywords: heme distortion, lipocalin, crystal structure, nitric oxide, Rhodnius prolixus
Nitrophorins are nitric oxide (NO) transport proteins that aid in an insect's blood meal. Nitrophorins transport NO from the insect saliva, using a ferric heme center, to a victim's tissue, where it is released upon dilution and pH elevation (reviewed in Montfort et al. 2000; Walker and Montfort 2000; Walker 2005). Downstream binding by soluble guanylate cyclase (sGC) results in vasodilation, reduced platelet aggregation, and improved blood feeding for the insect. The nitrophorins from the saliva of Rhodnius prolixus, a blood-sucking insect and vector for the spread of Chagas' disease in South America, consist of at least seven proteins: four in the adult insect (NP1–4, numbered according to their abundances in the insect saliva) (Champagne et al. 1995) and at least three additional nitrophorins in the earlier stages of development (NP5–7) (Moreira et al. 2003; Andersen et al. 2004; Ribeiro et al. 2004).
Previous structural studies reveal the adult Rhodinius nitrophorins to have a fold typical of the lipocalin family, with an eight-stranded antiparallel beta-sheet forming a continuously hydrogen bonded beta-barrel, with one end open and the other closed (▶). Heme is sequestered in the open end of the barrel, coordinating to a histidine and providing for NO binding. Two loops, the AB loop (connecting beta-strands A and B) and the GH loop, respond to lower pH and NO binding to yield a closed binding cavity with hydrophobic groups packed against the NO molecule and an extensive hydrogen-bonding network at one end of the cavity (Weichsel et al. 2000). The kinetics of NO binding and release is governed by these loops (Andersen et al. 2000; Maes et al. 2004), despite their inherently dynamic behavior, apparently through a mechanism involving enhanced geminate recombination to the heme iron (Kondrashov et al. 2004; Kondrashov and Montfort 2007).
Figure 1.
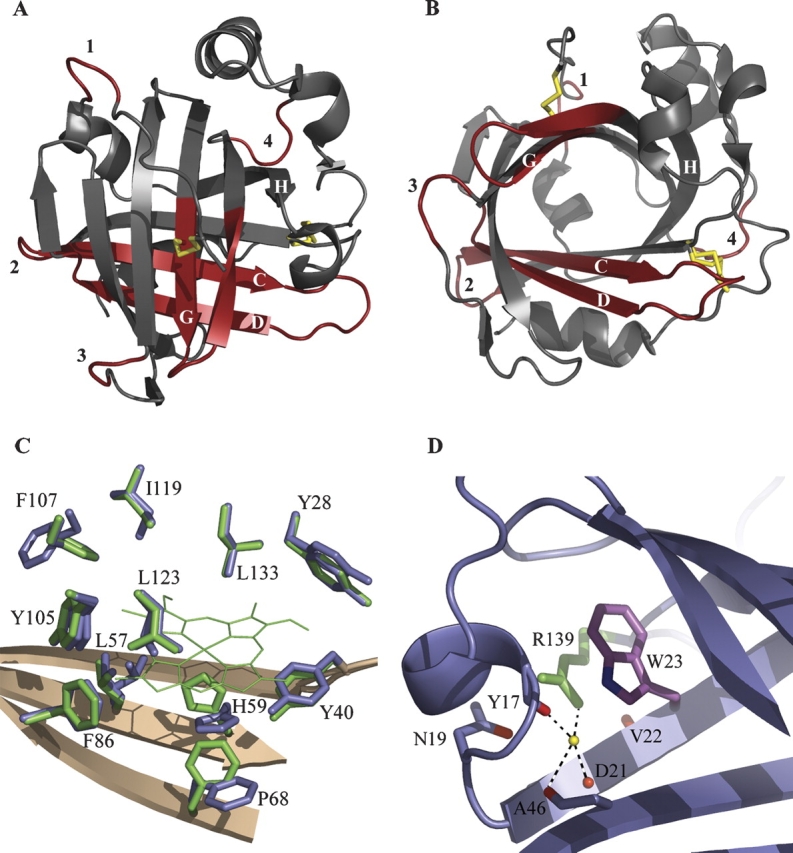
Crystal structure of apo-NP4. (A) Ribbon diagram highlighting changes. Subtle differences in the carbon-alpha backbone between apo- and holo-NP4 are highlighted in red. Labels 1–4 refer to the four loops displaying the largest changes (residues 9–12, 47–53, 100–103, and 140–143, respectively). Disulfide bonds are indicated with yellow bars. (B) As in A, emphasizing the open barrel. The disulfide bond between Cys 2 and Cys 122 (shown near the loop 1 label) occupies a single conformation, while that between Cys 41 and Cys 171 (shown near the loop 4 label) occupies two conformations. (C) Heme contacting residues in apo- and holo-NP4. Heme-pocket side chains of apo- and holo-NP4 are shown in blue and green, respectively. Heme in holo-NP4 is shown in green (thin lines) with heme propionates omitted for clarity. (D) Tryptophan–arginine interaction in NP4 (typical for the lipocalin fold). Tryptophan 23 and arginine 139 are in purple and green, respectively. Carbonyl oxygens in direct or water-mediated interaction (yellow sphere) with arginine 139 are in red.
The heme in the Rhodnius nitrophorins is both highly distorted (Andersen et al. 1998; Weichsel et al. 1998; Andersen and Montfort 2000; Roberts et al. 2001), and highly stabilized in the ferric (FeIII) state (E° ∼ −300 mV), (Andersen et al. 2000), leading to the suggestion that heme distortion is linked to the tuning of the heme reduction potential. Mutational analyses of residues contacting the heme further support this hypothesis (Shokhireva et al. 2003); however, point mutations to date give rise to only small changes in the heme geometry in both NP2, which is 42% identical with NP4 and displays the same fold (Andersen and Montfort 2000; Weichsel and Montfort, in preparation), and NP4 (A. Amoia and W. Montfort, in prep.), suggesting the protein, an inherently pliable and dynamic material, is stiffer than the conjugated heme molecule. To investigate this possibility, we have determined the crystal structure of heme deficient nitrophorin 4 (apo-NP4) and examined how the structure changes.
Results and Discussion
Crystal structure reveals an intact, well-ordered beta-barrel
We chose NP4 for pursuing the apo-structure since crystals of the intact protein diffract to 0.8 Å resolution (Kondrashov et al. 2004). Recombinant NP4 protein was isolated from inclusion bodies and renatured in the absence of heme. Crystals of the apo-protein appeared only under new conditions and grew slowly (3 wk vs. 24 h for the intact protein), but were isomorphous with crystals of the intact protein, yielding diffraction to 1.1 Å resolution. The high resolution of the data allowed for inclusion of hydrogen atoms at calculated positions, refinement of anisotropic temperature factors, and modeling of residues in multiple conformations, leading to an excellent structure (R free = 0.17; ▶).
Table 1.
Diffraction data measurement and refinement statistics
Apo-NP4 is remarkably similar to the intact protein. All secondary structure hydrogen bonding and both intramolecular disulfide bonds are intact (shown in ▶). Minor backbone conformational changes are observed in some loop regions and in residues previously in contact with the heme (▶). Visual inspection of the apo-NP4 electron density maps revealed weak but significant difference density peaks (3.5 σ) in the core of the beta-barrel, approximately where the heme lies in the intact protein. Modeling of the density was not possible, but likely represents weak binding of one or more buffer or cryoprotectant molecules. Unexplained electron density is common in the internal cavities of lipocalins (see, for example, apo-human plasma retinol binding protein) (Zanotti et al. 1993).
A root-mean-square deviation (RMSD) of 0.31 Å between apo- and holo-NP4 confirms that the beta-barrel remains rigid upon removal of the heme cofactor. Careful examination of the superimposed backbones of intact and apo-NP4 reveals a subtle flexibility in two general areas. The first involves four of the turn regions, including residues 9–12 (random coil between the N terminus and helix 1), 47–53 (BC Loop, the loop between beta-strands B and C), 100–103 (EF Loop), and 140–143 (random coil between strand H and helix 2) (▶). The backbones of these turns are now in two conformations, and both conformations are positioned slightly more to the center of the protein. The second area affected is locally at the point of alteration, particularly residues close to the proximal side of the heme (56–79 in sheet strands C and D) and those near the distal side of the heme (121–132 in sheet strand G and the GH loop). The backbone atoms of residues 56–79 now display two conformations in the apo-structure. Backbone stretches representing both the proximal and distal sides of the heme pocket have a similar shape to that of the intact protein, but the backbone shifts slightly into the abandoned heme pocket (▶).
In general, the side chains of apo-NP4 have more flexibility than holo-NP4. This is associated with the above-mentioned backbone motion. Overall, apo-NP4 has 69 of its 184 residues (35%) in multiple conformations, 38 more than in the intact protein. Although the side chains have more conformational freedom, they are not completely disordered, and thus modeling of the second conformation was possible.
Removal of cofactor slightly affects heme-contacting residues
In holo-NP4, the heme iron is coordinated to His 59 and is tightly packed into the lipocalin beta-barrel. The heme pocket is hydrophobic and rich in aromatic residues: Of the contact residues, six are aromatic and four of these form ring-face to heme-edge contacts (▶). Upon removal of the heme cofactor, the overall integrity of the area surrounding the heme is maintained. However, all side chains, except Leu 133, are slightly affected. Tyr 28, Leu 57, Tyr 105, Phe 107, Ile 119, and Leu 123 now display two conformations. The second conformation of Leu 57 and Ile 119, and both conformations of Tyr 105 and Leu 123, provide for a slight collapse of the heme pocket. The second conformation of Tyr 28 remains in the same plane but is translated. Tyr 40, which is proximal to the heme, remains in one conformation but shifts into the heme pocket. Val 36, within the AB loop, collapses into the heme pocket due to absence of the heme vinyl substituents (not shown).
Perhaps most interesting are the shifts due to the loss of heme coordination to proximal His 59. The His 59 side chain adopts a new predominant conformation in the apo-structure and is substantially more dynamic, with three atoms in the imidazole ring essentially unobserved in the final electron density map. The structural water linking Asp 70 to His 59, which is prominent in the intact structure, is retained in the apo-protein and is weakly associated with His 59. The new predominant position of His 59 occupies the space normally taken up by Phe 68, which rotates away from the pocket. As noted above, beta-strand C, which contains His 59, and the CD loop, become more dynamic and occupy two conformations when not ligated to heme.
Heme distortion upon binding to apo-NP4
Overall, the heme-binding pocket in the apo-structure is nearly unchanged from that of the intact structure, retaining a large, open cavity. Heme binding can therefore be readily accomplished through simple side-chain rearrangement. In the intact structure, the heme and the heme-contact residues are extremely well-ordered, displaying little of the dynamics observed in the apo-structure. The optimal fit leads to distortion of the heme, predominately through ruffling (rotation of the pyrrole rings about the Fe–N bonds). This distortion, which is likely to cost only a few kcal/mol, may be of functional importance for altering the heme reduction potential (Andersen et al. 2000; Roberts et al. 2001; Shokhireva et al. 2003), and is enabled in part through heme binding into the relatively stiff lipocalin beta-barrel.
AB and GH loops close at low pH
Intact NP4 adopts a closed conformation at low pH that brings loops AB and GH together, leading to an extensive hydrogen-bonding network (Weichsel et al. 2000). This transition is also observed in the apo-NP4 structure, measured at pH 5.6. Both open and closed conformations of the GH Loop (residues 125–133) are present in the structure but the closed conformation predominates. The AB loop (residues 31–37) is well-ordered in the crystal and displays only the closed conformer. In the closed conformation, Asp 30, Asp 129, and Leu 130 are hydrogen bonded, and the 130–131 peptide bond is flipped over with respect to the open conformation, bringing Leu 130 into the distal pocket, where it packs against NO in the intact protein. Thus, the rigid, intact, lipocalin fold functions normally even in the absence of heme.
Folding and stability of lipocalins
That apo-NP4 retains its open beta-barrel geometry in the absence of heme, rather than collapsing, is consistent with the general trend among lipocalins. Structures of several proteins with lipocalin folds have been determined in their holo- and apo-forms, a few of which are listed in ▶, and these too display a resilient beta-barrel. Thus, this appears to be a general feature of the fold even where permanent cofactor binding occurs, as in the nitrophorins.
Table 2.
Comparison of selected apo- and holo-lipocalins
The folding mechanism of beta-sheet containing proteins in general, and beta-barrel proteins in particular, are less well understood than those of other structural classes. However, investigations of calycins, which contain 10-stranded antiparallel beta-barrels, and lipocalins (eight-stranded beta-barrels), have been informative. Folding studies of cellular retinoic acid-binding protein 1, a calycin, indicate that hydrophobic collapse leads to formation of the ligand-binding cavity before consolidation of hydrogen bonding in the beta-barrel, suggesting that side-chain interactions rather than hydrogen bonding determine the beta-sheet topology (Clark et al. 1996, 1997). Stability studies of serum retinol binding protein, a true lipocalin, indicate that a conserved tryptophan/arginine motif is key for protein folding and stability in the lipocalin family (Greene et al. 2001). Apo-NP4 folds reversibly in vitro and displays minimal changes in the beta-barrel with respect to its holo-form, consistent with the above findings. NP4 also displays the conserved Trp/Arg motif (Flower et al. 2000), which organizes a key series of well-ordered hydrogen bonds around a structural water in both apo- and holo-NP4; this arrangement may serve to pin the closed end of the beta-barrel together (▶). Interestingly, the structure of the highly related protein NP2 has lost the arginine in this motif, but it is replaced with a histidine residue that links the same region together but in a new manner that does not involve water (Andersen and Montfort 2000). Both proteins are additionally stabilized through disulfide bond formation (▶).
Summary
Refolding and crystallizing apo-NP4 led to a well-ordered, high-resolution crystal structure that displays an overall shape nearly identical to that for the intact heme-containing protein. Two loops near the heme-binding pocket reside in the closed conformation found for the intact protein at the same pH (5.6). A conserved Trp/Arg interaction thought to be important in lipocalin folding remains intact in the apo-protein. The general stability of the fold and apparent rigidity of the beta-barrel provide the means for distorting the heme cofactor into a ruffled conformation that may serve to stabilize the ferric oxidation state in the nitrophorins.
Materials and Methods
Expression and purification of apo-NP4
NP4 protein was expressed in E. coli as inclusion bodies, denatured and refolded. Reductant was removed after refolding to allow for the air oxidation of two intramolecular disulfide bonds. The heme cofactor was not included. The protein was purified by ion-exchange chromatography followed by gel filtration as previously described (Andersen et al. 1998).
Crystallization, data collection, and data processing
Crystals were grown at room temperature (∼25°C) using the hanging-drop technique in 2.8 M ammonium sulfate, pH 5.6. After 1 d of equilibration, protein drops were seeded with wild-type NP4 crystals and large, single, apo-NP4 crystals appeared in ∼3 wk. A single crystal was transferred to a cryoprotectant solution of 3.2 M ammonium sulfate, pH 5.6, and allowed to equilibrate. Following equilibration, the crystal was flash frozen in liquid nitrogen for data collection.
Data were acquired at Brookhaven National Laboratory, New York, on beamline X29 with a Q315 detector at 100°K and processed with MOSFLM and SCALA (Leslie 2006) to 1.11 Å resolution. The crystal belongs to the C2 space group with cell constants typical of those found for intact NP4 (Roberts et al. 2001) (▶).
Structure determination
The initial apo-structure was built using difference Fourier methods, starting with wild-type NP4 model 1X8O (Kondrashov et al. 2004). The phases were subsequently refined with anisotropic temperature factors using REFMAC from the CCP4 package (Collaborative Computational Project Number 4 1994); model building was accomplished with the program COOT (Emsley and Cowtan 2004). Certain residues were found to occupy more than one conformation and were built and refined as such. In such cases, the occupancies were generally set to 0.5 for each conformer and left fixed when refined. Both disulfide bonds were clearly observed and modeled with full occupancy. Refinement converged to R factors of 15% and 17% for Rcryst and Rfree, respectively (▶).
Model quality was assessed using the program PROCHECK (Laskowski et al. 1993) as implemented in CCP4. All residues displayed phi/psi values in the favored or allowed regions of a Ramachandran plot. Structural figures were prepared using PyMOL (Delano Scientific, http://www.pymol.org/). Structural superpositioning was accomplished using the secondary-structure alignment approach and the program SSM Superimpose (Krissinel and Henrick 2004). Similar RMSD values were found using more traditional programs and just the carbon alpha-atoms for the beta-barrel.
Atomic coordinates
The atomic coordinates and structure factors have been deposited with the Protein Data Bank (PDB entry 2OFM).
Acknowledgments
We thank Jacquie Brailey for help in protein preparation, Abreeza Zegeer for help with crystallization, and Dr. Annie Héroux and Brookhaven National Laboratory for mail-in data collection. Data for this study were measured at beamline X29 of the National Synchrotron Light Source, supported principally from the Offices of Biological and Environmental Research and of Basic Energy Sciences of the U.S. Department of Energy, and from the National Center for Research Resources of the National Institutes of Health. This work was supported in part by National Institutes of Health grant HL62969 (to W.R.M.), T32 GM008659 (to A.M.A.), and the ARCS Foundation (to A.M.A.).
Footnotes
Reprint requests to: William R. Montfort, University of Arizona, Department of Biochemistry and Molecular Biophysics, 1041 East Lowell Street, Tucson, AZ 85721, USA; e-mail: montfort@email.arizona.edu; fax: (520) 621-1697.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.072981907.
References
- Andersen J.F. and Montfort, W.R. 2000. Crystal structures of nitrophorin 2: A trifunctional antihemostatic protein from the saliva of Rhodnius prolixus . J. Biol. Chem. 275 30496–30503. [DOI] [PubMed] [Google Scholar]
- Andersen J.F., Weichsel, A., Balfour, C.A., Champagne, D.E., and Montfort, W.R. 1998. The crystal structure of nitrophorin 4 at 1.5 Å resolution: Transport of nitric oxide by a lipocalin-based heme protein. Structure 6 1315–1327. [DOI] [PubMed] [Google Scholar]
- Andersen J.F., Ding, X.D., Balfour, C., Shokhireva, T.K., Champagne, D.E., Walker, F.A., and Montfort, W.R. 2000. Kinetics and equilibria in ligand binding by nitrophorins 1–4: Evidence for stabilization of a NO–ferriheme complex through a ligand-induced conformational trap. Biochemistry 39 10118–10131. [DOI] [PubMed] [Google Scholar]
- Andersen J.F., Gudderra, N.P., Francischetti, I.M., Valenzuela, J.G., and Ribeiro, J.M. 2004. Recognition of anionic phospholipid membranes by an antihemostatic protein from a blood-feeding insect. Biochemistry 43 6987–6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne D.E., Nussenzvieg, R.H., and Ribeiro, J.M.C. 1995. Purification, partial characterization, and cloning of nitric oxide-carrying heme proteins (nitrophorins) from salivary glands of the blood-sucking insect Rhodnius prolixus . J. Biol. Chem. 270 8691–8695. [DOI] [PubMed] [Google Scholar]
- Chaudhuri B.N., Kleywegt, G.J., Bjorkman, J., Lehman-McKeeman, L.D., Oliver, J.D., and Jones, T.A. 1999. The structures of alpha 2u-globulin and its complex with a hyaline droplet inducer. Acta Crystallogr. D Biol. Crystallogr. 55 753–762. [DOI] [PubMed] [Google Scholar]
- Clark P.L., Liu, Z.P., Zhang, J., and Gierasch, L.M. 1996. Intrinsic tryptophans of CRABPI as probes of structure and folding. Protein Sci. 5 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark P.L., Liu, Z.P., Rizo, J., and Gierasch, L.M. 1997. Cavity formation before stable hydrogen bonding in the folding of a beta-clam protein. Nat. Struct. Biol. 4 883–886. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project Number 4 1994. The CCP4 Suite: Programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. D50 760–763. [DOI] [PubMed] [Google Scholar]
- Emsley P. and Cowtan, K. 2004. COOT: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60 2126–2132. [DOI] [PubMed] [Google Scholar]
- Flower D.R., North, A.C., and Sansom, C.E. 2000. The lipocalin protein family: Structural and sequence overview. Biochim. Biophys. Acta 1482 9–24. [DOI] [PubMed] [Google Scholar]
- Greene L.H., Chrysina, E.D., Irons, L.I., Papageorgiou, A.C., Acharya, K.R., and Brew, K. 2001. Role of conserved residues in structure and stability: Tryptophans of human serum retinol-binding protein, a model for the lipocalin superfamily. Protein Sci. 10 2301–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrashov D.A. and Montfort, W.R. 2007. Nonequilibrium dynamics simulations of nitric oxide release: Comparative study of nitrophorin and myoglobin. J. Phys. Chem. B (in press). [DOI] [PubMed]
- Kondrashov D.A., Roberts, S.A., Weichsel, A., and Montfort, W.R. 2004. Protein functional cycle viewed at atomic resolution: Conformational change and mobility in nitrophorin 4 as a function of pH and NO binding. Biochemistry 43 13637–13647. [DOI] [PubMed] [Google Scholar]
- Krissinel E. and Henrick, K. 2004. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 60 2256–2268. [DOI] [PubMed] [Google Scholar]
- Laskowski R.A., MacArthur, M.W., Moss, D.S., and Thorton, J.M. 1993. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26 283–291. [Google Scholar]
- Leslie A.G. 2006. The integration of macromolecular diffraction data. Acta Crystallogr. D Biol. Crystallogr. 62 48–57. [DOI] [PubMed] [Google Scholar]
- Maes E.M., Weichsel, A., Andersen, J.F., Shepley, D., and Montfort, W.R. 2004. Role of binding site loops in controlling nitric oxide release: Structure and kinetics of mutant forms of nitrophorin 4. Biochemistry 43 6679–6690. [DOI] [PubMed] [Google Scholar]
- Montfort W.R., Weichsel, A., and Andersen, J.F. 2000. Nitrophorins and related antihemostatic lipocalins from Rhodnius prolixus and other blood-sucking arthropods. Biochim. Biophys. Acta 1482 110–118. [DOI] [PubMed] [Google Scholar]
- Moreira M.F., Coelho, H.S., Zingali, R.B., Oliveira, P.L., and Masuda, H. 2003. Changes in salivary nitrophorin profile during the life cycle of the blood-sucking bug Rhodnius prolixus . Insect Biochem. Mol. Biol. 33 23–28. [DOI] [PubMed] [Google Scholar]
- Newcomer M.E. 1993. Structure of the epididymal retinoic acid binding protein at 2.1 Å resolution. Structure 1 7–18. [DOI] [PubMed] [Google Scholar]
- Paesen G.C., Adams, P.L., Harlos, K., Nuttall, P.A., and Stuart, D.I. 1999. Tick histamine-binding proteins: Isolation, cloning, and three-dimensional structure. Mol. Cell 3 661–671. [DOI] [PubMed] [Google Scholar]
- Ribeiro J.M., Andersen, J., Silva-Neto, M.A., Pham, V.M., Garfield, M.K., and Valenzuela, J.G. 2004. Exploring the sialome of the blood-sucking bug Rhodnius prolixus . Insect Biochem. Mol. Biol. 34 61–79. [DOI] [PubMed] [Google Scholar]
- Roberts S.A., Weichsel, A., Qiu, Y., Shelnutt, J.A., Walker, F.A., and Montfort, W.R. 2001. Ligand-induced heme ruffling and bent NO geometry in ultra-high resolution structures of nitrophorin 4. Biochemistry 40 11327–11337. [DOI] [PubMed] [Google Scholar]
- Sacchettini J.C., Gordon, J.I., and Banaszak, L.J. 1989a. Crystal structure of rat intestinal fatty-acid-binding protein. Refinement and analysis of the Escherichia coli-derived protein with bound palmitate. J. Mol. Biol. 208 327–339. [DOI] [PubMed] [Google Scholar]
- Sacchettini J.C., Gordon, J.I., and Banaszak, L.J. 1989b. Refined apoprotein structure of rat intestinal fatty acid binding protein produced in Escherichia coli . Proc. Natl. Acad. Sci. 86 7736–7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokhireva T., Berry, R.E., Uno, E., Balfour, C.A., Zhang, H., and Walker, F.A. 2003. Electrochemical and NMR spectroscopic studies of distal pocket mutants of nitrophorin 2: Stability, structure, and dynamics of axial ligand complexes. Proc. Natl. Acad. Sci. 100 3778–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker F.A. 2005. Nitric oxide interaction with insect nitrophorins and thoughts on the electron configuration of the {FeNO}6 complex. J. Inorg. Biochem. 99 216–236. [DOI] [PubMed] [Google Scholar]
- Walker F.A. and Montfort, W.R. 2000. The nitric oxide-releasing heme proteins from the saliva of the blood-sucking insect Rhodnius prolixus . Adv. Inorg. Chem. 51 295–358. [Google Scholar]
- Weichsel A., Andersen, J.F., Champagne, D.E., Walker, F.A., and Montfort, W.R. 1998. Crystal structures of a nitric oxide transport protein from a blood-sucking insect. Nat. Struct. Biol. 5 304–309. [DOI] [PubMed] [Google Scholar]
- Weichsel A., Andersen, J.F., Roberts, S.A., and Montfort, W.R. 2000. Reversible nitric oxide binding to nitrophorin 4 from Rhodnius prolixus involves complete distal pocket burial. Nat. Struct. Biol. 7 551–554. [DOI] [PubMed] [Google Scholar]
- Winter N.S., Bratt, J.M., and Banaszak, L.J. 1993. Crystal structures of holo and apo-cellular retinol-binding protein II. J. Mol. Biol. 230 1247–1259. [DOI] [PubMed] [Google Scholar]
- Zanotti G., Berni, R., and Monaco, H.L. 1993. Crystal structure of liganded and unliganded forms of bovine plasma retinol-binding protein. J. Biol. Chem. 268 10728–10738. [PubMed] [Google Scholar]