Abstract
A protocol is described for the production of both intracellularly expressed and secreted selenomethionyl-derivatized recombinant proteins in baculovirus-infected insect cells. The method results in the production of recombinant soluble proteins with an SeMet occupancy of ∼75% and with a recovery of ∼20% that of native protein expression. The method is independent of the percentage methionine content of the protein and is reliable and consistent. Similar results are obtained using either Spodoptera frugiperda Sf9 or Trichoplusia ni High Five insect cells as the expression host, and when cultures are grown in either shake flasks or in Wave BioReactors.
Keywords: selenomethionine, baculovirus, insect cells, MAD phasing, multiwavelength anomalous diffraction
The incorporation of selenomethionine (SeMet) residues into proteins provides a means, utilizing multiwavelength anomalous diffraction (MAD), to phase solution in protein structure determinations by X-ray crystallography (Hendrickson et al. 1990; Hendrickson and Ogata 1997; see e.g., von Delft et al. 2003). Selenomethionine substitution is useful also for chain tracing in protein crystallography (see, e.g., Bushnell et al. 2001). The method was developed using Escherichia coli as the host for recombinant SeMet-substituted protein production (Hendrickson et al. 1990). Recent developments in structural proteomics, including high-throughput protein expression and purification strategies (Lesley 2001; Hosfield et al. 2003; McMullan et al. 2005; Brodsky and Cronin 2006) and high-throughput crystallographic approaches (Muchmore et al. 2000; Hosfield et al. 2003), have generated an increased need for selenomethionyl-substituted recombinant proteins suitable for X-ray structure solution using MAD phasing.
The incorporation of SeMet into recombinant proteins produced in E. coli often approaches 100% (Hendrickson et al. 1990; Guerrero et al. 2001; von Delft et al. 2003). Expression is typically carried out by using the E. coli methionine auxotrophs DL41 (Hendrickson et al. 1990) or B834 (Leahy et al. 1992). The production of SeMet-derivatized protein has also been reported in a cell-free system using E. coli extracts (Kigawa et al. 2002). Although E. coli is the preferred host for recombinant protein production, many eukaryotically derived DNA sequences do not yield efficient protein synthesis in that host, or the protein is often produced in an insoluble form (“inclusion bodies”). In such cases, the most widely used alternative for the production of recombinant soluble protein has been the baculovirus expression vector system (BEVS), which uses recombinant baculoviruses to infect insect cells, generally grown in suspension culture. This latter system also has the advantage of providing much of the post-translational modifications that occur in higher order cell types, such as phosphorylation and glycosylation (for a review, see O'Reilly et al. 1994).
There have been a number of reports describing the production of SeMet derivatized recombinant proteins in eukaryotic hosts. These have included Pichia pastoris with reported values of 40%–50% SeMet incorporation into secreted proteins (Larsson et al. 2002; Xu et al. 2002), Saccharomyces cerevisiae with 55% SeMet incorporation into an intracellularly produced protein (Bushnell et al. 2001), Chinese hamster ovary (CHO) cells with 93% incorporation for a secreted protein (Lustbader et al. 1995), HEK293 cells with 93% for a secreted protein (Barton et al. 2006), HEK293T cells with 60% for an intracellularly produced protein (Aricescu et al. 2006a), and Spodoptera frugiperda Sf9 and Sf21 insect cells using the BEVS with 40%–85% incorporation reported for secreted proteins (Chen and Bahl 1991a,b; Fremont et al. 1998; Bellizzi et al. 1999; McWhirter et al. 1999; Carfi et al. 2002;) and 40%–100% incorporation reported for intracellularly produced proteins (McWhirter et al. 1999; Liemann et al. 2002; Aricescu et al. 2006b). It is evident that there is a great deal of variability in the results obtained and it has been noted in some reports that the reproduction of some of the results has been difficult to obtain for other proteins (see, e.g., Aricescu et al. 2006b; and below). In addition, much of the previously reported methodology has been applied to a single protein, or to a limited number of either intracellularly expressed or secreted proteins. In addition, the impact of SeMet toxicity on the yield of recombinant protein has not often been reported.
In general, incorporation of SeMet into secreted proteins has been more successful than for intracellularly expressed proteins, which is readily explained since most experiments involving SeMet labeling in eukaryotic cells involves replacement of the initial growth medium with a SeMet-containing medium and, thus, early production of non-SeMet-labeled secreted recombinant protein is discarded. In light of the variability among previous reports, and of differences between SeMet incorporation into secreted and intracellulary expressed proteins, we sought to develop a reliable and consistent single protocol for the production of highly SeMet-derivatized proteins using the BEVS system. The protocol, reported here, yields SeMet derivatized proteins with a consistent SeMet occupancy of 70%–75% and at a production yield of ∼20% that of the non-SeMet derivatized proteins. The protocol is equally successful when using either Spodoptera frugiperda Sf9 or Trichoplusia ni High Five insect cells as the expression host, or when the expressions are carried out in shake flasks or in Wave BioReactors.
Results and Discussion
The initial target for SeMet substitution was the secreted enzyme dipeptidyl peptidase IV (DP4). The few published procedures (Chen and Bahl 1991a,b; Fremont et al. 1998; Bellizzi et al. 1999; Carfi et al. 2002) for producing secreted SeMet-substituted proteins in the BEVS were synthesized into a unified scheme (see Aertgerts et al. 2004) that was expected to produce recombinant DP4 with an SeMet substitution in the range 40%–85%, as reported in those earlier studies. These initial experiments utilized High Five insect cells grown in ESF-921 protein-free medium without added fetal bovine serum (FBS). The initial infection was allowed to proceed for 16 h prior to the replacement of the medium with ESF-921 protein-free methionine-free medium. After growth for an additional 4 h, SeMet was added to a final concentration of 50 mg/L (0.25 mM) and growth continued for a further 28 h. The amino acid analysis of purified DP4 indicated, however, that the selenomethionine incorporation was ∼35%, a little below the low end of the range reported in earlier studies. Although this level of incorporation was sufficient to aid in the structure solution of that enzyme (see Aertgerts et al. 2004), a refinement of the process was undertaken in order to improve the SeMet substitution for future projects.
Reports describing the production of SeMet-substituted secreted proteins in BEVS (Chen and Bahl 1991a,b; Fremont et al. 1998; Bellizzi et al. 1999; Carfi et al. 2002) utilized a 24–36-h growth period post-infection in met+ medium followed by a 0–12-h growth period in met− medium prior to the addition of SeMet to a final concentration of 50–100 mg/L. Such timelines are unsuitable, however, for the production of optimally SeMet substituted intracellularly produced proteins, since the synthesis of recombinant protein under control of the viral polyhedrin promotor in insect cells is known to occur between 12 and 24 h post-infection (O'Reilly and Miller 1988). Thus, for intracellularly produced proteins, such timelines would be expected to lead to a mixture of SeMet substituted and SeMet-free protein, an expectation borne out experimentally in our study. For example, when conditions similar to those of Bellizzi et al. (1999) were used to produce SeMet-substituted FGFR2 kinase domain intracellularly in either Sf9 or High Five insect cell lines (32 h infection followed by transfer to met− medium for 4 h followed by the addition of SeMet to 100 mg/L and with a total expression period of 72 h), the mass spectroscopy analysis indicated that appoximately half of the protein had no SeMet incorporation whatsoever, whereas the remainder had ∼55% incorporation (data not shown). As indicated earlier in this article, the isolation of such mixtures of unincorporated and incorporated material for secreted proteins is largely avoided in these earlier studies due to the exchange of the initial met+ growth medium for met− medium prior to the addition of SeMet, such that any SeMet-free secreted recombinant protein produced to that point is discarded. Therefore, a protocol for the production of intracellularly produced SeMet derivatized recombinant protein must use a timeline that, ideally, excludes the production of SeMet-free protein. It was considered that once this timeline was established, it should be readily applicable also to the production of SeMet-substituted secreted recombinant proteins.
Based on previous studies and on in-house strategies, the general guidelines for SeMet substitution were formulated as follows: Healthy dividing insect cells at high viability (>98%) and at densities of 2–4 × 106 cells/mL were infected with recombinant baculoviruses at a multiplicity of infection (moi) of ∼1 in ESF-921 protein-free medium without antibiotics. After a defined period of infection (to be optimized by experimentation), the cells were spun down and resuspended in an equal volume of ESF-921 protein-free methionine-free medium with antibiotics (50 μg/mL gentamycin). After a further defined period of infection (again, to be optimized by experimentation), SeMet was added to the desired concentration and infection continued for 48–96 h total. Using this general protocol, the effects of timeline on the incorporation of SeMet into the intracellularly produced FGFR2 kinase domain was examined with regard to the durations of infection in met+ and met− media prior to the addition of SeMet, and to total expression time. As shown in ▶, the critical point of SeMet addition is within the first 16 h following viral infection, since the addtion of SeMet at 24 h post-infection is too late to avoid the production of some SeMet-free recombinant protein, which reduces the overall level of incorporation. These observations are consistent with the timeline of recombinant protein production in BEVS reported by O'Reilly and Miller (1988), and indicates that the initiation of recombinant protein expression from the polyhedrin promoter in baculovirus-infected insect cells is circa 16–20 h post-infection. Under optimal SeMet concentrations (see below), the replacement of the met− medium with fresh met− medium at the time of SeMet addition was found to increase the incorporation of SeMet by about 5% (data not shown). This increase in SeMet incorporation was considered insufficient to justify the expense of the medium and the additional exposure of the cells to potential contamination when conducting large-scale expressions (≥5 L). However, it does provide a means to improve the SeMet incorporation in critical, or low-volume, experiments. The presence or absence of 5% FBS in the medium had little effect on the incorporation of SeMet (data not shown), nor did variations in the total time of expression between 48–96 h (data not shown).
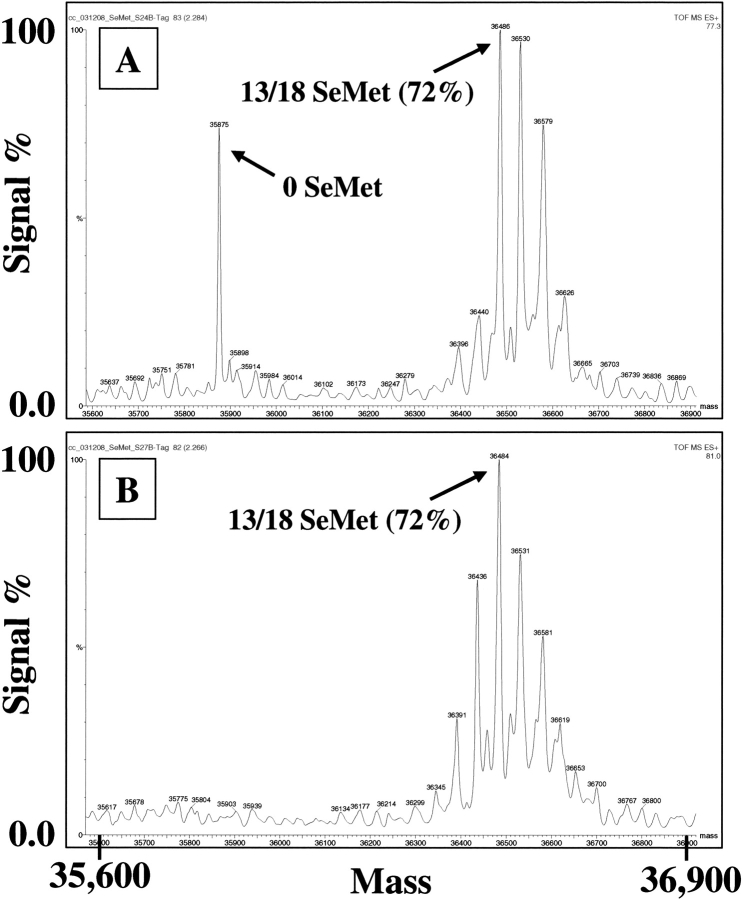
Figure 1.
Effect of time of addition of SeMet on SeMet incorporation into BEVS-produced recombinant FGFR2 kinase domain. High Five insect cells (0.5 L in 1-L shake flasks) were infected with a recombinant baculovirus overproducing the kinase domain of FGFR2 at an moi of ∼1 in ESF921 protein-free medium. After 16 h (A) or 8 h (B) the medium was replaced with ESF921 protein-free, methionine-free medium, and after a further 8 h, SeMet was added to a final concentration of 250 mg/L. Cells were harvested 48 h post-infection and the recombinant FGFR2 isolated from the cell pellet by IMAC chromatography. The His tag was removed by treatment with TEV protease and the protein further purified by reverse IMAC. The purified proteins were analyzed by mass spectrometry.
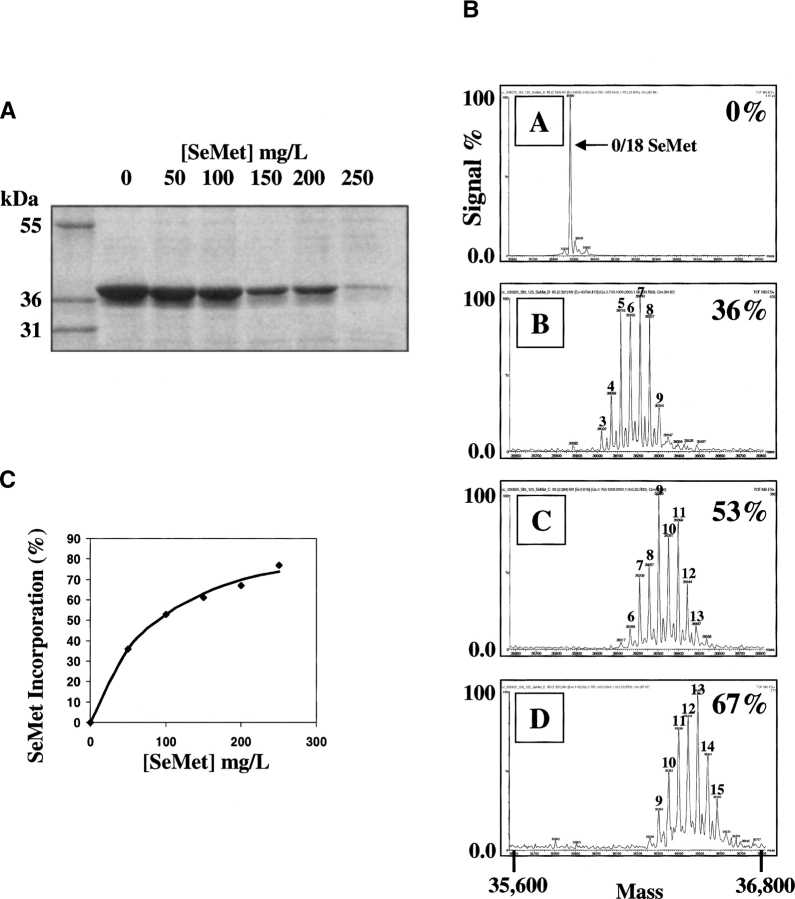
After establishing a timeline for infection and SeMet addition (see, e.g., ▶), the effects of SeMet concentration on the incorporation of SeMet, and on recombinant protein production, were examined. Reports describing the production of SeMet-substituted proteins in BEVS have used SeMet concentrations in the range 50–100 mg/L. ▶ shows that the recovery of recombinant protein in Sf9 cells is compromised by the presence of SeMet in the growth medium, with a reduction to ∼50% that of native protein recovery at a SeMet concentration of 100 mg/L (see ▶). This corresponded to a SeMet incorporation level of 53%. Greater incorporation of SeMet, to ∼70%, was obtained by increasing the SeMet concentration to 200 mg/L. Under such conditions, there was a significant drop in recombinant protein recovery to ∼20% that of the native protein. However, this was considered an acceptable trade-off in order to obtain protein with the greater SeMet incorporation. Increasing the SeMet concentration in the growth medium above 200 mg/L resulted in an unacceptable recovery of target protein (see ▶). However, if a lower level of SeMet incorporation is deemed sufficient for phase determination, the data in ▶ show that one may titrate the SeMet incorporation level in the target (i.e., to a maximum of ∼70%) in order to achieve a more desirable protein recovery. In High Five cells the balance of high SeMet incorporation (70%–80%; see below) with acceptable protein recovery (∼20%; see ▶) was achieved at an SeMet concentration of 250 mg/L (data not shown; see also ▶, ▶; ▶ below).
Figure 2.
Effect of SeMet concentration on SeMet incorporation and on recombinant protein recovery in BEVS-produced recombinant FGFR2 kinase domain. Sf9 insect cells (0.5 L in 1-L shake flasks) were infected with a recombinant baculovirus overproducing the kinase domain of FGFR2 at a moi of ∼1 in ESF921 protein-free medium. After 8 h the medium was replaced with ESF921 protein-free methionine-free medium, and after a further 8 h, SeMet was added to various final concentrations. Cells were harvested 48 h post-infection and the recombinant FGFR2 isolated from the cell pellet by IMAC chromatography. (A) SDS-PAGE analysis of material eluted from IMAC chromatography after expression in the presence of the indicated concentrations of SeMet. (B) Mass spectrogram traces of purified detagged FGFR2 kinase domains following expression in the presence of (A) 0, (B) 50, (C) 100, and (D) 200 mg/L SeMet. The % SeMet incorporation is indicated on the right in each trace (estimated by inspection as described in the Materials and Methods section). (C) Plot of % SeMet incorporation into recombinant FGFR2 kinase domain as a function of the concentration of SeMet used in the growth medium.
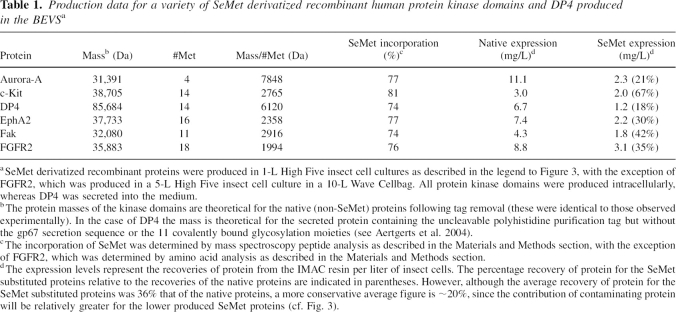
Table 1.
Production data for a variety of SeMet derivatized recombinant human protein kinase domains and DP4 produced in the BEVSa
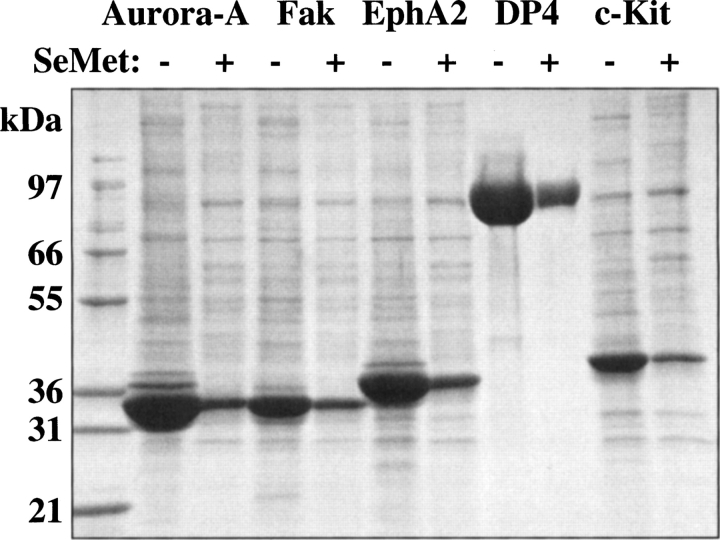
Figure 3.
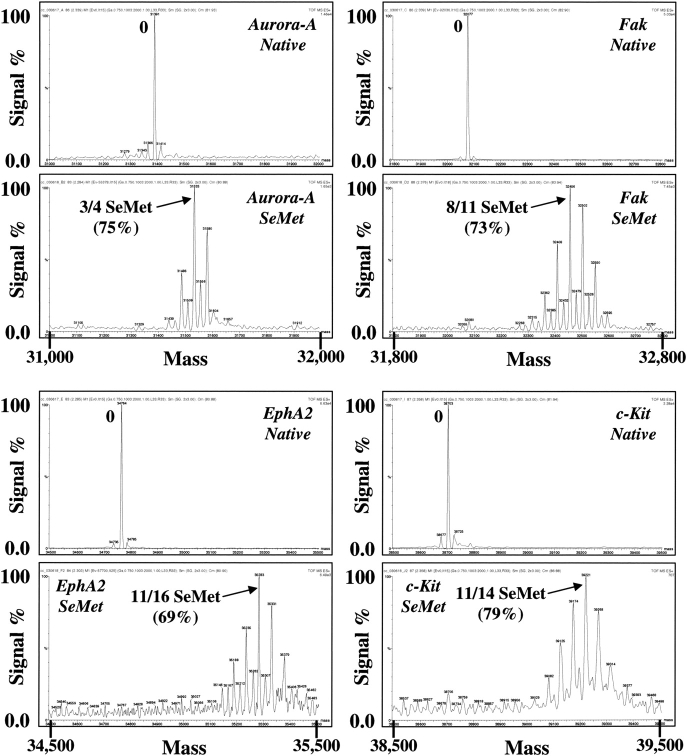
SDS-PAGE analysis of a selection of SeMet-derivatized proteins produced in the BEVS. SeMet-derivatized proteins were produced by infecting High Five insect cells (1 L in 3-L shake flasks) with recombinant baculoviruses overproducing various intracellularly expressed human protein kinase domains, or secreted DP4 peptidase, at a moi of ∼1 in ESF921 protein-free medium. After 8 h the medium was replaced with ESF921 protein-free methionine-free medium, and after a further 8 h, SeMet was added to a final concentration of 250 mg/L. The cells were harvested 48 h post-infection and the recombinant proteins isolated by IMAC chromatography. Native proteins were produced under identical conditions with the exception that SeMet was not added and ESF-921 protein-free medium was used throughout. The figure shows a comparison, by SDS-PAGE analysis, of the recoveries of the native proteins and their SeMet-derivatized counterparts as eluted from the IMAC chromatography columns.
Figure 4.
Mass spectroscopy analysis of a selection of SeMet-derivatized proteins produced in the BEVS. Shown are the mass spectroscopy traces of the proteins produced as described in the legend to ▶. Prior to the analysis, the proteins were further purified by removing the polyhistidine purification tags with TEV protease and repurifying over IMAC chromatography.
In order to test the generality of the SeMet incorporation protocol, the incorporation of SeMet into five different intracellularly expressed recombinant protein kinases was examined using High Five insect cells, in addition to the secreted enzyme DP4. These proteins vary greatly in their percentage of methionine content, as listed in ▶, and so the applicability of the protocol in this regard could be assessed. As shown in ▶ and ▶, and compiled in ▶, the incorporation of SeMet was found to be quite consistent, ranging from 74%–81%, irrespective of the methionine content of the protein, or whether the protein was produced intracellularly or in a secreted form. Also, as illustrated in ▶, the yield of recombinant protein produced under these optimized conditions for SeMet incorporation shows an average drop in recombinant protein recovery, to about 20% that of native protein recovery. Thus, the nature of the protein itself appears not to influence the incorporation of SeMet, although the recovery of recombinant protein is somewhat protein specific. This indicates that the protocol should produce SeMet-substituted proteins with a consistently high SeMet incorporation. The protocol was found also to be applicable to scaleup, since the production of a number of SeMet substituted recombinant proteins in 5L insect cell cultures using 10-L Wave Cellbags generated results that were similar to those obtained in the shake flask cultures (e.g., FGFR2 in ▶).
Conclusions
There have been a limited number of reports describing procedures for the incorporation of SeMet into recombinant proteins produced in baculovirus-infected insect cells. Among these, there is substantial variation in the conditions reported with regard to media type and components, the timeline of viral infection, and the results of SeMet incorporation, the latter ranging from 35%-100%. The present report describes a unified protocol that may be used to obtain a consistently high incorporation of SeMet (70%–75%) into both intracellularly produced and secreted SeMet derivatized recombinant proteins. The method should find broad application in crystallographic approaches to protein structure solution by MAD phasing.
Materials and Methods
Materials
ESF-921 protein-free cell culture medium and ESF-921 protein-free methionine-free cell culture medium were obtained from Expression Systems LLC. Spodoptera frugiperda Sf9 and Trichoplusia ni High Five insect cells that had been adapted to ESF-921 protein-free insect cell medium were obtained from Expression Systems LLC. Disposable polycarbonate Erlenmeyer flasks were supplied by Corning. Wave BioReactors (System 20/50EH) and Wave Cellbags (10/0) were obtained from Wave Biotech. L(+)-selenomethionine (SeMet) was purchased from Acros Organics.
Passaging of insect cells
Spodoptera frugiperda Sf9 and Trichoplusia ni High Five insect cells were passaged at 27°C using standard procedures (O'Reilly et al. 1994). Cell densities and viabilities (Trypan Blue dye method) were determined by using an Innovatis Cedex AS 20 cell counter.
Determination of SeMet incorporation
The incorporation of SeMet was initially determined by amino acid analysis carried out at AAA Service Laboratory. This procedure estimates SeMet substitution based on the disappearance of methionine from the amino acid analysis chromatogram. An alternative procedure, utilizing peptide analysis by mass spectrometry, was developed in-house (Lim et al. 2003), and was found to correlate well with the data generated from the amino acid analysis. The incorporation of SeMet could also be determined reasonably accurately by inspection of the mass spectrometry trace of the intact protein. For such determinations, the SeMet proteins were analyzed by using a LCT ESI-TOF mass spectrometer (Waters) coupled with an Agilent 1100 HPLC system (Agilent Technologies). The protein samples were injected by using an HTS-PAL autosampler (LEAP Technologies) onto a 2.1 × 75-mm Poroshell C18 column (Agilent Technologies) and eluted with a 3.5-min acetonitrile/TFA gradient at 400 μL/min. The raw MS data, acquired using positive electrospray ionization with mass range m/z 1000–2000 in Continuum mode, were converted to zero-charge spectra by using the MaxEnt deconvolution software (Waters). This latter approach was not suitable for the secreted DP4 protein, since post-translational derivatization by glycosylation did not allow for the mass spectrometry data to be deconvoluted, and the SeMet incorporation was determined by the peptide analysis procedure.
Generation of recombinant baculoviruses
Details of the cDNA cloning and construction of recombinant baculoviruses expressing Aurora-A, Fak, and EphA2 kinase domains were described previously (Nowakowski et al. 2002), as were those for cKit kinase domain (Mol et al. 2003) and secreted DP4 peptidase (Aertgerts et al. 2004). A recombinant baculovirus expressing the kinase domain of the human FGFR2 receptor tyrosine kinase, residues 450–759, was constructed by using identical procedures to those described previously (Nowakowski et al. 2002). The source of the FGFR2 kinase DNA was by PCR amplification from a mixture of prostrate, placenta, testis, fetal brain, and fetal liver cDNA libraries.
Acknowledgment
We thank Devon A. Thompson for generating the recombinant baculovirus expressing the FGFR2 kinase domain.
Footnotes
Reprint requests to: Ciarán N. Cronin, Pfizer, Inc., 10777 Science Center Drive, San Diego, CA 92121, USA; e-mail: ciaran.cronin@pfizer.com; fax: (858) 526-4240.
Abbreviations: BEVS, baculovirus expression vector system; IMAC, immobilized-metal affinity chromatography; moi, multiplicity of infection; SeMet, selenomethionine; TEV, tobacco etch virus (protease).
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.072931407.
References
- Aertgerts K., Ye, S., Tennant, M.G., Kraus, M., Rogers, J., Sang, B.-C., Skene, R.J., Webb, D.R., and Prasad, G.S. 2004. Crystal structure of human dipeptidyl peptidase IV in complex with a decapeptide reveals details on substrate specificity and tetrahedral intermediate formation. Protein Sci. 13 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricescu A.R., Lu, W., and Jones, E.Y. 2006a. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr. D62 1243–1250. [DOI] [PubMed] [Google Scholar]
- Aricescu A.R., Assenberg, R., Bill, R.M., Busso, D., Chang, V.T., Davis, D.A., Dubrovsky, S.J., Gustafsson, L., Hedfalk, K., Heinemann, U., et al. 2006b. Eukaryotic expression: Developments for structural proteomics. Acta Crystallogr. D62 1114–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton W.A., Tzvetkova-Robev, D., Erdjumentbromage, H., Tempst, P., and Nikolov, D.B. 2006. Highly efficient selenomethionine labeling of recombinant proteins produced in mammalian cells. Protein Sci. 15 2008–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellizzi J.J., Widom, J., Kemp, C.W., and Clardy, J. 1999. Producing selenomethionine-labeled proteins with a baculovirus expression vector system. Structure 7 R263–R267. [DOI] [PubMed] [Google Scholar]
- Brodsky O. and Cronin, C.N. 2006. Economical parallel protein expression screening and scale-up in Escherichia coli . J. Struct. Funct. Genomics 7 101–108. [DOI] [PubMed] [Google Scholar]
- Bushnell D.A., Cramer, P., and Kornberg, R.A. 2001. Selenomethionine incorporation in Saccharomyces cerevisiae RNA polymerase II. Structure 9 R11–R14. [DOI] [PubMed] [Google Scholar]
- Carfi A., Gong, H., Lou, H., Willis, S.H., Cohen, G.H., Eisenberg, R.J., and Wiley, D.C. 2002. Crystallization and preliminary diffraction studies of the ectodomain of the envelope glycoprotein D from herpes simplex virus 1 alone and in complex with the ectodomain of the human receptor HveA. Acta Crystallogr. D58 836–838. [DOI] [PubMed] [Google Scholar]
- Chen W. and Bahl, O.P. 1991a. Recombinant carbohydrate and selenomethionyl variants of human choriogonadotropin. J. Biol. Chem. 266 8192–8197. [PubMed] [Google Scholar]
- Chen W. and Bahl, O.P. 1991b. Selenomethionyl analog of recombinant human choriogonadotropin. J. Biol. Chem. 266 9355–9358. [PubMed] [Google Scholar]
- Fremont D.H., Crawford, F., Marrack, P., Hendrickson, W.A., and Kappler, J. 1998. Crystal structure of mouse H2-M. Immunity 9 385–393. [DOI] [PubMed] [Google Scholar]
- Guerrero S.A., Hecht, H.-J., Hofmann, B., Biebl, H., and Singh, M. 2001. Production of selenomethionyl-labelled proteins using simplified culture conditions and generally applicable host/vector systems. Appl. Microbiol. Biotechnol. 56 718–723. [DOI] [PubMed] [Google Scholar]
- Hendrickson W.A. and Ogata, C.M. 1997. Phase determination from multiwavelength anomalous diffraction measurements. Methods Enzymol. 276 494–523. [DOI] [PubMed] [Google Scholar]
- Hendrickson W.A., Horton, J.R., and LeMaster, D.M. 1990. Selenomethionyl proteins produced for analysis by multiwavelength anomalous diffraction (MAD): A vehicle for direct determination of three-dimensional structure. EMBO J. 9 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosfield D., Palan, J., Hilgers, M., Scheibe, D., McRee, D.E., and Stevens, R.C. 2003. A fully integrated protein crystallization platform for small-molecule drug discovery. J. Struct. Biol. 142 207–217. [DOI] [PubMed] [Google Scholar]
- Kigawa T., Yamaguchi-Nunokawa, E., Kodama, K., Matsuda, T., Yabuki, T., Matsuda, N., Ishitani, R., Nureki, O., and Yokoyam, S. 2002. Selenomethionine incorporation into a protein by cell-free synthesis. J. Struct. Funct. Genomics 2 29–35. [DOI] [PubMed] [Google Scholar]
- Larsson A.M., Stahlberg, J., and Jones, T.A. 2002. Preparation and crystallization of selenomethionyl dextranase from Penicillium minioluteum expressed in Pichia pastoris . Acta Crystallogr. D58 346–348. [DOI] [PubMed] [Google Scholar]
- Leahy D.J., Hendrickson, W.A., Aukhil, I., and Erickson, H.P. 1992. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science 258 987–991. [DOI] [PubMed] [Google Scholar]
- Lesley S.A. 2001. High-throughput proteomics: Protein expression and purification in the post-genomic world. Protein Expr. Purif. 22 159–164. [DOI] [PubMed] [Google Scholar]
- Liemann S., Chandran, K., Baker, T.S., Nibert, M.L., and Harrison, S.C. 2002. Structure of the reovirus membrane-penetration protein, μ1, in a complex with its protector protein, σ3. Cell 108 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K.B., Cronin, C.N., and Kassel, D.B. 2003. Proceedings of the 51st ASMS Conference Mass Spectrometry and Allied Topics, Montreal, Canada.
- Lustbader J.W., Wu, H., Birken, S., Pollak, S., Gawinowicz Kolks, M.A., Pound, A.M., Austen, D., Hendrickson, W.A., and Canfield, R.E. 1995. The expression, characterization, and crystallization of wild-type and selenomethionyl human chorionic gonadotropin. Endocrinology 136 640–650. [DOI] [PubMed] [Google Scholar]
- McMullan D., Canaves, J.M., Quijano, K., Abdubek, P., Nigoghossian, E., Haugen, J., Klock, H.E., Vincent, J., Hale, J., Paulsen, J., et al. 2005. High-throughput protein production for X-ray crystallography and use of size exclusion chromatography to validate or refute computational biological unit predictions. J. Struct. Funct. Genomics 6 135–141. [DOI] [PubMed] [Google Scholar]
- McWhirter S.M., Pullen, S.S., Holton, J.M., Crute, J.J., Kehry, M.R., and Alber, T. 1999. Crystallographic analysis of CD40 recognition and signaling by human TRAF2. Proc. Natl. Acad. Sci. 96 8408–8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol C.D., Lim, K.B., Sridhar, V., Zou, H., Chien, E.Y., Sang, B.C., Nowakowski, J., Kassel, D.B., Cronin, C.N., and McRee, D.E. 2003. Structure of a c-kit product complex reveals the basis for kinase transactivation. J. Biol. Chem. 278 31461–31464. [DOI] [PubMed] [Google Scholar]
- Muchmore S.W., Olson, J., Jones, R., Pan, J., Blum, M., Greer, J., Merrick, S.M., Magdalinos, P., and Nienaber, V.L. 2000. Automated crystal mounting and data collection for protein crystallography. Struct. Fold. Des. 8 R243–R246. [DOI] [PubMed] [Google Scholar]
- Nowakowski J., Cronin, C.N., McRee, D.E., Knuth, M.W., Nelson, C.G., Pavletich, N.P., Rogers, J., Sang, B.C., Scheibe, D.N., Swanson, R.V., et al. 2002. Structures of the cancer-related Aurora-A, FAK, and EphA2 protein kinases from nanovolume crystallography. Structure 10 1659–1667. [DOI] [PubMed] [Google Scholar]
- O'Reilly D.R. and Miller, L.K. 1988. Expression and complex formation of simian virus 40 large T antigen and mouse p53 in insect cells. J. Virol. 62 3109–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly D.R., Miller, L.K., and Luckow, V.A. 1994. Baculovirus expression vectors. A laboratory manual. Oxford University Press, New York.
- von Delft F., Inoue, T., Saldanha, S.A., Ottenhof, H.H., Schmitzberger, F., Birch, L.M., Dhanaraj, V., Witty, M., Smith, A.G., Blundell, T.L., et al. 2003. Structure of E. coli ketopantoate hydroxymethyl transferase complexed with ketopantoate and Mg2+, solved by locating 160 selenomethionine sites. Structure 11 985–996. [DOI] [PubMed] [Google Scholar]
- Xu B., Munoz, I.G., Janson, J.-C., and Stahlberg, J. 2002. Crystallization and X-ray analysis of native and selenomethionyl β-mannanase Man5A from blue mussel, Mytilus edulis, expressed in Pichia pastoris . Acta Crystallogr. D58 542–545. [DOI] [PubMed] [Google Scholar]