Abstract
Amperometry in chromaffin cells expressing green fluorescent protein (GFP) fused to synaptosome-associated protein of 25 kDa (SNAP-25) have been used to test the involvement of single amino acids in exocytotic function, overcoming some of the limitations of studies based on Botulinum neurotoxin cleavage, as this occurs at defined sites of the protein. Constructs containing either the whole SNAP-25 polypeptide or several deleted forms lacking its C-terminal domain were heavily overexpressed in transfected cells. All GFP-fusions were located in both the cytoplasm and the plasma membrane. Although a construct containing complete SNAP-25 sustained normal secretion, removal of four or more amino acids of its C terminus greatly altered the overall rate and extent of exocytosis. Further mutational analysis proved that Leu203, the fourth residue from the C terminus, is critical for secretion. Kinetics of single granule fusions from cells expressing truncated forms showed slow onset and decay times when compared with control cells expressing full SNAP-25. Thus, these data provide direct evidence for the involvement of a specific residue of SNAP-25 in exocytosis and show that overexpression of GFP-SNAP contructs combined with single vesicle fusion measurements constitutes a powerful approach to dissect the structural elements playing a role in individual steps of the exocytotic cascade.
Keywords: membrane fusion, amperometry, green fluorescent protein expression
In the nervous system, information is sent across synapses between individual cells by exocytosis of chemical neurotransmitters. For this reason, the elucidation of the molecular basis of exocytosis is essential to understanding neurotransmission. In this sense, the characterization of a variety of proteins [soluble N-ethylmaleimide-sensitive fusion protein, attachment protein receptors (SNAREs)], which constitute the complex involved in specific docking and fusion of neurotransmitter-containing vesicles, has stimulated an unprecedented progress in this matter (1–5). Assembly of the initial core complex between plasma membrane proteins (t-SNAREs) such as syntaxin (6) and synaptosome-associated protein of 25 kDa (SNAP-25) (7) and vesicle associated proteins (v-SNAREs) such as synaptobrevin (8) provides the specificity required for vesicle docking and, probably, the minimal machinery for membrane fusion (9) whereas the interaction between these proteins and other membrane-attached (synaptotagmin, munc-18, etc.) or cytosolic (SNAPs, ATP, etc) factors ensures the necessary control for the process. Cleavage of SNAREs by Clostridial neurotoxins has been established as an essential tool to investigate the role of these proteins in neurotransmission (10). More specifically, botulinum neurotoxins A and E (BoNT A and E) proteolize the C-terminal domain of SNAP-25 (11, 12), causing partial inhibition of secretion in both neuronal (11) and endocrine systems (13, 14). Given that the neurotoxins are unable to cleave SNAP-25 when this protein is forming part of mature trimeric complexes, as demonstrated in in vitro assays (15), it has been proposed that the initial release, which is relatively insensitive to BoNT A (16, 17), could be driven by complexes formed by the remaining uncleaved proteins. The domain restriction imposed by toxin cleavage prompted us to design a new approach to the involvement of specific SNAREs residues in discrete stages of the exocytotic process. Thus, our strategy was based in overexpressing native as well as altered forms of SNAP-25 coupled to green fluorescent protein (GFP) in bovine chromaffin cells and studying the effect produced by these constructs in the secretory properties of the cells. Our results indicate that expression of truncated forms of SNAP-25 cause inhibition of secretion, consistent with the effect reported for BoNT A in adrenomedullary cells, thus stressing the specificity of toxin action. Furthermore, a specific residue, Leu203, close to the C terminus, appears essential for the function of SNAP-25 in exocytosis.
MATERIALS AND METHODS
Chromaffin Cell Culture and Transfections.
Chromaffin cells were prepared from bovine adrenal glands and were maintained as described (18). The cDNA corresponding to the SNAP-25a isoform (19) was cloned into pEGFP-C3 (CLONTECH) to be expressed as in-frame fusion to the C terminus of GFP. Deletions and point mutations were generated by PCR of a fragment corresponding to the C terminus of SNAP-25 and a sense primer that corresponds to amino acids 80–86 of SNAP-25 (5′-AGATTTAGGGAAATGCTGTGG-3′). The different deletions and mutations were defined by the antisense primers that coded for the desired C terminus.
Chromaffin cells were transfected either by electroporation or by calcium phosphate. For the calcium phosphate procedure (20), cells plated the day before on 35-mm plates (Costar) were incubated with 6 μg of plasmid overnight. For electroporation, 107 cells were resuspended in 0.8 ml of electroporation buffer [in mM: 140 NaCl, 0.75 Na2HPO4, 25 Hepes (pH 7.0)], and linearized plasmid (50 μg in 60 μl H2O) was added. Electroporator (Bio-Rad Gene Pulser II) was set at 250 V and 950 μF. A time constant around 17 ms was obtained in a typical experiment. After leaving the cells 10 min to recover, 0.15 ml of electroporated cells were plated in each 35-mm plate. Cells were washed the next day. Both methods yielded low transfection efficiencies, although, in general, many cells per plate were available for amperometric measurements. In addition, seasonal variability in transfection efficiency was observed, with almost no expression during the summer months.
Amperometric Determination of Exocytosis.
Carbon-fiber electrodes were used to monitor catecholamine release from individual chromaffin granules in conditions previously described (17). Experiments were performed in cells stimulated by superfusion with depolarizing 59 mM high potassium. Electrode variations were alleviated by using the same electrode for measurements in control (nontransfected) and transfected cells in the same dish. Controls in nontransfected cells were taken before and after performing measurements in transfected cells, and peak analysis included experiments performed in 10–17 cells from each separated transfection experiment.
Confocal Microscopy Studies.
Analysis of the distribution of native SNAP-25 and GFP–S-25 constructs was performed by using immunostaining methods previously described (21). Fluorescence was investigated by using a Laser Scanning confocal True Confocal Scanner (Leica, Heidelberg, Germany) microscope. Usually, eight confocal layers covering the total cell volume were obtained [1.25-μm thickness (z)], and individual layers, projections, or three-dimensional reconstructions were used to study fluorescence distribution.
RESULTS
Constructs Containing GFP Fused to Native or Altered Forms of SNAP-25 Are Heavily Expressed in Chromaffin Cells.
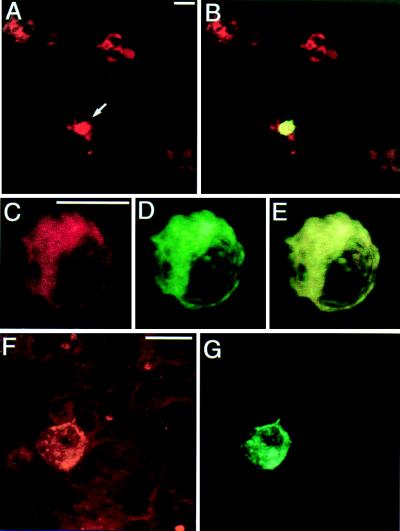
Our experimental strategy implied the overexpression of native and mutant SNAP-25 proteins in chromaffin cells with the aim of competing and, eventually, substituting the native SNAP-25, thus studying their effect on the secretory activity of individual cells. To know which cells were expressing the exogenous SNAP-25 protein, the latter was fused to GFP. For this purpose, we assembled constructs in which the C terminus of GFP was linked to the N terminus of SNAP-25 (GFP–S-25). We chose this region of SNAP-25 for the fusion because it seems to be less critical for protein function, as an N-terminal deletion of 24 residues did not affect binding to other SNAREs (18). Two days after transfection of chromaffin cells, a low proportion of them (Fig. 1 A and B) showed bright green fluorescence, indicating the strong expression of GFP–SNAP-25. All chromaffin cells were also immunoreactive to an anti-SNAP-25 antibody (Fig. 1A), which labeled both native and exogenous SNAP-25. Confocal analysis at higher magnification of a cell expressing the GFP–S-25 construct revealed that the distribution pattern of anti-SNAP-25 antibody (Fig. 1C) and GFP (Fig. 1D) were coincident, as demonstrated by almost total overlay (Fig. 1E). The fusion protein was heavily expressed in the cytoplasm and also was located in patches in the plasma membrane. This membrane location is consistent with the one reported for the native protein in chromaffin cells (21) whereas heavy cytoplasm labeling is typical of protein overexpression. A similar pattern of expression was observed when truncated SNAP-25 forms were used instead of the whole protein. We first tried to express a form of SNAP-25 lacking the last nine C-terminal residues (GFP–S-25 Δ 9) because the inhibitory action of BoNT A is based on the cleavage of the same residues. Overexpression of the truncated form is evidenced when the amount of anti-SNAP-25 immunoreactivity (Fig. 1F) present in a GFP-positive cell (Fig. 1G) is compared with the one from nonexpressing cells (Fig. 1F). These observations were common to the other truncated or mutated constructs that were used for functional studies and let us to conclude that GFP–SNAP-25 fusion proteins constituted the majority of the SNAP-25 protein present in the cells that have been transfected.
Figure 1.
Expression of GFP–S-25 constructs in cultured chromaffin cells. Transfected bovine chromaffin cells were incubated with anti-SNAP-25 antibody, followed by labeling with a second antibody coupled to rhodamine. (A and B) Low field magnification (200×) of stained chromaffin cells. In B, the green fluorescence attributable to GFP expression is observed as yellow (arrow) because of the overlap with the red labeling from the anti-SNAP-25 antibody. (C, D, and F) Three-dimensional reconstruction image of a GFP–S-25-expressing chromaffin cell showing the coincident distribution pattern obtained with anti-SNAP-25 antibody (C) and GFP (D). E is an overlay of C and D. (F and G). Expression of GFP–S-25 Δ9 in chromaffin cells. The level of SNAP-25, because of overexpression of the GFP–S-25 Δ9 construct, as detected by an anti-SNAP-25 antibody (F), is much higher in the GFP expressing cell (G) than in the nearby nonexpressing chromaffin cells. Fluorescence intensity was set to low gaining to better appreciate these differences. A similar distribution was observed with other truncated or mutated constructs. (Bars = 10 μm.)
Overexpression of SNAP-25 Truncated Forms Inhibits Catecholamine Secretion from Chromaffin Cells.
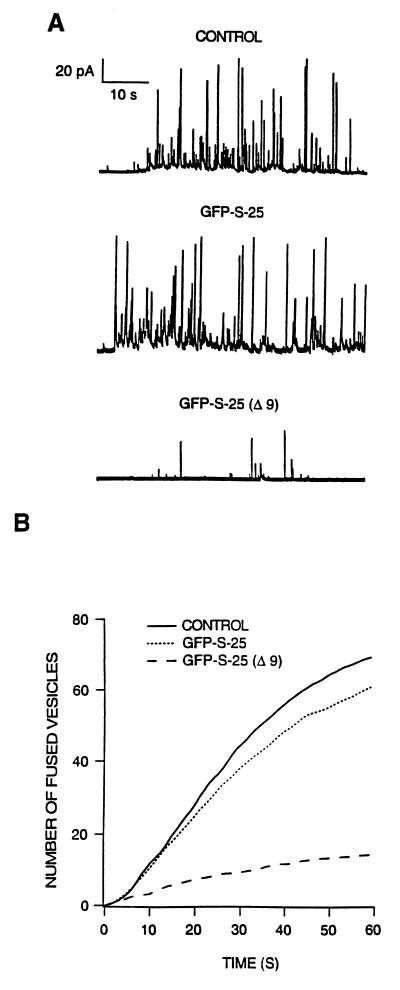
To explore the functionality of chromaffin cells expressing GFP–S-25 constructs, we monitored the focal release of catecholamine contents by amperometry (22) of strong fluorescent cells. We first tested whether the expression of the construct containing full SNAP-25 (GFP–S-25) affected secretion induced by a continuous depolarizing stimulus. The characteristics of vesicle release from transfected cells (Fig. 2A, GFP–S-25) did not differ much from the ones obtained for control nontransfected cells present in the same culture dish (Fig. 2A, CONTROL). Further analysis by averaging of cumulative event histograms for 14 control and 13 transfected cells revealed no significant differences in the amount (70 ± 9 vs. 61 ± 11 vesicles released per minute, respectively) and profile of catecholamine release (Fig. 2B). By contrast, overexpression of the truncated form GFP–S-25 Δ9, which, as mentioned above, would be similar to the product resulting from BoNT A action, dramatically affected secretory behavior (Fig. 2 A and B). The amount of released vesicles decreased strongly, and the overall rate of secretion was clearly affected. This effect was quantified by analyzing the rate of release during the first 10 s and the number of vesicles released during 1 min, providing direct evidence about the specificity of BoNT A in causing inhibition of neurotransmission through the cleavage of SNAP-25 C-terminal domain.
Figure 2.
Secretory activity of chromaffin cells expressing GFP–S-25 constructs. (A) Amperometric traces representative from experiments performed in cells expressing full length SNAP-25 (GFP–S-25) and a truncated form (GFP–S-25 Δ9). Shown are also traces obtained from control nontransfected cells (CONTROL) present in the same culture plates that transfected cells. (B) Cumulative event analysis. Conditions were as described in A, after building of event cumulative responses for individual cells. The average secretion was obtained from control nontransfected (n = 14, continuous line), GFP–S-25 (n = 12, pointed line), and GFP–S-25 Δ9-expressing cells (n = 13, dashed line).
A Minimal Deletion of Four Residues in the C Terminus of SNAP-25 Inhibits Secretion.
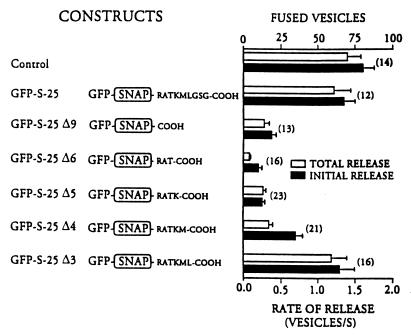
The type of experiments previously described would allow us to address a further question regarding SNAP-25 structure–function relationships: Are all nine amino acids cleaved by BoNT A from the SNAP-25 C terminus, and lacking in GFP–S-25 Δ9, equally needed for SNAP-25 function? We tried to answer this question by expressing different GFP–S-25 constructs lacking the last six, five, four, and three C-terminal residues from SNAP-25 and characterizing the secretory activity of cells transfected with them. As can be seen in Fig. 3, the rate of release and total secretion of cells expressing constructs lacking six, five, and four residues was greatly diminished compared with catecholamine release from control nontransfected and GFP–S-25-transfected cells. By contrast, the exocytotic properties of cells expressing GFP–S-25 Δ3 were similar to the controls. Therefore, Leu203, which is present in GFP–S-25 Δ3 but absent in GFP–S-25 Δ4, seems to have a decisive contribution in sustaining normal secretory activity in transfected chromaffin cells.
Figure 3.
Analysis of the minimal truncation in SNAP-25 C terminus that sustains normal secretory activity in chromaffin cells. After averaging cumulative event responses as indicated in Fig. 2, the rate of release during the initial 10 s (INITIAL RELEASE) and extent of secretion during 1 min of stimulation (TOTAL RELEASE) was measured for control nontransfected and cells expressing the full length and deletions of SNAP-25 constructs. The structure of each construct, regarding its C terminus, is shown at the left of the figure. Data are means ± SEM from experiments performed in a number (n) of cells.
Leu203 Mutations Greatly Affect Exocytosis.
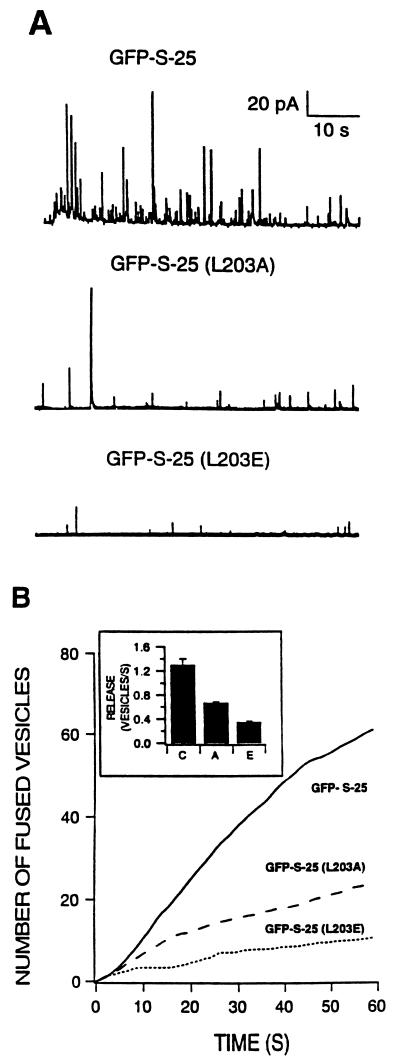
To further demonstrate the influence of Leu203 in the exocytotic function of SNAP-25, we tested the effect caused by specific mutations of this residue in the context of the complete SNAP-25 construct (GFP–S-25). The first mutation, which replaced leucine by alanine, was a conservative change that maintained the hydrophobic characteristics with a shorter lateral hydrocarbon chain. Overexpression of GFP–S-25(L203A) (Fig. 4) constructs clearly affects the secretory capability of transfected cells (n = 13 analyzed cells). Both the initial rate of release and total catecholamine secretion obtained after 1 min of continuous depolarization decreased to ≈50% of the values characteristic of cells expressing the GFP–S-25 construct. A second, less conservative mutant, in which Leu203 was replaced by an amino acid of similar size but negatively charged, such as glutamic acid [GFP–S-25 (L203E)], had more impact in decreasing the secretory activity of transfected chromaffin cells (Fig. 4). The analyzed parameters of the rate of release and cumulative secretion found in these cells (n = 7) were similar to those obtained for the construct lacking the 9 C-terminal residues (Fig. 3). Therefore, both the size and the physicochemical properties of the amino acid at position 203 of SNAP-25 affect its secretory function, although polarity seems to have a greater influence.
Figure 4.
Mutations in Leu203 affect secretory activity of transfected chromaffin cells. Role of SNAP-25 Leu203 residue in supporting secretory activity was analyzed by mutating it to alanine [GFP–S-25 (L203A)] or to glutamic acid [GFP–S-25 (L203E)]. (A) Representative traces obtained from depolarization responses of GFP–S-25-, GFP–S-25 (L203A)-, and GFP–S-25 (L203E)-expressing cells. (B) Averaged cumulative responses. The averaged responses from 12 control cells (GFP–S-25, solid line), 13 cells transfected with the Ala mutation (dashed line), and 7 cells expressing the Glu mutation (pointed line) are shown. Rate of secretion during the initial 10 s of stimulation for the control (C), and Ala (A), and Glu (E) mutations is shown in the inset.
Secretory Stages Altered by the Truncation of the C-Terminal Domain of SNAP-25.
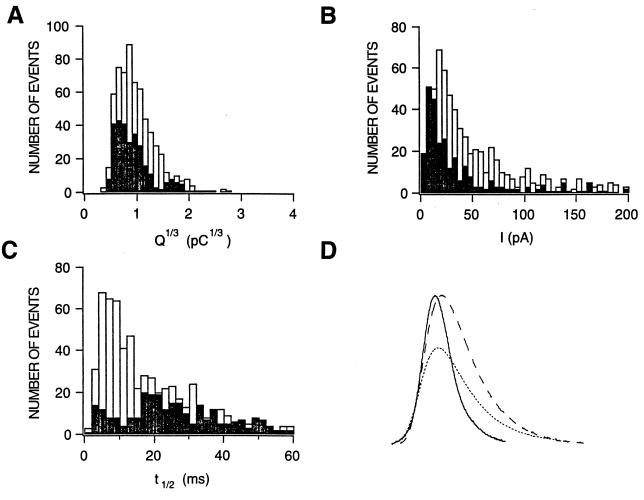
If the very final events of membrane fusion and catecholamine release were affected, it is conceivable that single event amperometric characteristics would reflect such modification because every step of the granule fusion process is represented in spike shape and kinetics (23). A careful analysis of secretory events occurring in the proximity of the carbon fiber electrode was performed searching for narrow and well separated spikes with amplitudes >5 pA. Selected fusions from cells transfected with GFP–S-25 Δ6 (285 events) and control nontransfected cells (627 spikes) present in the same culture dishes were analyzed by measuring amplitude, half-width, and event charge. Data were binned to generate the distribution histograms shown in Fig. 5. No significant difference was detected in the average charge (Q1/3) released per vesicle (0.98 ± 0.04 and 0.91 ± 0.06 pC1/3 for control and GFP–S-25 Δ6-expressing cells, respectively), and both distributions centered around the modal values of 0.7 to 0.8 pA (Fig. 5A). By contrast, this analysis revealed significant differences in both the distribution of amplitude values and kinetic parameters affecting the half-width distribution (Fig. 5 B and C). Cells expressing the truncated form of SNAP-25 released catecholamines in events having a mean amplitude of 47 ± 5 pA versus the value of 75 ± 6 pA for control cells whereas the distributions had maximum probability values around 9 and 20 pA, respectively (Fig. 5B). In addition, single event kinetics were clearly affected: The mean half-width value changed from 22 ± 1 ms in control cells to 30 ± 1 ms in cells expressing the truncated construct. In fact, the distribution pattern of this parameter evidenced these changes by passing from a narrow distribution with maximum value at 7 ms to a broad distribution with a maximum at 21 ms (Fig. 5C). Further analysis revealed that expression of the truncated form slowed considerably both the onset and the decay times contributing to event kinetics, changing from average values of 4.9 ± 2 and 30 ± 3 ms, respectively, in control conditions to 8.8 ± 0.6 and 42 ± 2 ms in cells expressing the truncated construct. In additional experiments, we found no significant differences when comparing events occurring in nontransfected and GFP–S-25-expressing cells (n = 398, 0.91 ± 0.02 pC1/3 mean charge, 76 ± 6 pA averaged amplitude, and 19 ± 2 ms mean half-width). These results are summarized in Fig. 5D, which depicts fusions with characteristics of an average event in control nontransfected and GFP–S-25 Δ6-expressing cells, the latter having slower single event kinetics, as it is observed when normalized events are compared. It is interesting to notice that amperometric events after BoNT A treatment had t1/2 values very similar to those of control (17) whereas GFP–S-25 Δ6-expressing cells exhibited longer and smaller events. This apparent contradiction could be explained if we assume that after BoNT A incubation, there is still a population of ≈10% of toxin-unaffected SNAP-25 (14), which would be responsible for reduced release (10–15% of the release in control cells). This release would have similar kinetic properties as the one in control cells. By contrast, in GFP–S-25 Δ6-expressing cells, the truncated SNAP-25 overwhelms the native protein because of its strong overexpression, substituting it in the few events that have success. In this case, the kinetic properties would be determined by the truncated protein.
Figure 5.
Effect of the expression of SNAP-25 constructs on single event characteristics. Individual “spikes” were analyzed from depolarization responses of GFP–S-25 Δ6-expressing (n = 285) and control nontransfected cells (n = 627). Distributions for fusion event histograms parameter are from cells expressing the truncated form (solid bars) and control cells (open bars). (A) Single fusion charge distributions. Event charge was calculated by trapezoidal integration, and histograms of Q1/3 were built by using 0.1 (pC)1/3 bin size. (B) Amplitude distributions. Amplitude was measured, and data was binned at 5-pA intervals. (C) Spike half-width time distributions. Half-width was measured by subtraction of the half-height times corresponding to the rising and falling portions of each spike. Data were binned at 4-ms intervals. (D) Examples of amperometric events. Shown are events with the average charge, amplitude, and kinetic characteristics similar to the average found for control nontransfected cells (solid line event) and cells expressing GFP–S-25 Δ6 (pointed line spike). For better kinetic comparison, GFP–S-25 Δ6 event was normalized to the amplitude of the control event (dashed line peak), and the events were digitally filtered.
DISCUSSION
The use of BoNT A and E in biochemical (14, 24, 25) and biophysical (16, 17) studies of exocytosis have provided an evolving picture of the complex role of SNAP-25 in docking and post-docking exocytotic stages. These studies also evidenced some deficits of the neurotoxin approach, specially the restriction to protein domains delimited by toxin cleavage. The studies described here overcome some of these limitations in different ways: First, molecular biology methods allow us to study the functional impact of deletions that are not confined to the limits imposed by toxin cleavage and also can address the effect of single amino acid changes. And second, the expression of large populations of altered SNAREs in combination with single-cell biophysical measurements establishes an extremely useful method to define the role of SNAP-25 at the very final membrane fusion stages of exocytosis.
However, several potential limitations of the present approach should be discussed: (i) In some cases, the fusion of GFP with a protein can modify its properties, resulting in an impairment of protein localization and/or function. This, however, does not seem to be the case with SNAP-25 because GFP–S-25, the fusion containing the complete protein, showed a membrane localization similar to the one of native SNAP-25, and its overexpression in the cytoplasm did not affect exocytosis. By contrast, fusion proteins with four or more amino acids deleted from the C terminus, which also showed the same localization, had, however, a deleterious effect on exocytosis. (ii) Cells are transfected at very low efficiency, which has precluded the use of biochemical methods to demonstrate in a more direct way (i.e., coimmunoprecipitation of complexes formed by GFP-constructs and SNAREs) the interaction between the endogenous SNAREs and the transfected proteins. (iii) In transfected cells, it is not possible to directly assess the origin of “individual” fusion events as coming from altered or normal remaining complexes. However, the individual spike event characteristics from fusions happening in cells expressing deleted SNAP-25 are clearly different from the ones in control nontransfected or GFP-whole SNAP-25 expressing cells (Fig. 5). This indicates that the secretion observed in cells expressing deleted SNAP-25 may come from fusion complexes formed with the altered protein. The modification in individual spike event characteristics should not be a technical artifact arising from measuring a small amount of secreted vesicles because kinetic properties obtained in conditions of reduced secretion, for instance, by using a small area electrode with control untreated cells (17) are similar to the ones obtained in the standard conditions of normal secretion. This means that the different kinetic characteristics observed in cells transfected with the deleted constructs are actually a consequence of the presence of truncated SNAP-25 and not caused by reduced secretion. Differences in spike kinetics also could be caused by electrode variability. However, significant differences in the average amplitude and t1/2, with respect to control cells, were only observed in certain deleted constructs but not in cells expressing the whole SNAP-25 protein. (iv) Regarding the differences in inhibitory efficiency observed between the different constructs, a possibility would be that they arise from different stabilities and/or levels of expression of the transfected proteins. However, only cells showing strong fluorescence and, therefore, expressing high levels of GFP–SNAP-25 fusions at the time of the amperometric measurements were chosen for exocytotic studies. Possible variations among strongly fluorescent cells were lessened by performing amperometric measurements in a sufficient number of different cells. Moreover, one of the reasons to choose the strategy of using GFP–SNAP-25 fusions, instead of cotransfections of separated GFP and SNAP-25 expressing vectors, was to be sure that transfected and, therefore, strong GFP expresser and fluorescent cells also had a high amount of exogenous SNAP-25.
Concerning SNAP-25 structure-function relationships, the present study demonstrates that the fourth amino acid from the C terminus, Leu203, is a critical residue for sustaining normal SNAP-25 activity because its substitution for alanine or glutamic acid produced a substantial decrease in the initial rate of catecholamine secretion. It has been proposed that the sequence between Asn153 and Leu203 forms an extended α-helix, characteristic of coiled-coil domains (26). Recently, the crystal structure of a core SNARE complex, probably representing the final form of the SNARE assembly, has been elucidated (27). Crystallographic data reveal a highly twisted and parallel four-helix bundle. The C-terminal fragment of SNAP-25 (Val120–Gly206) forms one of these helices. Of interest, Leu203 would be located in the region that, on interacting with synaptobrevin, is closer to the synaptic vesicle and, therefore, might be essential for joining the plasmalemma and vesicular membranes at the step prior to membrane fusion. Our findings suggest that Leu203, the first residue from the C-terminal end included in one of these zipper structures, would act as the final fastener piece, necessary for tight assembly, in the zippering mechanism. Thus, it could occur that the SNARE complex begins to zip from the N-terminal region of SNAP-25 and proceeds to reach the final link, Leu203, near the membrane. The lack (in SNAP-25 deleted constructs) or substitution (in mutated SNAP-25) of this amino acid might reduce the likelihood of completing the process, alter the rate of membrane fusion, or modify the properties of fusion pore dilation (28), inducing inhibition of exocytosis. Regarding the latter possibility, our single event alterations are remarkably similar to the changes observed in evoked postsynaptic potentials when synapsin function was interfered (29), which constituted one of the first indications for a direct role of this protein in neurotransmitter release. Accordingly, SNAP-25 and, probably, the other associated SNAREs that form mature docking complexes might be participating in different exocytotic stages, such as productive docking (18) or ATP-dependent priming (30) and other early postdocking events (24), and finally can remain associated [for example, modulating the activity of the calcium sensor (31)] or can constitute the membrane fusion machinery (ref. 9 and this work).
In summary, the present study provides evidence about the involvement of a specific residue of SNAP-25 on exocytosis. In this sense, the experimental approach presented here represents a unique opportunity to establish, at the amino acid level, how SNAREs are designed to sustain critical functions in the secretory pathway.
Acknowledgments
We thank S. Ingham for art work. The kind supply of SNAP-25a cDNA by Dr. Christina Bark from Karoliska Institut (Stockhom, Sweeden) and Igor-based programs by Drs. Ricardo Borges and Fernando Segura from the Universidad de la Laguna (Tenerife, Spain) is greatly acknowledged. We also thank Eva Martínez and Mar Francés for technical assistance and Carmen Carrasco-Serrano for her help in some transfection experiments. This work was supported by grants from the Spanish Dirección General de Investigación Científica y Técnica to S.V. and L.M.G. (DGICYT, PM 95-0110) and M.C. (DGICYT PB95–0690) and Generalitat Valenciana (GV-D-vs.-20-158-96) to M.C. and S.V.
ABBREVIATIONS
- BoNT
botulinum neurotoxin
- GFP
green fluorescent protein
- SNAP-25
synaptosome-associated protein of 25 kDa
- SNARE
soluble N-ethylmaleimide-sensitive fusion protein, attachment protein receptors
References
- 1.Bennett M, Scheller R. Proc Natl Acad Sci USA. 1993;90:2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Söllner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 3.Bark I C, Wilson M C. Proc Natl Acad Sci USA. 1994;91:4621–4624. doi: 10.1073/pnas.91.11.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Südhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 5.Hanson P I, Heuser J E, Jahn R. Curr Opin Neurobiol. 1997;7:310–315. doi: 10.1016/s0959-4388(97)80057-8. [DOI] [PubMed] [Google Scholar]
- 6.Bennett M K, Calakos N, Scheller R H. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 7.Oyler G A, Higgins G A, Hart R A, Battenberg E, Billigsley M, Bloom F E, Wilson M C. J Cell Biol. 1989;109:3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trimble W S, Cowan D M, Scheller R H. Proc Natl Acad Sci USA. 1988;85:4538–4542. doi: 10.1073/pnas.85.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber T, Zemelman B V, McNew J A, Westermann B, Gmachl M, Parlati F, Sollner T H, Rothman J E. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 10.Montecucco C, Schiavo G. Mol Microbiol. 1994;13:1–8. doi: 10.1111/j.1365-2958.1994.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 11.Blasi J, Chapman E R, Link E, Binz T, Yamasaki S, De Camilli P, Südhoff T C, Niemann H, Jahn R. Nature (London) 1993;365:160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- 12.Schiavo G, Santucci A, Dasgupta B R, Mehta P P, Jontes J, Benfenati F, Wilson M C, Montecucco C. FEBS Lett. 1993;335:99–103. doi: 10.1016/0014-5793(93)80448-4. [DOI] [PubMed] [Google Scholar]
- 13.Knight D E. FEBS Lett. 1986;207:222–226. doi: 10.1016/0014-5793(86)81492-2. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence G W, Weller U, Dolly J O. Eur J Biochem. 1994;222:325–333. doi: 10.1111/j.1432-1033.1994.tb18871.x. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhoff T C, Niemann H. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu T, Binz T, Niemann H, Neher E. Nat Neurosci. 1998;1:192–200. doi: 10.1038/642. [DOI] [PubMed] [Google Scholar]
- 17.Gil A, Viniegra S, Gutiérrez L M. Eur J Neurosci. 1998;10:3369–3378. doi: 10.1046/j.1460-9568.1998.00341.x. [DOI] [PubMed] [Google Scholar]
- 18.Gutiérrez L M, Viniegra S, Rueda J, Ferrer-Montiel A-V, Cànaves J, Montal M. J Biol Chem. 1997;272:2634–2639. doi: 10.1074/jbc.272.5.2634. [DOI] [PubMed] [Google Scholar]
- 19.Bark I C, Wilson M C. Gene. 1994;139:291–292. doi: 10.1016/0378-1119(94)90773-0. [DOI] [PubMed] [Google Scholar]
- 20.Graham F L, van der Eb A J. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 21.Gutiérrez L M, Cànaves J, Ferrer-Montiel A-V, Reig J A, Montal M, Viniegra S. FEBS Lett. 1995;372:39–43. doi: 10.1016/0014-5793(95)00944-5. [DOI] [PubMed] [Google Scholar]
- 22.Wightman R M, Jankowski J A, Kennedy R T, Kawagoe K T, Schroeder T J, Leszczyszyn D J, Near J A, Diliberto E J Viveros OH. Proc Natl Acad Sci USA. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroeder T J, Borges R, Finnegan J M, Pihel K, Amatore C, Wightman R M. Biophys J. 1996;70:1061–1068. doi: 10.1016/S0006-3495(96)79652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee A, Kowalchy J A, DasGupta B R, Martin T F J. J Biol Chem. 1996;271:20227–20230. doi: 10.1074/jbc.271.34.20227. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence G W, Foran P, Mohammed N, DasGupta B R, Dolly J O. Biochemistry. 1997;36:3061–3067. doi: 10.1021/bi9622478. [DOI] [PubMed] [Google Scholar]
- 26.Chapman E R, An S, Barton N, Jahn R. J Biol Chem. 1994;269:27427–25432. [PubMed] [Google Scholar]
- 27.Sutton R B, Fasshauer D, Jahn R, Brunger A T. Nature (London) 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 28.Monk J R, Fernandez J M. Neuron. 1994;12:707–716. doi: 10.1016/0896-6273(94)90325-5. [DOI] [PubMed] [Google Scholar]
- 29.Hilfiker S, Schweizer F E, Kao H-T, Czernig A J, Greengard P, Augustine G J. Nat Neurosci. 1998;1:29–35. doi: 10.1038/229. [DOI] [PubMed] [Google Scholar]
- 30.Otto H, Hanson P I, Jahn R. Proc Natl Acad Sci USA. 1997;94:6197–6201. doi: 10.1073/pnas.94.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tahara M, Coorssen J R, Timmers K, Blank P S, Whalley T, Scheller R, Zimmerberg J. J Biol Chem. 1998;273:33667–33673. doi: 10.1074/jbc.273.50.33667. [DOI] [PubMed] [Google Scholar]