Abstract
There is a great deal of evidence for synergistic interactions between G protein-coupled signal transduction pathways in various tissues. As two specific examples, the potent effects of the biogenic amines norepinephrine and dopamine on sodium transporters and natriuresis can be modulated by neuropeptide Y and atrial natriuretic peptide, respectively. Here, we report, using a renal epithelial cell line, that both types of modulation involve recruitment of receptors from the interior of the cell to the plasma membrane. The results indicate that recruitment of G protein-coupled receptors may be a ubiquitous mechanism for receptor sensitization and may play a role in the modulation of signal transduction comparable to that of the well established phenomenon of receptor endocytosis and desensitization.
There are many examples documenting that the cellular response to catecholamines and other small molecules can be enhanced by peptide hormones. In the nervous system, the efficacy of synaptic transmission is modulated both by peptide neurotransmitters and by biogenic amines (1, 2). In the kidney, the potent effects of the biogenic amines norepinephrine and dopamine (DA) on sodium transporters and natriuresis can be modulated by neuropeptide Y (NPY; refs. 3 and 4) and atrial natriuretic peptide (ANP; refs. 5–8), respectively. Little is known about the molecular basis for such heterologous sensitization. Here, we report that one cellular mechanism by which peptide hormones induce heterologous sensitization involves recruitment of catecholamine receptors from the interior of the cell to the plasma membrane. We show by the use of confocal microscopy and subcellular fractionation that the well established ability of ANP to potentiate the effects of DA and the ability of NPY to potentiate the effects of norepinephrine are attributable to recruitment of these two classes of receptors to the plasma membrane.
MATERIALS AND METHODS
Na+,K+-ATPase Activity in Single Proximal Tubular Segments.
Renal proximal tubular segments were microdissected from a collagenase-treated rat kidney. Individual segments were incubated with the indicated drugs for 30 min at room temperature. Na+,K+-ATPase activity was measured in single segments by ouabain-sensitive ATP hydrolysis (9). Assays were performed in the presence of 70 mM Na+ (Vmax conditions). This method permits direct measurements of Na+,K+-ATPase activity in intact permeable cells, wherein effects of changes in Na+ influx are eliminated. Results were calculated relative to the length of the tubular segments.
Antibodies.
Na+,K+-ATPase, a plasma membrane marker enzyme, was probed with a monoclonal antibody (a kind gift from M. Caplan, Yale University, New Haven, CT). D1 DA receptors were probed with affinity-purified rabbit anti-D1 rat DA receptor antibodies, which recognize the third extracellular loop of the receptor (a kind gift from R. M. Carey, University of Virginia, Charlottesville, VA; ref. 10). α1A-Adrenergic receptors were probed with affinity-purified rabbit anti-human α1A-adrenergic receptor antibodies, which recognize the second extracellular loop of the receptor (11). Commercially available goat anti-rat α1A-adrenergic receptor antibodies purchased from Santa Cruz Biotechnology gave results (not shown) virtually identical to those shown here. Goat anti-rabbit Texas Red-X antibodies (Molecular Probes) and donkey anti-goat Texas Red antibodies (Jackson ImmunoResearch) were used as secondary antibodies.
Confocal Microscopy.
D1 DA receptor and α1A-adrenergic receptor immunofluorescence were visualized in LLCPK cells by confocal microscopy as described (12). Briefly, cells were incubated with drugs or vehicle for desired times, then fixed in ice-cold 4% (vol/vol) paraformaldehyde. After a brief exposure to sodium borohydride (2 mg/ml), cells were blocked and made permeable with 7% (vol/vol) normal goat serum, 5% (vol/vol) nonfat dry milk, and 0.1% Triton X-100 in PBS. The cells were then incubated overnight at 4°C with primary antibodies. After washing, cells were incubated for 1 h at room temperature with secondary antibodies and mounted in Prolong antifade (Molecular Probes). The cells were examined with a Zeiss LSM410 inverted confocal scanning laser microscope with excitation at 543 nm and detection at >570 nm. Negative controls included cells incubated with primary antibodies preabsorbed to their immunizing peptides and cells incubated with secondary antibody alone.
Subcellular Fractionation of Renal Cortical Tissue.
Slices (250 μm) from outer cortex of rat kidney, consisting predominantly of proximal tubular cells, were incubated with the indicated drugs and protease inhibitors (Complete, Boehringer Mannheim) for 15 min at 37°C, homogenized, and centrifuged at 2,000 × g for 10 min at 4°C. The supernatant was layered on top of a prechilled 10–40% (wt/vol) sucrose gradient with 1 ml of 65% (wt/vol) sucrose cushion and centrifuged at 96,500 × g for 16 h at 4°C (12). Fractions (1 ml) were collected from the bottom of the tubes. Each fraction (1–10) was electrophoresed and blotted on poly(vinylidene difluoride) membrane. The unspecific sites were blocked with 5% (vol/vol) nonfat dry milk in PBS/0.1% Tween 20. Horseradish peroxidase-conjugated secondary antibodies were used to probe the primary antibodies. The specific bands were visualized with the chemiluminescence method (ECL Plus Kit, Amersham Pharmacia).
Each fraction from vehicle-treated tissue was probed for Na+,K+-ATPase, which was used as a marker for the plasma membrane fraction. The amount of Na+,K+-ATPase was highest in fraction 1 (plasma membrane fraction) and absent from fractions 3–10. Fraction 7, which contained the greatest concentration of α1A-adrenergic receptors and D1 DA receptors, was used as a reference. After treatment with vehicle or indicated drug, fractions 1 and 7 were probed for the indicated receptor, and the abundance of the receptor was measured semiquantitatively with a PhosphoImager.
RESULTS
Heterologous Sensitization of D1 DA Receptors by ANP.
To study heterologous receptor sensitization, we used a renal epithelial cell line, LLCPK cells, as a model. These cells retain many of the characteristics of renal proximal tubular epithelial cells, including their capacity to synthesize and metabolize DA. Recruitment of the D1 DA receptor subtype to the plasma membrane of LLCPK cells was observed after incubation with the DA precursor l-dopa and after inhibition of the DA-metabolizing enzyme catechol-O-methyl transferase (12). Several lines of evidence suggest that DA may serve as a permissive agent for the natriuretic effect of ANP (5–7).
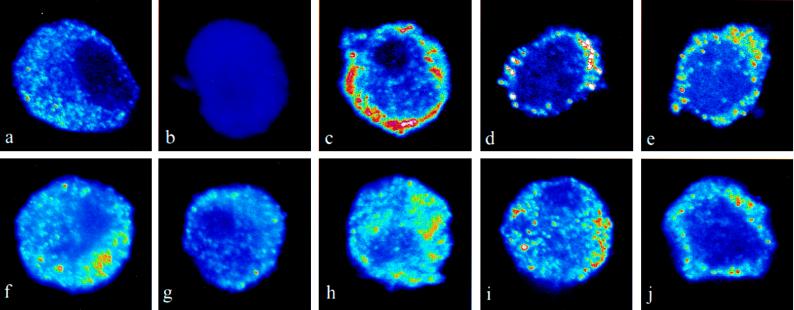
To examine whether heterologous receptor recruitment might explain this phenomenon, we tested the effect of ANP on the localization of the D1 subtype of DA receptors. Under basal conditions, a large number of D1 DA receptors are located intracellularly (Fig. 1 a and b). ANP at concentrations between 10−9 M and 10−6 M (Fig. 1 c and d) translocated the D1 DA receptors from the interior of the cell toward the plasma membrane. This effect was observed within 1 min and was sustained for at least 5 min. cGMP, the second messenger for ANP, mimicked the effect of ANP and translocated the D1 DA receptors toward the plasma membrane (Fig. 1e). ANP-induced translocation of D1 DA receptors was abolished by a D1 DA receptor antagonist, SCH 23390 (10−6 M; Fig. 1f). D1 DA receptors are positively coupled to adenylyl cyclase, and we have previously reported that forskolin recruits D1 DA receptors toward the plasma membrane (12). DDA, a specific inhibitor of adenylyl cyclase, abolished the effect of ANP on D1 DA receptor translocation (Fig. 1g). DA (10−10 M) and ANP (10−11 M), each added alone, had no effect on D1 DA receptor trafficking, but when added together, they caused translocation of the D1 DA receptors toward the plasma membrane (Fig. 1 h–j). These results indicate an interaction between the cAMP and cGMP pathways in the translocation process.
Figure 1.
Effect of ANP on localization of D1 DA receptors. Confocal recordings of D1 DA receptors were taken from midsections of LLCPK cells. The color-scale used is a pseudo-color-scale, with blue indicating low fluorescence signal and red–white indicating high fluorescence signal. The images shown are representative for at least three separate experiments. (a) The D1 DA receptor signal is found throughout untreated cells. (b) In samples in which the primary antibody had been preabsorbed with the peptide against which the antibody was raised, there was no detectable fluorescence signal. (c–j) Cells were treated with drugs for 1 min. Treatment with ANP at 10−9 M (c) or 10−6 M (d) resulted in a distinct signal in the region of the plasma membrane. This effect was sustained for at least 5 min (not shown). (e) cGMP (10−4 M) also resulted in an enhanced signal in the region of the plasma membrane. (f) In cells treated with the D1 DA receptor antagonist SCH 23390 (10−6 M), the effect of ANP (10−6 M) was abolished. (g) Dideoxyadenosine (DDA; 10−4 M) also abolished the effect of ANP (10−6 M) on D1 DA receptor localization. DA (10−10 M; h) and ANP (10−11 M; i) had no effect on receptor localization when added alone but did, in combination, enhance the D1 DA receptor signal in the region of the plasma membrane (j). SCH 23390 or DDA alone had no effect on D1 DA receptor localization (data not shown).
The localization of D1 DA receptors after exposure to vehicle or ANP was evaluated with another approach. Slices from outer cortex of rat kidney were incubated with vehicle or ANP, homogenized, and subjected to sucrose gradient centrifugation. Receptor abundance in various fractions was detected by immunoblot. ANP caused a significant increase of D1 DA receptor abundance in the plasma membrane fraction (Fig. 2). The ratio of D1 DA receptor abundance in fraction 1 (plasma membrane fraction) to fraction 7 (reference fraction) was significantly higher (P < 0.005) in the ANP-treated slices (0.16 ± 0.04) than in the vehicle-treated slices (0.083 ± 0.02; n = 8).
Figure 2.
Localization of the D1 DA receptor by subcellular fractionation. Rat renal outer cortical tissue slices were incubated with or without ANP (10−6 M) for 15 min, subjected to subcellular fractionation, and immunoblotted for D1 DA receptor. The amount of the integral membrane protein Na+,K+-ATPase, used as a marker for the plasma membrane, was highest in fraction 1 and not detectable in fractions 3–10 in vehicle-treated tissue. The plasma membrane fraction was therefore identified as fraction 1. In vehicle-treated tissue, the D1 DA receptor subtype was located mainly in fraction 7 (reference fraction).
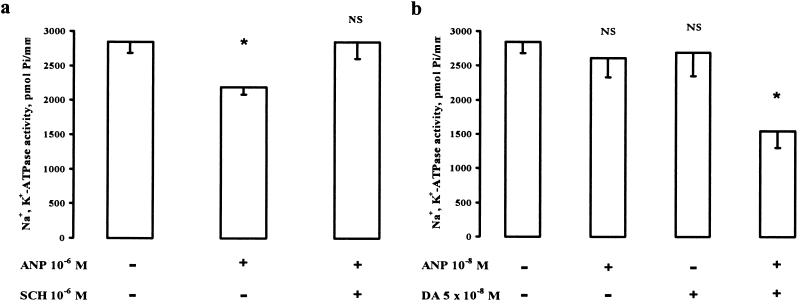
Because the natriuretic effect of ANP is blocked by D1 DA receptor antagonists (5–8) and because inhibition of the activity of Na+,K+-ATPase is an important component of the mechanism by which DA produces its natriuretic effect (13), we examined the possibility that ANP and DA may interact in the regulation of tubular Na+,K+-ATPase activity. Incubation of rat renal proximal tubular segments with ANP (10−6 M) caused a 25 ± 2% inhibition of Na+,K+-ATPase activity (P < 0.05; Fig. 3a). This effect was abolished by the specific D1 DA receptor antagonist, SCH 23390 (10−6 M). The D1 DA receptor antagonist alone had no effect on Na+,K+-ATPase activity. Subthreshold doses of ANP (10−8 M) and DA (5 × 10−8 M) had no effect added singly, but added together, they significantly decreased Na+,K+-ATPase activity (Fig. 3b). These studies indicate that the D1 DA receptors recruited by ANP to the plasma membrane are physiologically active.
Figure 3.
Effect of ANP and DA on the activity of Na+,K+-ATPase in single permeable rat proximal tubule segments. ANP (10−6 M) significantly decreased Na+,K+-ATPase activity. (a) This effect was abolished by the D1 DA receptor antagonist SCH 23390 (10−6 M). (b) In tubules incubated with both ANP (10−8 M) and DA (5 × 10−8 M), Na+,K+-ATPase activity was decreased by 48 ± 10%. In each experiment, 5–8 tubules from the same rat were studied in each protocol (vehicle or drug incubation). Average values from these tubules were used as one data point. Data in a and b are means ± SEM for data from three rats. ∗, P < 0.05 vs. control; NS, not significant vs. control. Statistical analysis was performed with paired and unpaired Student’s t test.
Heterologous Sensitization of α1A-Receptors by NPY.
The antinatriuretic effect of renal sympathetic nerve stimulation is mediated primarily by the α1A-adrenergic receptor subtype (14). Moreover, norepinephrine and NPY are colocalized in the renal sympathetic nerve endings (3). To examine whether heterologous receptor sensitization via recruitment may be a generalized phenomenon for synergism between first messengers acting via G protein-coupled receptors, the effect of NPY on the localization of α1A-adrenergic receptors was examined.
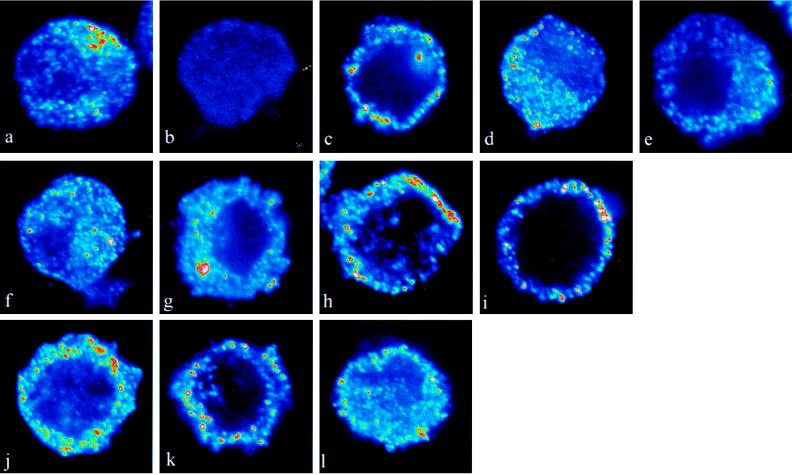
In unstimulated LLCPK cells, the α1A-adrenergic receptors are localized to a large extent in the cytoplasm (Fig. 4 a and b). After 10 s of exposure to 10−8 M (not shown) or 10−6 M (Fig. 4c) phenylephrine, the α1-adrenergic receptor agonist, the α1A adrenergic receptors were translocated toward the plasma membrane; 60 s later, the receptors had returned to the cytoplasm (Fig. 4d). This rapid internalization is consistent with the fast desensitization phenomena described for several G protein-coupled receptors (15, 16). The recruitment of the α1A-adrenergic receptors at 10 s was not observed in cells pretreated with the α1A-adrenergic receptor antagonist prazosin (10−6 M; Fig. 4e). Incubation with phenylephrine (10−10 M) alone (Fig. 4f) or NPY (10−10 M) alone (Fig. 4g) for 10 s had no effect on receptor localization, but when applied in combination (Fig. 4h), there was a marked and rapid increase of the α1A-adrenergic receptor signal in the region of the plasma membrane. This effect was sustained for at least 5 min (Fig. 4i). Thus, the presence of NPY prevented the rapid internalization and desensitization of the α1A-adrenergic receptors. Incubation with NPY (10−8 M) alone for 10 s caused a rapid movement of the α1A-adrenergic receptors to the region of the plasma membrane (Fig. 4j). This effect was sustained for at least 5 min (Fig. 4k). The effect of NPY (10−8 M) at 10 s was not seen in cells pretreated with the α1A-adrenergic receptor antagonist prazosin (10−6 M; Fig. 4l).
Figure 4.
Effect of NPY on localization of α1A-adrenergic receptors. Confocal micrographs show the localization of the α1A-adrenergic receptor in LLCPK cells. (a) In untreated cells, the α1A-adrenergic receptor immunofluorescence signal was found throughout the cell. (b) In sections in which the primary antibody had been preabsorbed with the peptide against which the antibody was raised, there was no detectable fluorescence signal. (c) After treatment with the α1A-adrenergic receptor agonist phenylephrine (10−6 M) for 10 s, a distinct signal was observed in the region of the plasma membrane. (d) After 60 s, α1A-adrenergic receptor immunofluorescence signal was again observed throughout the cell. (e) The relocalization of the α1A-adrenergic receptors at 10 s was not observed in cells pretreated with the α1A-adrenergic receptor antagonist prazosin (10−6 M). Incubation with phenylephrine (10−10 M) alone (f) or NPY (10−10 M) alone (g) for 10 s had no effect on receptor localization, but when applied in combination (h), there was a marked and rapid increase of the α1A-adrenergic receptor signal in the region of the plasma membrane. (i) This effect was sustained for at least 5 min. (j) Incubation with NPY (10−8 M) alone for 10 s caused a rapid relocalization of the α1A-adrenergic receptors to the region of the plasma membrane. (k) This effect was sustained for at least 5 min. (l) The effect of NPY (10−8 M) at 10 s was not seen in cells pretreated with the α1-adrenergic receptor antagonist prazosin (10−6 M).
The translocation of α1A-adrenergic receptors toward the plasma membrane by NPY plus phenylephrine was confirmed by subcellular fractionation (Fig. 5). Thus, the ratio of α1A-adrenergic receptor abundance in fraction 1 (plasma membrane fraction) to fraction 7 (reference fraction) was significantly greater (P < 0.003) in tissue treated with NPY plus phenylephrine (3.9 ± 0.5), than in vehicle-treated tissue (2.1 ± 0.3; n = 6).
Figure 5.
Localization of the α1A-adrenergic receptor by subcellular fractionation. Rat renal outer cortical tissue slices were incubated with or without NPY (10−6 M) plus phenylephrine (10−6 M) for 15 min, subjected to subcellular fractionation, and immunoblotted for α1A-adrenergic receptors. For experimental details, see legend to Fig. 2.
Effect of Phenylarsine Oxide (PAO), an Inhibitor of Endocytosis, on Receptor Localization.
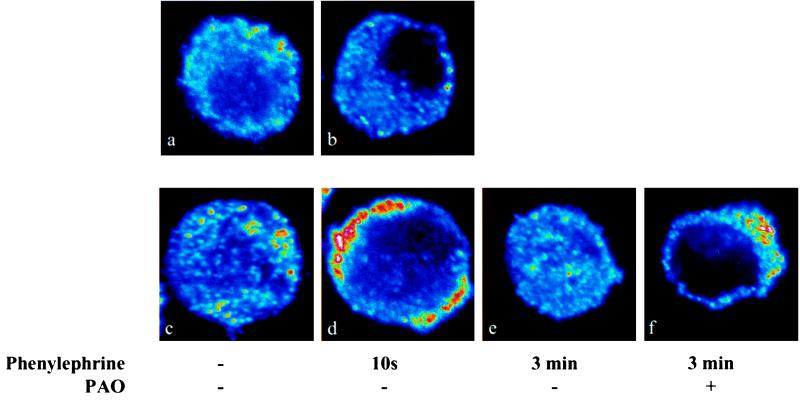
We tested whether the accumulation of D1 DA receptor and α1A-adrenergic receptor signal in the region of the plasma membrane was due to recruitment of receptors from the cytoplasm or to inhibition of internalization of receptors. Cells were incubated for 3 min with or without PAO to inhibit the receptor internalization process (17). PAO did not increase the D1 DA receptor (Fig. 6a) or α1A-adrenergic receptor (Fig. 6b) signal in the region of the plasma membrane. To test whether PAO has the ability to inhibit receptor internalization in LLCPK cells, we examined the effect of phenylephrine on the α1A-adrenergic receptor signal in PAO-treated cells. In untreated cells (Fig. 6c), the α1A-adrenergic receptor signal is found throughout the cell. Treatment for 10 s with phenylephrine (10−6 M) resulted in an increased α1A-adrenergic receptor signal in the region of the plasma membrane (Fig. 6d). After a 3-min exposure to phenylephrine, the α1A-adrenergic receptor signal was again observed throughout the cell (Fig. 6e). This internalization of α1A-adrenergic receptors was abolished in PAO-treated cells (Fig. 6f).
Figure 6.
Peptide-induced accumulation of D1 DA and α1A-adrenergic receptors at the plasma membrane is attributable to increased receptor recruitment. Control cells immunolabeled for D1 DA receptor (a) or α1A-adrenergic receptor (b) did not show an increased signal in the region of the plasma membrane after pretreatment with the endocytosis inhibitor PAO (10−5 M) for 3 min. (c and d) In cells treated with phenylephrine (10−6 M) for 10 s, an increased α1A-adrenergic receptor signal was found in the region of the plasma membrane. (e) After a 3-min treatment with phenylephrine, the α1A-adrenergic receptor signal was observed throughout the cell. (f) This internalization of α1A-adrenergic receptors was abolished after PAO treatment.
DISCUSSION
It is well established that catecholamines and peptide hormones can desensitize their cognate receptors by endocytosis and that heterologous desensitization also can be achieved by this mechanism (15, 16). The present study provides evidence that heterologous sensitization can be achieved by recruitment of receptors to the plasma membrane.
The kidney is the main organ responsible for the maintenance of stable water and electrolyte homeostasis. Transport of salt and water in the renal tubules has to be modulated precisely, to avoid fluctuations in blood pressure, hypovolemia and hypervolemia, and hyponatremia and hypernatremia. Heterologous sensitization by peptide hormones allows the kidney to integrate different stimuli. The results from the present study indicate that peptide hormones can regulate sodium homeostasis indirectly via sensitization of catecholamine receptors.
Many studies have unequivocally shown that the natriuretic effects of ANP are, to a large extent, mediated by DA (5–8). The present findings—that ANP recruits silent DA receptors to the plasma membrane—provide a plausible explanation for this phenomenon.
Synergism between NPY and α-adrenergic receptors has been described in many tissues (3, 18–22). In renal proximal tubular cells, NPY acts synergistically with α-adrenergic receptors to increase Na+,K+-ATPase activity. NPY alone, in high concentrations, stimulates Na+,K+-ATPase activity, and this effect is abolished by α-adrenergic antagonists (3). The interaction between NPY and α-adrenergic receptors can now be explained by the finding that NPY causes a sustained recruitment of α-adrenergic receptors to the plasma membrane. It would be interesting to determine whether NPY recruitment of α-adrenergic receptors also occurs in other tissues, where physiological functions are also regulated by an interaction between NPY and α-adrenergic receptors.
The present study, which describes a mechanism for interaction between G protein-coupled receptors, has implications for a variety of biological responses. Heterologous receptor recruitment provides a general mechanism for interactions between neuropeptides and small-molecule first messengers and may account for the activation of silent synapses (23–26). For instance, it was recently reported that insulin recruits γ-aminobutyric acid type A receptors to postsynaptic and dendritic membranes (23). Catecholamine receptors are often likely to be saturated with their ligands. The described interaction between peptide hormones and catecholamine receptors would be an efficient way of increasing the overall catecholaminergic cellular response. It would also provide for the fine-tuned regulation of cell homeostasis, neurotransmission, ion transport in epithelial cells, and other specialized functions involved in cell homeostasis.
Acknowledgments
This work was funded by grants from the Swedish Medical Research Council to H.B. and A.A.; by a grant from the Märta and Gunnar V. Philipssons Foundation to H.B.; by a scholarship from Frimurare Barnhuset to U.H.; by National Institutes of Health Grants MH40899 and DA 10044 to P.G. as well as DK 15843, DK 52617, HL 55006, and FO6 TW 02286 to G.D.; a grant from the Department of Veterans Affairs to G.D.; and a grant from the Nobel Foundation to G.D.
ABBREVIATIONS
- DA
dopamine
- NPY
neuropeptide Y
- ANP
atrial natriuretic peptide
- DDA
dideoxyadenosine
- PAO
phenylarsine oxide
References
- 1.Illes P, Regenholt J T. Nature (London) 1990;344:62–63. doi: 10.1038/344062a0. [DOI] [PubMed] [Google Scholar]
- 2.Noel M B, Gratton A. Synapse. 1995;21:110–122. doi: 10.1002/syn.890210204. [DOI] [PubMed] [Google Scholar]
- 3.Ohtomo Y, Meister B, Hökfelt T, Aperia A. Kidney Int. 1994;45:1606–1613. doi: 10.1038/ki.1994.211. [DOI] [PubMed] [Google Scholar]
- 4.Holtbäck U, Ohtomo Y, Förberg P, Sahlgren B, Aperia A. Am J Physiol. 1998;275:F1–F7. doi: 10.1152/ajprenal.1998.275.1.F1. [DOI] [PubMed] [Google Scholar]
- 5.Katoh T, Sophasan S, Kurokawa K. Am J Physiol. 1989;257:F300–F309. doi: 10.1152/ajprenal.1989.257.2.F300. [DOI] [PubMed] [Google Scholar]
- 6.Webb R L, Della Puca R, Manniello J, Robson R D, Zimmernam M B, Ghai R D. Life Sci. 1986;38:2319–2327. doi: 10.1016/0024-3205(86)90639-9. [DOI] [PubMed] [Google Scholar]
- 7.Winaver J, Burnett J C, Tyce G M, Dousa T P. Kidney Int. 1990;38:1133–1140. doi: 10.1038/ki.1990.323. [DOI] [PubMed] [Google Scholar]
- 8.Hegde S, Chen C-J, Lokhandwala M F. Clin Exp Hypertens A. 1991;13:357–369. doi: 10.3109/10641969109045056. [DOI] [PubMed] [Google Scholar]
- 9.Doucet A, Katz A I, Morel F. Am J Physiol. 1979;237:F105–F113. doi: 10.1152/ajprenal.1979.237.2.F105. [DOI] [PubMed] [Google Scholar]
- 10.O’Connell D P, Botkin S J, Ramos S I, Sibley D R, Ariano M A, Felder R A, Carey R M. Am J Physiol. 1995;268:F1185–F1197. doi: 10.1152/ajprenal.1995.268.6.F1185. [DOI] [PubMed] [Google Scholar]
- 11.Fu M L X, Wallukat G, Hjalmarson Å, Hoebeke J. J Clin Exp Immunol. 1994;97:146–151. doi: 10.1111/j.1365-2249.1994.tb06593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brismar H, Asghar M, Carey R M, Greengard P, Aperia A. Proc Natl Acad Sci USA. 1998;95:5573–5578. doi: 10.1073/pnas.95.10.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aperia A. Curr Opin Nephrol Hypertens. 1994;3:39–45. doi: 10.1097/00041552-199401000-00005. [DOI] [PubMed] [Google Scholar]
- 14.DiBona G F, Kopp U C. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 15.Lefkowitz R J. J Biol Chem. 1998;273:18677–18689. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 16.Caron M C, Lefkowitz R J. Recent Prog Horm Res. 1993;48:277–290. doi: 10.1016/b978-0-12-571148-7.50014-2. [DOI] [PubMed] [Google Scholar]
- 17.Knutson V P, Ronnett G V, Lane M D. J Biol Biochem. 1983;258:12139–12142. [PubMed] [Google Scholar]
- 18.Häggblad J, Fredholm B B. Neurosci Lett. 1987;82:211–216. doi: 10.1016/0304-3940(87)90132-7. [DOI] [PubMed] [Google Scholar]
- 19.Andriantsitohaina R, Stoclet J C. Br J Pharmacol. 1990;99:389–395. doi: 10.1111/j.1476-5381.1990.tb14714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.al-Arabi A, Andrews J F. Biomed Sci Instrum. 1997;33:216–225. [PubMed] [Google Scholar]
- 21.Wahlestedt C, Edvinsson L, Ekblad E, Hakanson R. J Pharmacol Exp Ther. 1985;234:735–741. [PubMed] [Google Scholar]
- 22.Vila E, Tabernero A, Fernandes F, Salaices M. Br J Pharmacol. 1992;107:66–72. doi: 10.1111/j.1476-5381.1992.tb14464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan Q, Xiong Z G, Man H Y, Ackerley C A, Braunton J, Lu W Y, Becker L E, MacDonald J F, Wang Y T. Nature (London) 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- 24.Malenka R C, Nicoll R A. Neuron. 1997;19:473–476. doi: 10.1016/s0896-6273(00)80362-1. [DOI] [PubMed] [Google Scholar]
- 25.Lledo P M, Zhang X, Sudhof T C, Malenka R C, Nicoll R A. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- 26.Liao D, Hessler N A, Malinow R. Nature (London) 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]