Full text
PDF

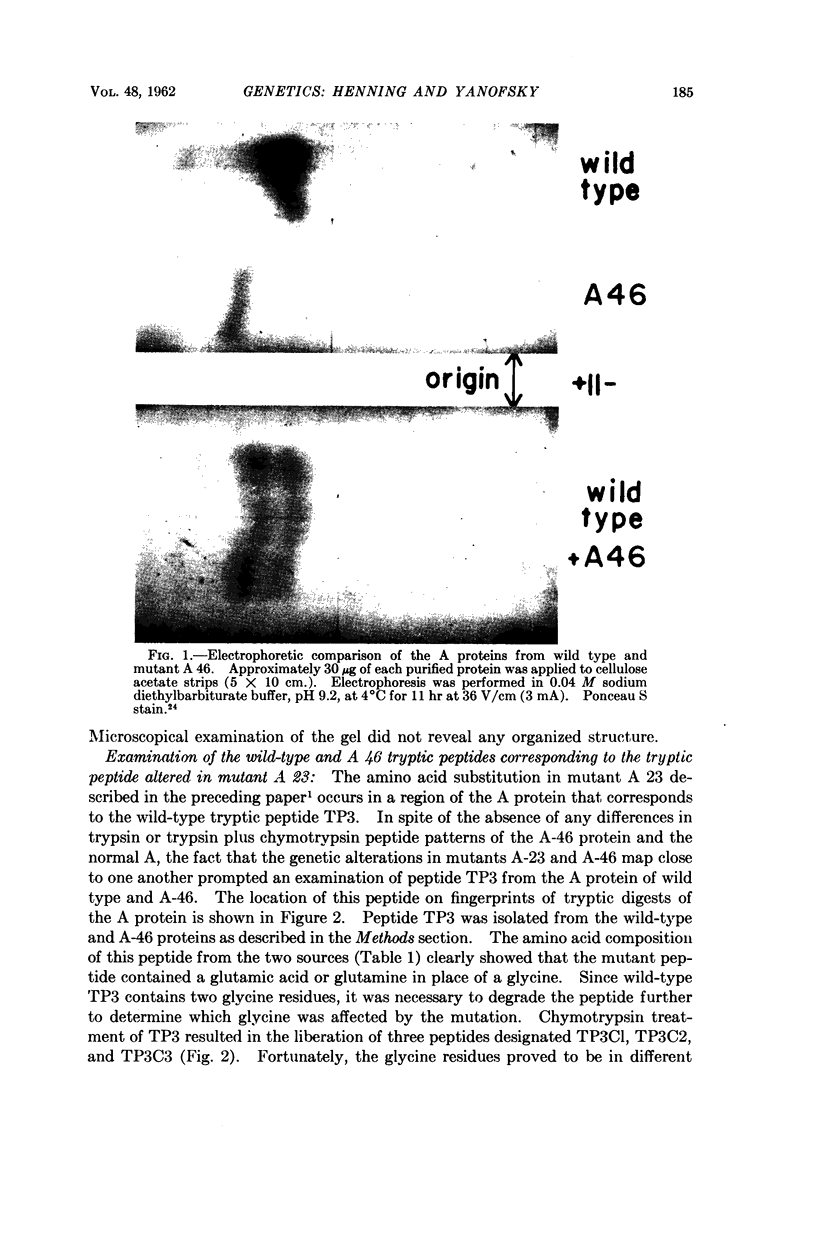
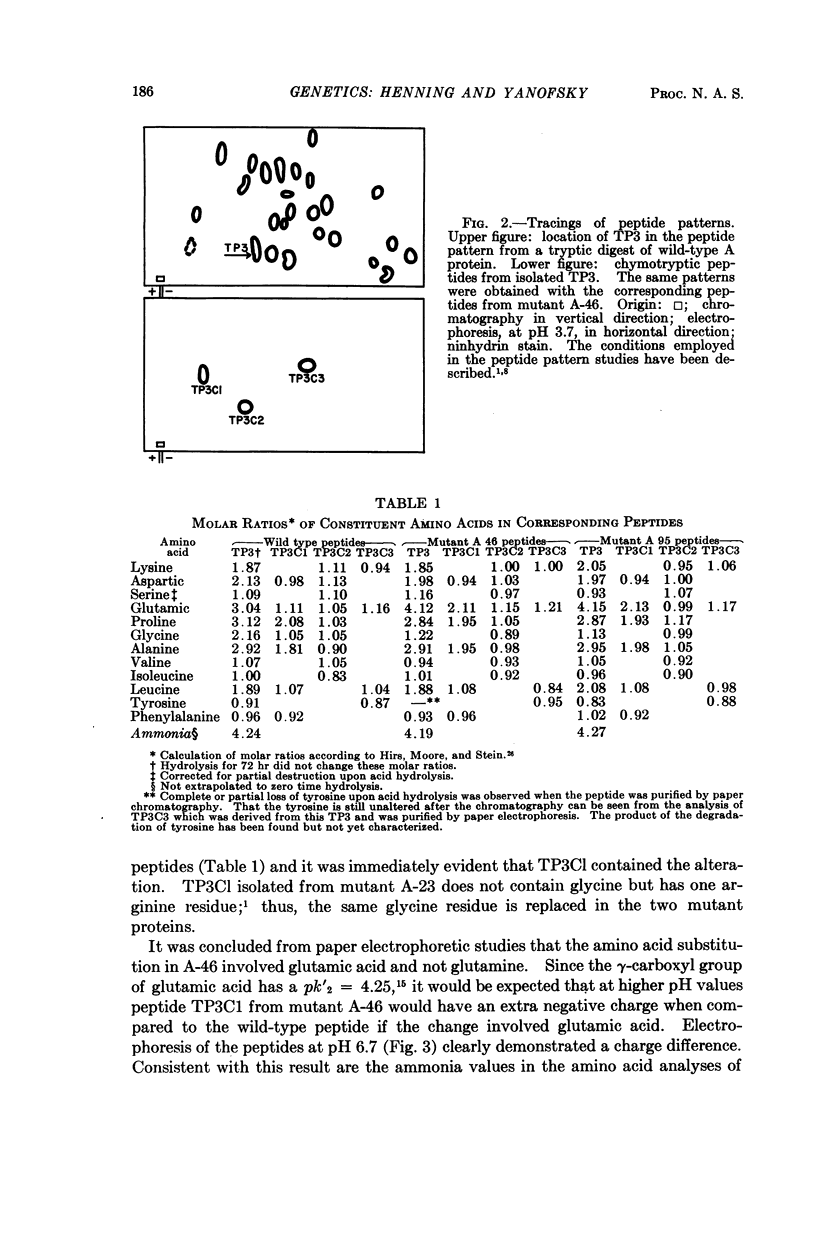




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., MYERS J. W. Modification of the penicillin technique for the selection of auxotrophic bacteria. J Bacteriol. 1953 Mar;65(3):348–353. doi: 10.1128/jb.65.3.348-353.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERER F. A., UHLIG H., WEBER E., SCHRAMM G. Primary structure of the protein of tobacco mosaic virus. Nature. 1960 Jun 18;186:922–925. doi: 10.1038/186922a0. [DOI] [PubMed] [Google Scholar]
- HELINSKI D. R., YANOFSKY C. Correspondence between genetic data and the position of amino acid alteration in a proein. Proc Natl Acad Sci U S A. 1962 Feb;48:173–183. doi: 10.1073/pnas.48.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H., MOORE S., STEIN W. H. Peptides obtained by tryptic hydrolysis of performic acid-oxidized ribonuclease. J Biol Chem. 1956 Apr;219(2):623–642. [PubMed] [Google Scholar]
- HIRS C. H. Studies on the structure of ribonuclease. Enzymatic hydrolysis of the four large peptides formed in the tryptic hydrolysis of the oxidized protein. J Biol Chem. 1960 Mar;235:625–632. [PubMed] [Google Scholar]
- KATZ A. M., DREYER W. J., ANFINSEN C. B. Peptide separation by two-dimensional chromatography and electrophoresis. J Biol Chem. 1959 Nov;234:2897–2900. [PubMed] [Google Scholar]
- KOHN J. An immuno-electrophoretic technique. Nature. 1957 Nov 9;180(4593):986–987. doi: 10.1038/180986a0. [DOI] [PubMed] [Google Scholar]
- KOSHLAND D. E., Jr Enzyme flexibility and enzyme action. J Cell Comp Physiol. 1959 Dec;54:245–258. doi: 10.1002/jcp.1030540420. [DOI] [PubMed] [Google Scholar]
- MALING B. D., YANOFSKY C. The properties of altered proteins from mutants bearing one or two lesions in the same gene. Proc Natl Acad Sci U S A. 1961 Apr 15;47:551–566. doi: 10.1073/pnas.47.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDLOFF V., BRAUNITZER G. [On hemoglobin. VI. A method for the preparative production of natural peptides. The isolation of tryptic cleavage products of human hemoglobin A with Dowex 1X2 using a ninhydrin-negative volatile buffer]. Hoppe Seylers Z Physiol Chem. 1961 May 3;323:129–144. doi: 10.1515/bchm2.1961.323.1.129. [DOI] [PubMed] [Google Scholar]
- Tsugita A., Gish D. T., Young J., Fraenkel-Conrat H., Knight C. A., Stanley W. M. THE COMPLETE AMINO ACID SEQUENCE OF THE PROTEIN OF TOBACCO MOSAIC VIRUS. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1463–1469. doi: 10.1073/pnas.46.11.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE F. H., Jr Regeneration of native secondary and tertiary structures by air oxidation of reduced ribonuclease. J Biol Chem. 1961 May;236:1353–1360. [PubMed] [Google Scholar]
- WITTMANN H. G., BRAUNITZER G. Isolation and composition of all tryptic peptides of TMV. Virology. 1959 Dec;9:726–728. doi: 10.1016/0042-6822(59)90170-9. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C. The tryptophan synthetase system. Bacteriol Rev. 1960 Jun;24(2):221–245. doi: 10.1128/br.24.2.221-245.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Crawford I. P. THE EFFECTS OF DELETIONS, POINT MUTATIONS, REVERSIONS AND SUPPRESSOR MUTATIONS ON THE TWO COMPONENTS OF THE TRYPTOPHAN SYNTHETASE OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1959 Jul;45(7):1016–1026. doi: 10.1073/pnas.45.7.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]