Abstract
Bone morphogenetic proteins (BMPs) comprise a large group of polypeptides in the transforming growth factor β superfamily with essential physiological functions in morphogenesis and organogenesis in both vertebrates and invertebrates. At present, the role of BMPs in the reproductive system of any species is poorly understood. Here, we have established the existence of a functional BMP system in the ovary, replete with ligand, receptor, and novel cellular functions. In situ hybridization histochemistry identified strong mRNA labeling for BMP-4 and -7 in the theca cells and BMP receptor types IA, IB, and II in the granulosa cells and oocytes of most follicles in ovaries of normal cycling rats. To explore the paracrine function of this BMP system, we examined the effects of recombinant BMP-4 and -7 on FSH (follicle-stimulating hormone)-induced rat granulosa cytodifferentiation in serum-free medium. Both BMP-4 and -7 regulated FSH action in positive and negative ways. Specifically, physiological concentrations of the BMPs enhanced and attenuated the stimulatory action of FSH on estradiol and progesterone production, respectively. These effects were dose- and time-dependent. Furthermore, the BMPs increased granulosa cell sensitivity to FSH. Thus, BMPs have now been identified as molecules that differentially regulate FSH-dependent estradiol and progesterone production in a way that reflects steroidogenesis during the normal estrous cycle. As such, it can be hypothesized that BMPs might be the long-sought “luteinization inhibitor” in Graafian follicles during their growth and development.
The development of preovulatory Graafian follicles that secrete a fertilizable oocyte at midcycle is the basis of fertility in female mammals (1). This process is under the control of the pituitary hormone follicle-stimulating hormone (FSH). During folliculogenesis, FSH interacts with receptors on the granulosa cells to evoke signal transduction pathways that result in the stimulation and inhibition of estradiol (E) and progesterone (P) production, respectively (2, 3). An important concept to emerge in the past decade is that growth factors produced by the follicle itself modulate, either amplifying or attenuating, FSH action (4). The current challenge is to understand how specific growth factors exert control of follicle function and how these modulations are integrated into the overall pattern of ovary physiology.
Of all the growth factors in the ovary, the members of the transforming growth factor β (TGF-β) superfamily figure most prominently in the regulatory events of folliculogenesis (4). Bone morphogenetic proteins (BMPs) comprise one of the largest subgroups of ligands in the TGF-β superfamily, with 15 BMPs having been described to date (5–7). BMPs are expressed in a tissue-specific manner in many different cell types during embryonic and adult life in both vertebrates and invertebrates (5–7). The importance of BMPs in regulating crucial events in morphogenesis, organogenesis, and cytodifferentiation has been established clearly from studies of BMP-deficient animals (5, 8). The biological effects of BMPs are mediated by specific cell-surface receptors. BMP receptors exist as two subtypes: type I and type II (9). The two types are structurally similar and both possess intrinsic serine/threonine kinase activity. Two BMP type I receptors, BMPR-IA (or ALK-3) and BMPR-IB (or ALK-6), and one type II receptor, BMPR-II, have been identified. Individually type I and II BMP receptors are able to bind ligand, but signal transduction by BMP receptors requires the formation of a heteromeric complex between type I and type II receptors (10). Once the BMPR-ligand complex is formed, the type II receptor, which has constitutive kinase activity, phosphorylates and activates the type I receptor, which, in turn, triggers downstream events in the BMP-signaling pathway.
Much is known about the cellular function and biological importance of BMP signaling in a large number of embryonic and adult tissues; however, the role of BMPs in the reproductive system is poorly understood, and no experimental evidence on BMP action in reproductive cells is available for any species. The mRNAs encoding BMP-2, -3, -3b, -6, and -15 have been identified in mammalian ovaries (7, 11–14), and BMP-6 and -15 mRNAs have been localized to oocytes (7, 12). Although these results support a potential function of BMPs in the ovary, nothing is known about the expression of receptors for BMPs and the functional consequences of BMP receptor activation in the ovary.
As a first step toward understanding the role of BMPs in female reproduction, we have characterized the cellular sites of expression of the BMP type IA, IB, and II receptors and BMP-4 and -7 mRNAs in the rat ovary. BMP-4 and -7 were investigated because they are the most extensively studied members of the BMP family, and the recombinant BMP-4 and -7 proteins are available for research. Here we have established the existence of a functional BMP ligand/receptor system in the ovary and linked BMP bioactivity to the regulation of FSH action in ovarian granulosa cells.
MATERIALS AND METHODS
Reagents and Supplies.
The recombinant proteins Xenopus BMP-4, human BMP-7, and human activin-A were prepared as described previously (15–17). Ovine FSH (4,453 units/mg of NIDDK-oFSH-S1) was supplied by the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases (Rockville, MD). McCoy’s 5a medium, Medium 199, and dinucleotide triphosphates were purchased from GIBCO/BRL. Cell culture plates were purchased from Falcon. Reagents for reverse transcription–PCR were obtained from Perkin–Elmer.
Cell Culture.
Twenty-three-day-old Sprague–Dawley rats (Harlan Breeders, Indianapolis) were implanted, by Harlan, with silastic capsules containing 10 mg of diethylstilbestrol to increase granulosa cell number (18). Ovaries were removed and the granulosa cells were isolated and cultured as described previously (19). Granulosa cells (5 × 104 viable cells) were pipetted into 96-well culture plates containing 200 μl (final volume) of tissue culture medium (McCoy’s 5a medium containing 100 units/ml penicillin, 100 mg/ml streptomycin sulfate, 2 mM l-glutamine, and 1 μM androstenedione). Granulosa cells were cultured for up to 48 h at 37°C in water-saturated atmosphere containing 5% CO2 in air, with the indicated concentrations of FSH, BMP-4, BMP-7, and/or activin-A. After culture, the levels of P and E in the medium were measured by radioimmunoassay as described previously (20). The animal protocols were approved by the University of California at San Diego Institutional Animal Care and Use Committee.
Construction of Probe Plasmids.
Total RNA from 27-day-old rat ovaries was prepared, and single-stranded cDNA was synthesized by reverse transcriptase and then subjected to PCR as described previously (21). To design primers for PCR, DNA sequences of rat BMP-4 and BMPR-IA were obtained from GenBank. Because DNA sequences of rat BMP-7, BMPR-IB, and BMPR-II were not available from GenBank, we designed these PCR primers by choosing the homologous DNA sequence regions between human and mouse homologues of BMP-7, BMPR-IB, and BMPR-II cDNAs, which were available from GenBank. Specifically, these primers are derived from the cDNA clones at the nucleotide numbers of 737–757 and 1181–1200 (accession no. of the cDNA clone is Z22607) for BMP-4 (22), 497–514 and 865–882 (accession no. X56906) for BMP-7 (23), 441–460 and 876–895 (accession no. D38082) for BMPR-IA (24), 528–547 and 965–984 (accession no. U89326) for BMPR-IB (A. K. Astrom, unpublished data from GenBank), and 525–544 and 895–904 (accession no. AF003942) for BMPR-II (25). These primers were selected from different exons of the corresponding genes to discriminate PCR products that might arise from possible chromosome DNA contaminants. PCR was performed under the following conditions: 35 cycles, annealing at 50°C for 30 sec; extension at 72°C for 30 sec; and denaturation at 94°C for 30 sec. All PCR products were cloned into pBluescript SK(+) plasmid, and their DNA sequences were confirmed.
In Situ Hybridization.
The in situ hybridization experiments were performed as described previously with minor modifications (26, 27). Eight consecutive sections (8 μm) were cut from each ovary and mounted onto poly-l-lysine-coated glass slides. The sections were digested with proteinase K, acetylated, washed, and dehydrated. Each antisense and sense cRNA probe was prepared by means of in vitro transcription by using T3 or T7 RNA polymerase. Hybridization was carried out with the 35S-labeled RNA probe (4–6 × 106 cpm/ml) in a solution containing 50% (vol/vol) deionized formamide, 0.3 M NaCl, 10 mM Tris⋅HCl (pH 8.2), 1 mM EDTA, 0.05% yeast tRNA, 10 mM DTT, 1× Denhardt’s solution, and 10% dextran sulfate. Hybridization solution (20 μl) was placed over each section and covered with a 60 × 22-mm acid-washed, silane-treated coverslip. Coverslips were sealed with liquid DPX (Gallard-Schlesinger Industries, Inc., Carle Place, NY). Sections were hybridized for 16 h at 58–60°C in a humidified chamber. After hybridization, the sections were treated with ribonuclease A and washed in 15 mM NaCl/1.5 mM sodium citrate at 60–62°C for 30 min. Dehydrated slides were exposed to x-ray film for several days. After adequate x-ray film images were obtained, the ovary sections were treated with xylene, rinsed in 100% ethanol, air-dried, and then coated with Kodak NTB-2 liquid autoradiograph emulsion. Slides were exposed for 4 weeks at 4°C in a desiccated, dark box. After exposure, the slides were developed (Kodak D19, 3.5 min at 14°C), rinsed briefly in distilled water, and fixed. After washing in distilled water for 1 h, slides were lightly counterstained with hematoxylin and eosin. After an autoradiography and counterstaining, the sections were analyzed microscopically. The in situ experiments were performed at least two times for each BMP ligand and receptor by using one ovary from six different animals in each experiment.
Statistical Analysis.
The E and P results shown are averages (mean ± SE) of at least three separate experiments, with triplicate determination for each treatment. Mean values from independent experiments were analyzed statistically by unpaired t test. Values were determined to be significant when P < 0.05.
RESULTS
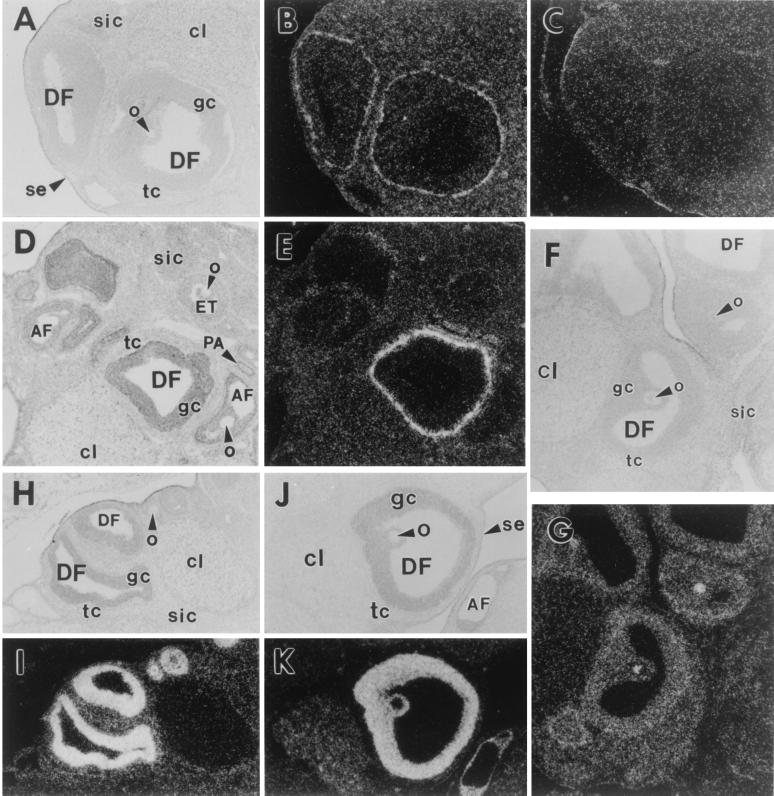
The mRNAs for BMP-4, -7 and the type IA, IB, and II BMP receptors were expressed in a tissue-specific manner in the adult rat ovary. Fig. 1 A and B shows that BMP-7 mRNA was present in the theca interstitial cells of healthy Graafian follicles, but was undetectable in other ovarian cell types. Hybridization with the control sense BMP-7 cRNA probe showed a nonspecific background signal (Fig. 1C); this was true for the other control sense probes used in these experiments (data not shown). Fig. 1 D and E shows that BMP-4 mRNA also was expressed strongly in the theca cells of healthy Graafian follicles, being present in both the theca interstitial and theca external cells. A weak but variable BMP-4 signal was observed in some corpora lutea and surface epithelial cells (data not shown). BMP-4 mRNA was not detectable in the other ovarian cell types.
Figure 1.
In situ hybridization of BMP-4 and -7 and type IA, IB, and II BMP receptors in adult rat ovaries. (A–C) BMP-7. (A) Bright field. DF, dominant follicle; o, oocyte; gc, granulosa cells; tc, theca cells; cl, corpus luteum; sic, secondary interstitial cells; se, surface epithelium. (B) Dark field of the same section hybridized with antisense cRNA probe. (C) Dark field hybridized with sense cRNA probe. (D and E) BMP-4. (D) Bright field. AF, atretic follicle; ET, early tertiary follicle; PA, preantral follicles. (E) Dark field of same section. (F and G) BMPR-IA. (F) Bright field. (G) Dark field of same section. (H and I) BMPR-IB. (H) Bright field. (I) Dark field. (J and K) BMPR-II. (J) Bright field. (K) Dark field of same section.
The mRNAs for BMPR-IA and -IB appeared to be widely expressed in the rat ovary, with the strongest hybridization signals being observed in the granulosa cells and oocytes of developing follicles (Fig. 1 F–I). The intensity of the signal for BMPR-IB was higher than that of BMPR-IA (compare Fig. 1 G with I). Hybridization signals for BMPR-II were most intense in the granulosa cells of all growing follicles (healthy and atretic) after the secondary stage (Fig. 1 J and K). The BMPR-II message was expressed weakly in some corpora lutea. A weak BMPR-II signal was observed in growing oocytes of primary follicles (those with a single layer of cuboidal granulosa cells), but none was observed in oocytes in late preantral and Graafian follicles (Fig. 1 J and K). No BMPR-II signal above background was observed in the other ovary cell types.
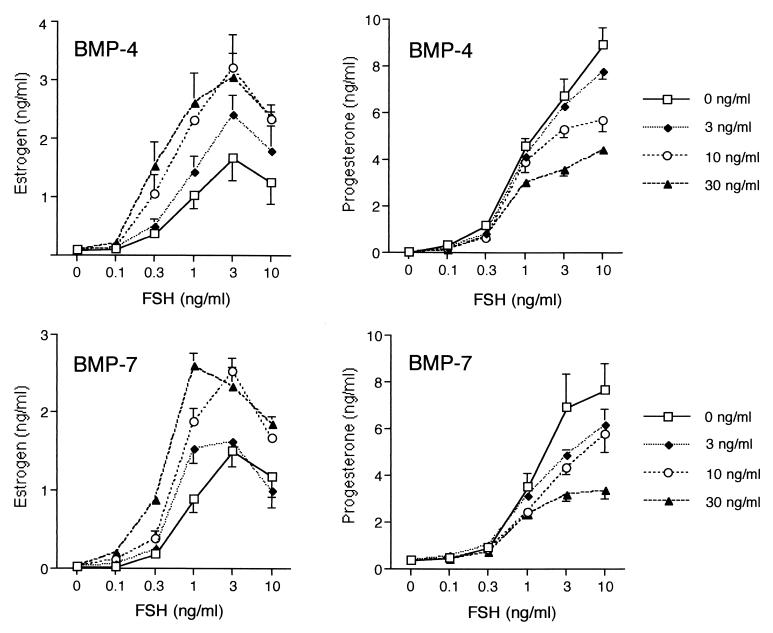
These in situ hybridization results suggest that BMP-4 and -7 produced by theca cells may interact with BMP receptors in the granulosa cells to regulate biological responses. To examine this possibility, we tested the effects of BMP-4 and -7 on FSH-induced steroidogenesis in primary cultures of rat granulosa cells grown in serum-free medium. When granulosa cells were cultured for 48 h as controls (no additions), there was no detectable E or P in the medium (Fig. 2). As expected (18), FSH markedly increased E and P production in a dose-dependent manner. Interestingly, these effects of FSH were changed markedly by BMP-4 and -7. The data in Fig. 2 show that the levels of FSH-induced E were increased substantially (≈2- to 3-fold) by both BMPs, and the effects were dose-dependent (ED50 for BMP-4 = 8.9 ± 0.4 ng/ml; ED50 for BMP-7 = 11.0 ± 1.0 ng/ml). In contrast to the positive effects on E production, both BMP-4 and -7 caused marked decreases (average ≈ 60%) in FSH-induced P production (Fig. 2), and the effects were dose-dependent (ED50 for BMP-4 = 10.9 ± 1.5 ng/ml; ED50 for BMP-7 = 11.6 ± 3.2 ng/ml). Treatment with BMP-4 and -7 alone had no effect on basal E and P production (Fig. 2).
Figure 2.
Effects of BMP-4 and -7 on E and P production by granulosa cells. Granulosa cells (5 × 104 viable cells per well/200 μl) were cultured for 48 h in serum-free medium containing androstenedione (1 μM) and either no additions (control), BMP-4 (3, 10, or 30 ng/ml), BMP-7 (3, 10, or 30 ng/ml), FSH (0.1, 0.3, 1, 3, or 10 ng/ml), or FSH in the presence of BMPs. After culture, E and P levels in the conditioned medium were measured by RIA.
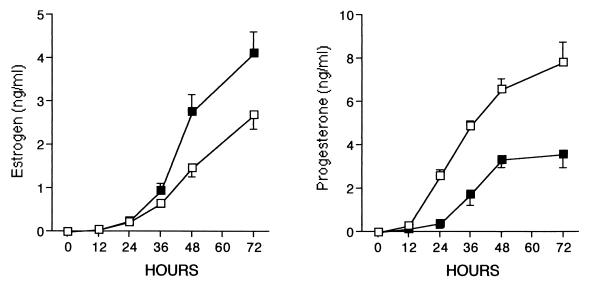
We next determined the time course of the effects of BMP on FSH action. After a 24-h lag phase, FSH induced a progressive increase in E production up to 72 h of culture (Fig. 3). This delay reflects the time needed for FSH to induce P450AROMATASE activity (3). Cotreatment of FSH with a saturating dose of BMP-7 (30 ng/ml) further increased the levels of E (≈2-fold) at each time point, but produced no change in the lag phase of FSH-induced E accumulation throughout the 72-h period. In contrast, the BMP-7 delayed the timing of FSH-stimulated P accumulation by approximately 12 h. The data in Fig. 3 show that BMP-7 completely inhibited the FSH stimulation of P at 24 h and then continued to suppress P levels (>50%) at 48 and 72 h of culture. These findings suggest that the role of BMP-7 is not only to suppress the maximal levels of FSH-induced P production, but also to delay the induction by FSH.
Figure 3.
Time-course effect of BMP-7 on E and P production by granulosa cells. Granulosa cells (5 × 104 viable cells per well/200 μl) were cultured for 48 h in serum-free medium containing androstenedione (1 μM) and FSH (3 ng/ml) in the absence (□) or presence of BMP-7 (30 ng/ml, ■). After culture, E and P levels in the conditioned medium were measured by RIA.
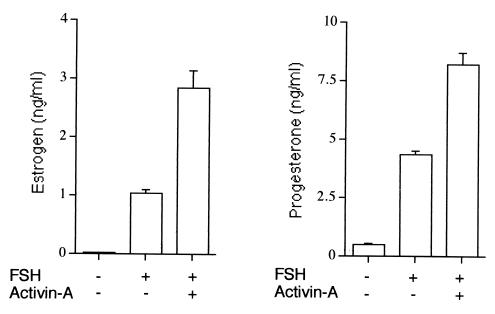
To investigate the specificity of the BMP response, we tested the effect of another TGF-β ligand, activin-A, on FSH-stimulated E and P production. In contrast to the effects of BMP-4 and -7, activin-A increased (2- to 3-fold) the FSH stimulation of both E and P production (Fig. 4). Thus, the effects of BMP-4 and -7 on FSH-dependent steroidogenesis are clearly different from those of activin-A.
Figure 4.
Effect of activin-A on E and P production by granulosa cells. Granulosa cells (5 × 104 viable cells per well/200 μl) were cultured for 48 h in serum-free medium containing androstenedione (1 μM) in the absence or presence of FSH (1 ng/ml) and activin-A (30 ng/ml). After culture, E and P levels in the conditioned medium were measured by RIA.
DISCUSSION
We present compelling evidence for a functional BMP system in the ovary. The results demonstrate that (i) the genes encoding BMP-4 and -7 and the family of BMP receptors, BMPR-IA, -IB, and –II, are expressed in a cell-type-specific manner in the normal cycling rat ovary and (ii) the interaction of physiological concentrations of BMP-4 and -7 with their receptors causes marked stimulatory and inhibitory effects on FSH-induced steroidogenesis. Accordingly, we propose that an intrinsic ovarian BMP system is involved in determining FSH activity and sensitivity in the granulosa cells during follicle growth and development.
This study demonstrated that BMP-4 and -7 are expressed strongly in the ovary, being prominent in thecal cells. This finding, together with the high levels of BMP receptor expression in granulosa cells, suggests a paracrine role of BMP-4 and -7 in regulating folliculogenesis. The evidence for coexpression of BMP-4 and -7 mRNAs supports the concept of coordinate regulation of these BMPs in the theca cells. Interestingly, the expression of BMP-4 and -7 appears to be cell-fate-specific in the cycle of folliculogenesis. BMP expression appeared very high in healthy follicles but barely detectable in follicles undergoing atresia. This suggests that the coordinate expression of BMP-4 and -7 is subject to different patterns of regulation in healthy and atretic follicles. With respect to follicle atresia, it is noteworthy that BMP-7-deficient mice die because of renal failure as a result of increased apoptosis in the embryonic kidney (28, 29). Collectively, these results suggest that regulatory stimuli interact directly or indirectly with theca cells to control the level of BMP-4 and -7 expression and that such changes are coupled in some manner to follicle fate. It will be important to investigate the mechanisms that regulate BMP-4 and -7 expression in theca cells with respect to follicle fate. In this regard, it has been reported recently that growth hormone and insulin-like growth factor I (IGF-I) are potent stimulators of BMP-4 mRNA in human dental pulp fibroblasts cultured in vitro (30). It is also known that IGF-I is a potent stimulator of rat theca cell function (31, 32) and that IGF-I expression is strong and weak in healthy and atretic follicles, respectively (33). Thus, exploring the possibility that IGF-I may be a physiological stimulus for BMP-4 and -7 expression during folliculogenesis should be investigated.
The observation that BMPR-IA, -IB, and -II mRNA expression is strongest in the granulosa cells and oocytes suggests that these cells are targets for BMPs. It is apparent that the BMP receptor mRNAs are expressed uniformly at high levels in all granulosa cells in all follicles, healthy as well as atretic. This finding indicates that granulosa cells constitutively express BMP receptor mRNAs regardless of whether the follicle is destined to live or die by apoptosis. By contrast, the BMP ligands appear to be expressed in healthy but not atretic follicles. The basis for and the significance of the receptor and BMP-4 and -7 ligand uncoupling remain to be determined. With respect to the oocyte, it has been reported that BMP-6 and BMP-15 mRNAs are expressed (7, 12), indicating the possibility of additional autocrine/paracrine BMP ligand–receptor interactions in the follicle. At present, nothing is known about the biological responses of oocyte-derived BMPs; however, it is noteworthy that factors from oocytes suppress granulosa cell expression of luteinizing hormone receptor mRNA and P production (34). These functions generally are considered key indicators of granulosa cell differentiation and, perhaps, luteinization. The oocyte factors having these effects have not been identified but may be BMPs. It seems possible, based on structural relationships, that BMPs from the theca might affect the mural granulosa cells, whereas oocytes affect the differentiation of cumulus cells.
The results of our tissue culture experiments demonstrate that BMP-4 and -7 act directly on granulosa cells and cause important changes in FSH action. Specifically, the BMPs initiated a time- and dose-dependent amplification and attenuation of FSH-induced E and P production, respectively. The evidence that the ED50 of the BMP responses (≈10 ng/ml or ≈300 pM) is equivalent to the reported Kd (254 pM) of the BMP receptor (35) indicates that the effects of the BMPs on the granulosa cells are receptor-mediated and physiologically relevant. The mechanisms underlying the divergent actions of BMP-4 and -7 are unknown. Given that Smad proteins and cyclic AMP, respectively, mediate BMP (36) and FSH (3) signaling, it is likely that the differential regulation might involve crosstalk between these key pathways. It is well established that the mechanism by which FSH stimulates E and P production involves the induction of the expression of specific steroidogenic enzymes (3). Thus, the simplest explanation for the BMP enhancement of E production is that FSH signaling is amplified by the Smad-signaling cascade, resulting in increased P450AROMATASE activity. In contrast, BMP inhibition of P production could result from Smad-induced down-regulation of FSH signals that stimulate enzymes in the P biosynthetic pathway (37), such as StAR (steroidogenic acute regulatory protein), P450scc (side-chain cleavage), and/or 3β-hydroxysteroid dehydrogenase. Further experiments are required to elucidate the underlying mechanisms by which BMPs regulate FSH action and to investigate to what extent these two BMPs cooperate in their effect on granulosa cells.
It has been shown that BMP-7 can bind receptors for both BMP (BMPR-IA, BMPR-IB, and BMPR-II) and activin (ActR-I, ActR-II, and ActR-IIB) and mimic some of the biological responses of activin, depending on the cell type (38). Because of this phenomenon, the question of specificity of the BMPs is linked to the issue of apparent redundancy in the ligand binding of the TGF-β family of receptors (9). With respect to the rat granulosa cells, there is evidence indicating that the genes encoding ActR-II and -IIB are expressed (39) and that activin-A is a potent regulator of cytodifferentiation (40). In the present study, we showed that the effects of BMP-4 and -7 on FSH-induced steroidogenesis were different from those of activin-A. Therefore, it seems likely that the BMPs and activin-A use different and distinct cell-surface receptors in the granulosa cells.
What is the physiological relevance of these BMP activities? A major concept in ovarian physiology is that FSH action in the granulosa cells of healthy Graafian follicles results in increased E production during the follicular phase of the cycle (1, 2), whereas P production is delayed until the periovulatory period. It has been postulated that there is a luteinization inhibitor that selectively prevents follicular P but not E production (41, 42). Although the existence and nature of this regulatory molecule remain hypothetical, our data support the hypothesis that BMP-4 and -7 function as luteinizing inhibitors. It is worthy to mention that granulosa luteinization appears to be associated with atresia of murine Graafian follicles (43). Viewed in conjunction with our present functional data in granulosa cells, the loss of BMP-4 and -7 expression within theca cells associated with atretic follicles may be important in explaining the occurrence of abortive luteinization during atresia.
In conclusion, these findings provide strong support for the concept that BMPs play an important physiological role in regulating FSH action in the mammalian ovary. Because a precise temporal and quantitative pattern of FSH action is obligatory for normal folliculogenesis and cyclicity, this ovarian BMP system could have implications for fertility and infertility.
Acknowledgments
We gratefully acknowledge the technical assistance of Lynn Imson and Jeff Wong (University of California at San Diego, Department of Reproductive Medicine) and Andrea Hartgrove for typing the manuscript. This work was supported in part by National Institute of Child Health and Human Development (NICHD) Grant R01 HD35383, National Institutes of Health (NIH) Center Training Grant 33130A, and NICHD/NIH Cooperative Agreement U54HD12303 as part of the Specialized Cooperative Centers Program in Reproduction Research.
ABBREVIATIONS
- BMP
bone morphogenetic protein
- BMPR
BMP receptor
- E
estradiol
- FSH
follicle-stimulating hormone
- P
progesterone
- TGF-β
transforming growth factor β
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Erickson G F. In: Endocrinology and Metabolism. Felig P, Baxter J D, Frohman L A, editors. New York: McGraw–Hill; 1995. pp. 973–1015. [Google Scholar]
- 2.Smith M S, Freeman M E, Neill J D. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 3.Richards J S. Endocr Rev. 1994;15:725–751. doi: 10.1210/edrv-15-6-725. [DOI] [PubMed] [Google Scholar]
- 4.Adashi E, Leung P C K. The Ovary: Comprehensive Endocrinology. New York: Raven; 1993. [Google Scholar]
- 5.Hogan B L M. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 6.Wozney J M, Rosen V. Clin Orthop. 1998;166:26–37. [PubMed] [Google Scholar]
- 7.Dube J L, Wang P, Elvin J, Lyons K M, Celeste A J, Matzuk M M. Mol Endocrinol. 1998;12:1809–1817. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- 8.Hogan B L M. Curr Opin Genet Dev. 1996;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- 9.Massagué J. Cell. 1996;85:947–950. doi: 10.1016/s0092-8674(00)81296-9. [DOI] [PubMed] [Google Scholar]
- 10.Liu F, Ventura F, Doody J, Massagué J. Mol Cell Biol. 1995;15:3479–3486. doi: 10.1128/mcb.15.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takao M, Hino J, Takeshita N, Konno Y, Nishizawa T, Matsuo H, Kangawa K. Biochem Biophys Res Commun. 1996;219:656–662. doi: 10.1006/bbrc.1996.0289. [DOI] [PubMed] [Google Scholar]
- 12.Lyons K M, Pelton R W, Hogan B L M. Genes Dev. 1989;3:1657–1668. doi: 10.1101/gad.3.11.1657. [DOI] [PubMed] [Google Scholar]
- 13.Hino J, Takao M, Takeshita N, Konno Y, Nishizawa T, Matsuo H, Kangawa K. Biochem Biophys Res Commun. 1996;223:304–310. doi: 10.1006/bbrc.1996.0889. [DOI] [PubMed] [Google Scholar]
- 14.Jaatinen R, Rosen V, Tuuri T, Ritvos O. J Clin Endocrinol Metab. 1996;81:3877–3882. doi: 10.1210/jcem.81.11.8923832. [DOI] [PubMed] [Google Scholar]
- 15.Natsume T, Tomita S, Iemura S, Kinto N, Yamaguchi A, Ueno N. J Biol Chem. 1997;272:11535–11540. doi: 10.1074/jbc.272.17.11535. [DOI] [PubMed] [Google Scholar]
- 16.Sampath T K, Maliakal J C, Hauschka P V, Jones W K, Sasak H, Tucker R F, White K H, Coughlin J E, Tucker M M, Pang R H, et al. J Biol Chem. 1992;267:20352–20362. [PubMed] [Google Scholar]
- 17.Kubo T, Shimasaki S, Kim H, Li D, Erickson G F. Biol Reprod. 1998;58:712–718. doi: 10.1095/biolreprod58.3.712. [DOI] [PubMed] [Google Scholar]
- 18.Erickson G F. Mol Cell Endocrinol. 1983;29:21–49. doi: 10.1016/0303-7207(83)90003-5. [DOI] [PubMed] [Google Scholar]
- 19.Erickson G F, Hsueh A J W. Endocrinology. 1978;102:1275–1282. doi: 10.1210/endo-102-4-1275. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Hsueh A J W, Erickson G F. J Biol Chem. 1979;254:11330–11336. [PubMed] [Google Scholar]
- 21.Shimasaki S, Shimonaka M, Zhang H-P, Ling N. J Biol Chem. 1991;266:10646–10653. [PubMed] [Google Scholar]
- 22.Chen D, Feng J Q, Feng M, Harris M A, Mundy G R, Harris S E. Biochim Biophys Acta. 1993;1174:289–292. doi: 10.1016/0167-4781(93)90200-w. [DOI] [PubMed] [Google Scholar]
- 23.Ozkaynak E, Schnegelsberg P N, Oppermann H. Biochem Biophys Res Commun. 1991;179:116–123. doi: 10.1016/0006-291x(91)91342-a. [DOI] [PubMed] [Google Scholar]
- 24.Takeda K, Oida S, Ichijo H, Iimura T, Maruoka Y, Amagasa T, Sasaki S. Biochem Biophys Res Commun. 1994;204:203–209. doi: 10.1006/bbrc.1994.2445. [DOI] [PubMed] [Google Scholar]
- 25.Beppu H, Minowa O, Miyazono K, Kawabata M. Biochem Biophys Res Commun. 1997;235:499–504. doi: 10.1006/bbrc.1997.6816. [DOI] [PubMed] [Google Scholar]
- 26.Nakatani A, Shimasaki S, Erickson G F, Ling N. Endocrinology. 1991;129:1521–1529. doi: 10.1210/endo-129-3-1521. [DOI] [PubMed] [Google Scholar]
- 27.Nakatani A, Shimasaki S, DePaolo L V, Erickson G F, Ling N. Endocrinology. 1991;129:603–611. doi: 10.1210/endo-129-2-603. [DOI] [PubMed] [Google Scholar]
- 28.Dudley A T, Lyons K M, Robertson E J. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 29.Luo G, Hofmann C, Bronckers A L, Sohocki M, Bradley A, Karsenty G. Genes Dev. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Bartold P M, Zhang C Z, Clarkson R W, Young W G, Waters M J. Endocrinology. 1998;139:3855–3862. doi: 10.1210/endo.139.9.6211. [DOI] [PubMed] [Google Scholar]
- 31.Magoffin D A, Erickson G F. In: Molecular Biology of the Female Reproductive System. Findlay J K, editor. New York: Academic; 1994. pp. 39–65. [Google Scholar]
- 32.Erickson G F, Li D, Shimasaki N, Ling N, Magoffin D. Endocrine. 1995;3:525–531. doi: 10.1007/BF02738828. [DOI] [PubMed] [Google Scholar]
- 33.Oliver J E, Aitman T J, Powell J F, Wilson C A, Clayton R N. Endocrinology. 1989;124:2671–2679. doi: 10.1210/endo-124-6-2671. [DOI] [PubMed] [Google Scholar]
- 34.Eppig J J, Chesnel F, Hirao Y, O’Brien M J, Pendola F L, Watanabe S, Wigglesworth K. Hum Reprod. 1997;12:127–132. [PubMed] [Google Scholar]
- 35.Koenig B B, Cook J S, Wolsing D H, Ting J, Tiesman J P, Correa P E, Olson C A, Pecquet A L, Ventura F, Grant R A, et al. Mol Cell Biol. 1994;14:5961–5974. doi: 10.1128/mcb.14.9.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heldin C-H, Miyazono K, ten Dijke P. Nature (London) 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 37.Miller W L. Endocr Rev. 1988;9:295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita H, ten Dijke P, Huylebroeck D, Sampath T K, Andries M, Smith J C, Heldin C-H, Miyazono K. J Cell Biol. 1995;130:217–226. doi: 10.1083/jcb.130.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Findlay J K, Xiao S, Shukovski L, Michel U. In: The Ovary. Adashi E Y, Leung P C K, editors. New York: Raven; 1993. pp. 413–432. [Google Scholar]
- 40.Cameron V A, Nishimura E, Mathews L S, Lewis K A, Sawchenko P E, Vale W W. Endocrinology. 1994;134:799–808. doi: 10.1210/endo.134.2.8299574. [DOI] [PubMed] [Google Scholar]
- 41.Falck B. Acta Physiol Scand. 1959;47:1–101. doi: 10.1111/j.1748-1716.1960.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 42.Nalbandov A V. In: Symposium on Oogenesis. Biggers J D, Schuetz A W, editors. Baltimore: Univ. Park Press; 1970. pp. 513–522. [Google Scholar]
- 43.Deane H W. Am J Anat. 1952;91:363–413. doi: 10.1002/aja.1000910303. [DOI] [PubMed] [Google Scholar]