Abstract
Somatic gene therapies require targeted transfer of the therapeutic gene(s) into stem cells that proliferate and then differentiate and express the gene in a tissue-restricted manner. We have developed an approach for gene therapy using marrow cells that takes advantage of the osteoblast specificity of the osteocalcin promoter to confine expression of chimeric genes to bone. Adherent marrow cells, carrying a reporter gene [chloramphenicol acetyltransferase (CAT)] under the control of a 1.7-kilobase rat osteocalcin gene promoter, were expanded ex vivo. After transplantation by intravenous infusion, engrafted donor cells in recipient mice were detected by the presence of the transgene in a broad spectrum of tissues. However, expression of the transgene was restricted to osteoblasts and osteocytes, as established by biochemical analysis of CAT activity and immunohistochemical analysis of CAT expression at the single cell level. Our data indicate that donor cells achieved long-term engraftment in various tissues of the recipients and that the CAT gene under control of the osteocalcin promoter is expressed specifically in bone. Thus, transplantation of multipotential marrow cells containing the osteocalcin promoter-controlled transgene provides an efficacious approach to deliver therapeutic gene expression to osteoblasts for treatment of bone disorders or tumor metastasis to the skeleton.
Keywords: osteocalcin, engraftment, mesenchymal stem cells, osteoprogenitors
Gene therapy by bone marrow transplantation is an advantageous approach for treatment of genetic and age-related bone disorders that compromise the entire skeleton (1), provided that the therapeutic gene can be targeted specifically into bone forming osteoblasts. Presently, this therapeutic application is limited by the inability to identify an osteoprogenitor cell with competency for both self-renewal and restricted differentiation into osteoblast lineage cells. In these studies, we have experimentally addressed the feasibility of using the bone-specific osteocalcin (OC) promoter to direct osteoblast-restricted expression of a transgene following transplantation of unfractionated marrow cells. Traditionally, transplantation-based gene therapies for the treatment of inherited and noninherited diseases have involved targeting the hematopoietic system (2, 3). Candidate diseases for treatment by gene therapy have included, for example, hemophilias, hemoglobinopathies, primary immunodeficiencies, and hematological malignancies and those that affect multiple organs and/or tissues (e.g., lysosomal storage diseases). Such approaches have been possible by the isolation and ex vivo expansion of CD34+ selected hematopoietic stem cells, introduction of a therapeutic gene(s) into the cells ex vivo, and return of the genetically modified cells to the patient (4–6).
In addition to hematopoietic stem cells, bone marrow contains mesenchymal stem cells that differentiate into cell lineages that form bone, cartilage, muscle, and adipose tissues (7–15). This pluripotent property of mesenchymal stem cells necessitates selection of lineage-specific cells or restricted expression of therapeutic genes to cells that commit to specific phenotypes for gene therapy. The characterization of mesenchymal stem cells has been hampered by heterogeneity of the marrow stromal cell population (16), uncertainty of their precise location (9, 17), limited representation (10, 13, 18), and lack of unique markers for the early-stage osteoprogenitor cells (11, 19–22). As an alternative to isolation of a purified committed osteoprogenitor population, we developed a strategy for targeting gene expression to bone cells to support skeletal gene therapy by using heterogeneous marrow cells. Our approach is to restrict activity of therapeutic/marker genes that are transfected into unfractionated marrow cells to bone by controlling gene expression with the bone tissue-specific osteocalcin promoter (23).
Over the past several years, we have achieved a significant understanding of the structure, function, and in vivo expression of the bone-specific osteocalcin gene (reviewed in ref. 24). Osteoblast-restricted expression of the OC promoter–reporter constructs in vivo has been well documented (23, 25–30). We have transplanted by intravenous infusion or expanded marrow cells from transgenic animals with an OC promoter–chloramphenicol acetyltransferase (CAT) reporter construct (27). Recipient mice were examined for expression of CAT in bone and nonosseous tissues biochemically and histochemically at the single cell level within the context of tissue organization. Our results demonstrate that the OC promoter can restrict gene expression to engrafted cells located in osseous tissues. A basis is thereby provided for developing strategies for transplantation-mediated gene therapy without the requirement to isolate committed lineage-specific osteoprogenitors.
MATERIALS AND METHODS
Preparation of Donor Cells.
Ex vivo-expanded bone marrow cells prepared from femur, tibia, and iliac bones of donor mice were used for transplantation. Bones were aseptically removed from 4- to 6-week-old transgenic mice (lineage SR62) harboring the 1.7-kilobase rat OC-CAT gene (27). Marrow was harvested, and cells from different bone sources were plated separately in media as described (27).
Nonadherent cells were removed on day 4, and the adherent population was expanded for 4–8 days in the same media under nondifferentiating conditions until used for transplantation. For transplantation, cultured cells were retrieved by digestion with 0.25% trypsin and 0.1 M EDTA at 37°C for 15 minutes and were resuspended and passed through a 40-μm sterile filter. To monitor cell viability and differentiation potential, an aliquot of the prepared donor cells to be used for transplantation was replated to culture in medium with addition of 50 μg/ml ascorbic acid and 10 mM β-glycerolphosphate to induce osteogenic differentiation (27).
Transplantation.
Our radiation and transplantation procedures are based on published protocols (15, 18). The recipient animals were 4- to 6-week-old C57B1/6XSJL mice (The Jackson Laboratory), the strain from which the SR62 transgenic was derived (27). Mice were irradiated in a 137Cs Gammacell 40 Irradiator (Atomic Energy, Ottawa) with two dosages of 5.5 Gy at 5-hour intervals before transplantation. Recipient mice received from 2 × 105 to 14 × 106 cultured marrow cells in different experiments and, in several studies, additionally received 1 × 106 fresh marrow cells (equivalent to ≈1/8 of the total marrow cells derived from one donor mouse). Cells were resuspended in 0.5 ml of culture media, and the entire volume was transplanted by tail vein injection. Animals were maintained and used in accordance with the Federal Animal Welfare Act and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Histological Procedures.
Mice were anesthetized with Metofane (Mallinkrodt), and 10 ml of cold PB solution (0.1 M NaH2PO4/NaHPO4, pH 7.3) were infused into the left ventricle, followed by 10 ml of cold 4% paraformaldehyde in PB solution manually at 3 ml/min (31). Soft tissues and femur and tibia, cut in half to expose their marrow cavities, were further fixed by immersion in paraformaldehyde/PB solution at 4°C for 3 hours. The fixed osseous samples were decalcified in 12–18% sodium EDTA (pH 6.6–7.2) at 4°C for 4 days, after which the samples were equilibrated in PBS (0.1 M NaCl and 0.008 M NaH2PO4/NaHPO4, pH 7.3) at 4°C for 1 day to remove the EDTA (31). The samples were dehydrated through standard graded isopropanol and xylene solutions at 4°C (32). Paraffin embedding was carried out under vacuum in low-melting-point media (Paraplast X-TRA, Electron Microscopy Sciences, Fort Washington, PA) at 56°C for 4 hours. Samples were cut into 5-μm sections by using a model 820 microtome (American Optical). The paraffin films were floated on 48°C water for 10 seconds and then were mounted onto positively charged microscope slides (Superfrost/Plus, Fisher Scientific) (33, 34).
Immunostaining for CAT was performed by using a Vectastain Elite kit (Vector Laboratories). Before applying antibodies, the deparaffinized and rehydrated tissue sections were treated with 0.6% H2O2 in methanol for 20 minutes to quench endogenous peroxidase activity and with 3% normal goat serum for 30 minutes to block nonspecific staining. Prepared slides were incubated with a polyclonal rabbit anti-CAT antibody (1:100–200, 5 Prime → 3 Prime, Boulder, CO) for 60 minutes at room temperature, were washed with PBS, and were stained with a biotinylated goat-anti-rabbit antibody (1:200) for 30 minutes. Excess antibody was removed by washing in PBS, and avidin and biotinylated horseradish peroxidase solutions were applied according to manufacturer’s recommendations. A diamino benzidine substrate kit for peroxidase (Vector Laboratories) was used for color development. Sections then were counterstained with Harris hematoxylin to identify bone tissue structure and cells, were dehydrated through graded isopropanol and toluene, and were mounted with Shandon-Mount (Shandon, Pittsburgh). For negative controls, the primary antibody (anti-CAT) was omitted from the reaction.
DNA Assays.
PCR was used to identify and locate engrafted donor cells that carry the OC-CAT transgene. Tissues dissected from recipient mice, transgenic donors (positive control), and nontransgenic (negative control) mice were frozen in liquid nitrogen, were crushed in a prechilled pulverizer, and were digested with 0.5 mg/ml proteinase K at 56°C overnight. Genomic DNA then was extracted by using the QIAamp Tissue Kit (Qiagen, Chatsworth, CA). The PCR primers used amplify a single 361-bp DNA fragment that spans the junction between the OC promoter 5′ primer (GTTTGACCTATTGCGCACATGACCC) and the CAT reporter 3′ primer (GGGCAAGAATGTGAATAAAGGCCGG). Conditions for PCR reactions were 1 minute at 94°C, 2 minutes at 60°C, and 1.5 minutes at 72°C for 25 cycles. PCR products were separated on acrylamide gels. Identity of the amplification products was verified by restriction digestions.
RESULTS
Ex Vivo Expansion of Marrow Cells That Support Osteoprogenitor Differentiation and Tissue-Restricted Gene Expression.
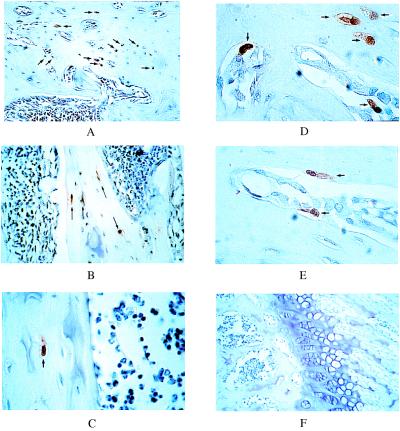
Initially, we determined that the plastic adherent marrow cells can be expanded in culture and retain competency for differentiation to osteoblasts as well as tissue-restricted activity of the bone-specific osteocalcin promoter. Bone marrow cultures were prepared from transgenic mice (lineage SR62) that were constructed with the proximal 1.7 kilobases of the rat OC gene promoter fused to a CAT reporter gene. We previously showed by enzyme analysis of whole tissue homogenates that expression of the CAT gene in the transgenic mice was largely restricted to osseous tissues including calvaria, femora, and tail vertebrae (27). To further define in vivo specificity of the OC promoter at the single cell level, we examined tissue sections from 6-week old transgenic mice by immunohistochemical staining using an anti-CAT antibody. Fig. 1 shows numerous CAT-positive cells in representative sections of cortical (Fig. 1A) and trabecular (Fig. 1B) bone from the femora of four transgenic animals (Fig. 1 A, B, D, and E). Under higher magnification, CAT protein reflecting OC promoter activity was detected in osteoblasts lining the bone surfaces and in mature osteocytes inside well-formed lacunae (Fig. 1 C–E). Neither the marrow cells (Fig. 1 A–C) nor chondrocytes [in the growth plate (Fig. 1F)] show CAT expression. Not all osteoblasts and osteocytes are positive for CAT immunostaining. The representation of cells expressing the CAT gene reflects the component of the osteoblast population that is at a developmental stage when OC gene expression occurs. Immunostaining did not detect CAT expression in nonosseous tissues and organs including lung, kidney, muscle, spleen, thymus, heart, liver, and intestine (data not shown). These data are consistent with biochemical analysis of CAT in whole tissue extracts (27). Taken together, these findings indicate that expression of the CAT reporter gene under control of the osteocalcin promoter is specific to osteoblasts and osteocytes in bone tissues of transgenic mice. Cells from these animals provide a viable vehicle for evaluating effectiveness of the bone-restricted osteocalcin promoter to confine gene expression to bone after transplantation.
Figure 1.
Expression of the bone-specific osteocalcin-CAT transgene in osteoblast lineage cells of bone or mice. Immunohistochemical detection of CAT-positive osteocytes (arrows) are seen in cortical bone (A, ×25) and in trabecular bone (B, ×40). At higher magnification (C, ×100), the mature osteocyte inside of a well formed lacunae is observed with an absence of positive cells in marrow. D and E show CAT-expressing cells at a higher magnification (×100) from different mice revealing positive surface osteoblasts, osteocytes in lacunae (D), and a preosteocyte in E. F shows the growth plate region (×25) of donor bone with an overall absence of OC-CAT-expressing cells in the cartilage.
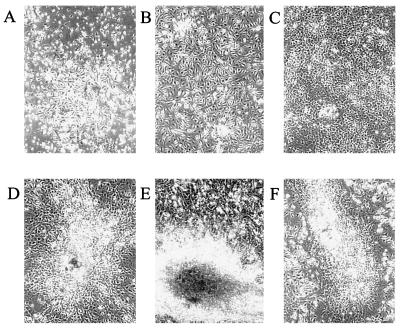
It has been well documented that the adherent marrow cell population is enriched in stromal derived cells including osteoprogenitors (refs. 8 and 11 and reviewed in refs. 10 and 16). We experimentally determined conditions for expansion of the adherent marrow population that would retain competency for engraftment and subsequent osteogenic differentiation (Table 1). For optimal engraftment (see below), adherent marrow cells were cultured under conditions that promote cell proliferation but do not permit expression of bone phenotypic properties (Fig. 2 A–C). Our methodology was to culture marrow cells in the absence of media supplements that facilitate differentiation (e.g., ascorbate and β-glycerophosphate). To maximize the representation of osteogenic stem cells in the cultures, marrow cells were harvested from both long bones and iliac bones. Cells from crushed iliac bones grew faster and formed more adherent cell colonies in culture compared with those obtained from flushed marrow cells of long bones. As shown in Fig. 2 A–C, under these conditions, we observed expansion of the adherent marrow cell population with formation of stromal cell colonies that histochemically do not express alkaline phosphatase, an early marker of osteogenesis (data not shown). This population of bone marrow derived cells supports reconstitution of the hematopoietic system and osteoblast differentiation in lethally irradiated mice that survive for 1.5 years posttransplantation (Table 1). Ex vivo culture did not compromise differentiation potential of osteogenic stem cells (Fig. 2 D–F). The cell colonies resumed proliferation and differentiate when cultured at the end of the ex vivo expansion period on day 8 in complete medium supplemented with ascorbate and β-glycerophosphate (differentiation medium) (Fig. 2E). As a control for viability and growth potential, we show that a replated mixture of ex vivo expanded and undifferentiated femur and iliac marrow cells that were used for transplantation retains its potential to differentiate (Fig. 2F). The importance of maintaining the cultures under conditions that preclude osteoprogenitor differentiation is demonstrated by a dramatic decline in in vivo engraftment of differentiated transplanted cells (Table 1 and Fig. 3A).
Table 1.
Variables contributing to the frequency of engraftment of transplanted ex vivo-expanded marrow cells in irradiated recipient mice
| Type and source of transplant | Culture conditions* | Cultured cells injected per mouse | Engraftment efficiency† |
|---|---|---|---|
| Adherent cells only | |||
| Long bone marrow | Differentiation | 4 × 106 | 0/2 |
| Differentiation | 8 × 106 | 0/1 | |
| Nondifferentiation | 1 × 106 | 0/3 | |
| Nondifferentiation | 4 × 106 | 4/4 | |
| Nondifferentiation | 15 × 106 | 6/6 | |
| Iliac and long bone marrow | Nondifferentiation | 15 × 106 | 6/7 |
| Adherent cells and supporting fresh marrow | |||
| Long bone marrow | Nondifferentiation | 4.5 × 106 | 12/12 |
| Nondifferentiation | 7.6 × 106 | 3/3 | |
| Iliac and long bone marrow | Nondifferentiation | 15 × 106 | 3/6‡ |
Differentiation = + ascorbate mice.
Total positive engrafted per total recipient mice; positive mice were identified by PCR analysis of the transgene in tissues.
Only three of the six transplanted mice survived after 11 days, and these three were positive for engrafted cells.
Figure 2.
Ex vivo expansion of adherent marrow stromal cells in nondifferentiation conditions. Shown are phase contrast micrographs of cultures of whole marrow harvested from long bones and plated at a concentration of 5 × 106 cells/ml. (A) Day-4 adherent cell colony. (B and C) Days 6 and 8 of culture in αMEM with 20% FCS. D shows day-8 culture in which ascorbate and β-glycerolphosphate are included in media from day 4, stimulating osteogenic colony formation; E and F show that the culture process does not alter the osteogenic potential of the cells. E shows that addition of ascorbate and β-glycerophosphate cultures at the end of the ex vivo expansion period from day 8 (C) to day 18 results in mineralized nodules (dark area in center). F shows donor cells harvested on day 8 and replated in differentiation media to develop osteogenic nodules (shown at day 13 after replating). D, E, and F show mineralized nodules.
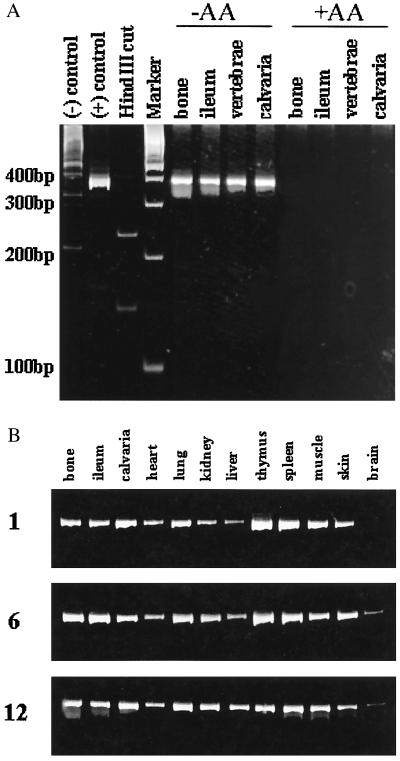
Figure 3.
Engraftment of donor cells in osseous and nonosseous tissues of recipient animals. A shows influence of the differentiation states of transplanted adherent stromal cells on engraftment by detection of the rOC-CAT transgene in control mice and bone tissues of recipient transplanted mice. Lanes: 1, DNA from a nontransgenic mouse [(−) control]; 2, a positive transgenic donor [(+) control]; 3, the selected PCR primers (described in Materials and Methods) give rise to the single product transgene of expected size 369 bp, which comigrated close to the 400-bp molecular marker in the 5% polyacrylamide gel. The identity and actual size of the PCR product is verified by restriction enzymes with HindIII (lane indicated), which cuts the DNA at the OC-CAT junction resulting in two PCR products. Lane 4 shows size markers (Boehringer Mannheim); in lanes 5–8, four bone tissues as indicated from transplanted animals with ex vivo-transplanted donor cells expanded in nondifferentiating conditions (−AA, ascorbic acid) show engraftment in mice 6 weeks posttransplant. In lanes 9–12, the transgene was not detected in the tissues of recipient animals transplanted with ex vivo-expanded cells differentiated in the presence of ascorbate and β-glycerolphosphate (+AA). B shows detection of the transgene 1, 6, and 12 months postimplantation in osseous and nonosseous tissues of recipient mice as indicated.
Bone-Tissue Specific Activity of the Osteocalcin Gene Promoter After Stem Cell-Mediated Engraftment.
We determined that a critical number of transplanted cells, >3 million per mouse, is required for engraftment (Table 1). We also show that the ex vivo-expanded adherent marrow cell population can support survival of the irradiated mice after transplant of the nondifferentiated cells. The poor rate of survival of mice when flushed marrow from long bone is combined with marrow from crushed iliac bone (Table 1) may reflect dilution of an adherent hematopoietic stem cell population. To establish long-term engraftment of ex vivo expanded bone marrow cells, PCR analysis (DNA PCR) was carried out on genomic DNA prepared from various tissues of transplanted animals. The PCR primers were designed to hybridize with sequences present in the transgene and produce only one prominent DNA band. The expected 369-bp PCR product was observed with DNA from the transgenic control donor and not in nontransgenic control mice (Fig. 3A, lanes 1 and 2). Identity of the PCR product was verified by restriction enzyme digestion with HindIII, which cleaved the DNA at the OC-CAT junction to yield two fragments of 139 and 229 bp (Fig. 3A, lane 3). Using this assay, we detected engraftment of transgenic donor cells that were maintained as undifferentiated expanded cells before transplant (Fig. 3A, −AA group). Cells that were differentiated ex vivo in the presence of ascorbate and β-glycerolphosphate before transplantation showed minimal or no engraftment (Fig. 3A, +AA group). Transplanted cells were detected in bone and nonosseous tissues of recipient mice as early as 1 month after transplantation and remained engrafted for as long as 12 months (Fig. 3B).
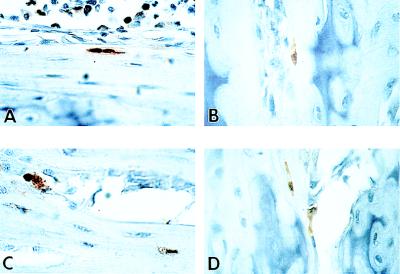
We next determined bone-specific activity of the osteocalcin promoter in engrafted cells of the transplanted recipient at the single cell level within the context of skeletal tissue organization. The principal objective was to evaluate whether an OC-CAT reporter gene that is carried in the germ line and expressed only in osseous cells of donor mice retains bone-restricted expression after transplantation and engraftment of marrow cells in recipient mice. Immunohistochemical staining of mouse tissue sections from recipient mice 4–5 weeks after transplantation was carried out by using an anti-CAT antibody. CAT-positive osteoblasts and osteocytes (Figs. 4) were present in bone sections of transplanted mice, indicating that donor marrow-derived cells engrafted in bone tissue in an environment that supports maturation to the developmental stage when the bone-specific osteocalcin promoter is transcriptionally active. Osteoblasts expressing CAT were observed in the primary spongiosa (Fig. 4 A and B) and cortical bone (Fig. 4 C and D) of the metaphysis. Osteocytes (Fig. 4 C and D) were found inside well formed lacunae in recipient bone sections, suggesting that the transplanted donor cells contained a population of immature osteogenic cells (e.g., osteoprogenitors) that were capable of further differentiation into mature and active bone cells. In contrast, systematic examination of nonosseous tissues from transplanted mice did not reveal CAT-positive cells, either by biochemical assay of homogenized tissue (Fig. 5) or by immunohistochemistry of tissue sections (data not shown). Fig. 5 shows bone-specific CAT activity in a recipient mouse 6 weeks posttransplant. The level of expression of OC-CAT in osteoblasts and osteocytes of transplanted mouse bone is high, albeit at an expected lower level than the transgenic donor. In nonosseous tissues, background CAT ctivity is detected, similar to that observed in brain and spleen tissue extracts from a negative control nontransgenic mouse (data not shown). These findings demonstrate that activity of the OC promoter is restricted to donor cells that have engrafted and undergone differentiation in osseous tissues of the recipient mouse after transplantation.
Figure 4.
Expression of the transgene by immunohistochemical detection of OC-CAT in osteoblasts from bone of recipient mice. Bone was harvested from two different recipient mice after 4 weeks (A and B) and 6 weeks (C and D). A and C show cortical bone, and B and D show bone trabeculae in which cartilage remnants stain deeper blue. Newly formed positive osteocytes (brown) in the well formed lacunae are evident (A and C) in the cortical bone. A positive osteoblast on the surface (stained brown) is seen in B and C. Toluidine blue counterstain; ×100.
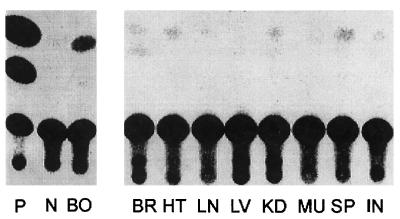
Figure 5.
Bone-specific expression of the transgene in recipient mice by tissue assay of CAT activity. CAT activity in homogenates of calvaria from a positive control pSR62 transgenic mouse (P), a nontransgenic negative mouse (N), and a 6-week-posttransplant mouse (BO), is compared (Left). CAT activity in soft tissues of the same marrow-transplanted recipient mouse and assayed with the same reagents as in the left panel is shown in the right panel for brain (BR), heart (HT), lung (LN), liver (LV), kidney (KD), muscle (MU), spleen (SP), and intestine (IN). Biochemical analyses for CAT activity were performed with equal amounts of protein extracts for each tissue as described in (27).
DISCUSSION
We have demonstrated that an adherent, pluripotent marrow cell population that has been expanded ex vivo can be transplanted into irradiated mice and remain competent to engraft and differentiate into bone tissue forming cells, osteoblasts, and osteocytes. At the time of transplantation, these donor cells did not express mature osteoblast marker genes. However, development of osteoblast and osteocyte properties subsequent to engraftment indicates that osteogenic stem cells are a component of the transplanted cell population. Bone marrow from many species contains multipotential precursor cells that can differentiate into mesenchymal lineage cells that include osteoblasts, chondroblasts, adipocytes, and myoblasts. On this basis, the mesenchymal population enriched in vitro by their adherence property to plastic surfaces has been designated a mesenchymal stem cell (35). Despite significant efforts to identify and purify mesenchymal stem cells or osteoprogenitors (21, 35), it remains to be established whether the cells that have been isolated to date are primitive or partially committed. However, the ability of this pluripotent population of mesenchymal stem cells to engraft after intravenous transplantation and become tissue-restricted differentiated cells (refs. 15, 36, and 37; these studies) provides evidence for stem cell properties. Bruder and colleagues (38) have shown that human mesenchymal stem cells can be expanded in vitro during extensive subcultivation and cryopreservation. Furthermore, Keating et al. (39) demonstrated that the long term cultured adherent stromal cells can be transplanted in man. Thus, manipulation of the adherent marrow population for integration of a bone-specific controlled transgene is feasible. Our studies have established that, by using the osteoblast-specific osteocalcin gene promoter to restrict gene expression to bone forming cells, the requirement to isolate a pure population of osteoprogenitor cells targeting genes to bone is effectively circumvented. In transplanted marrow cells that contain a transgene under control of the osteocalcin promoter, fidelity of bone tissue-specific transcription is retained. Genes that regulate osteoblast growth and differentiation or genes that support bone structure can be targeted to impaired skeletal tissue for facilitating repair of bone tissues in diseases such as osteoporosis and osteogenesis imperfecta (1, 35, 36).
The potential for therapeutic application of promoters with tissue-restricted activity is supported by demonstrations that the albumin and tyrosinase promoters can target gene expression to hepatoma and melanoma cells, respectively, to treat solid tumors (40, 41). Ferrari et al. have recently reported facilitation of muscle regeneration by transplanting unfractionated bone marrow cells (42). However, although expression of the reporter gene appeared to be muscle-specific when the cells were injected locally, it remains to be determined whether expression is restricted to the muscle when the cells are injected systemically. Pereira et al. (15) have transplanted cultured adherent marrow cells carrying the collagen type I (COLI) marker gene and have examined its expression in various tissues in recipient animals by PCR-based assays. As expected, the marker gene expression was detected in the lungs but not cartilage. The limitation is that the COLI promoter used in their study is active in lung, spleen, and bone. In contrast, several studies have documented bone-specific expression of osteocalcin in vivo (25–28, 30). The study by Ko et al. (29) used the OC promoter for in vivo targeting to bone tumors. Intratumoral injection of a vector that harbors an OC-thymidine kinase construct into a subcutaneous tumor of rat or human osteosarcoma abolished tumor growth in a host treated subsequently with acyclovir. However, this approach does not permit treatment of metastatic bone tumors.
We report osteoblast-targeted gene expression controlled by a bone-specific osteocalcin promoter after systemic transplantation of a heterogeneous mouse marrow cells. Although other studies have shown that bone marrow cells injected or implanted directly into specific regions of bone can engraft (37, 43), our experiments demonstrate that an adherent and expanded marrow stromal population transplanted systemically can retain competency to (i) engraft, (ii) differentiate into mature osteoblasts and osteocytes after transplantation, and (iii) support reconstitution of hematopoiesis in radiation-ablated mice. Hematopoietic cells are found in close association with stromal cells (reviewed in ref. 44). Earlier studies have shown that myeloid and lymphoid progenitors do adhere to plastic within hours and can support survival of lethally irradiated mice (45). Recently, Phinney et al. (46) demonstrated that hematopoietic lineage progenitors persist in plastic adherent mouse marrow population and 20 days of culture. In one other study in mice, hematopoietic recovery from total body irradiation was found after transplantation of a bone marrow stromal cell line (47). Our 10-day ex vivo-expanded adherent marrow population, maintained as nondifferentiated cells, support survival of fully marrow-ablated irradiated mice. Control-irradiated mice not receiving transplanted cells expired after 10 days. We also observed that 50% of the mice died [3 of 6 (Table 1)] in one experiment when marrow cells were used for transplantation from crushed iliac bone, which likely contained a lower percentage of adherent stem cells because, in the microenvironment of trabecular bone, contaminating surface osteoblasts may have diluted the representation of undifferentiated cells.
Our findings validate the strategy to use the bone tissue specificity of the osteocalcin gene promoter for restricting expression of therapeutic genes to skeletal cells. The efficacy of using the osteocalcin gene promoter in transplanted bone marrow cells for targeting expression of therapeutic genes to bone clinically would be compromised by a necessity to precede transplantation by radiation or chemoablation. However, Nilsson et al. (48) have recently demonstrated by Y chromosome painting that unfractionated marrow cells transplanted from a male to a nonablated female mouse engraft and differentiate into osteocytes. It may, therefore, be realistic to combine stem cell-mediated transplantation with tissue-restricted activity of the osteocalcin gene promoter to deliver therapeutic genes to bone for correcting imbalances between bone formation and resorption or treating heritable disorders and tumor metastasis to the skeleton.
Acknowledgments
We thank Dr. Edith Gardner (Garran Institute, Sydney, Australia) for helpful discussions and J. Rask for editorial assistance. This work was supported by a Translational Research Grant from the University of Massachusetts Medical Center and the National Institutes of Health Grant DE12528. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- CAT
chloramphenicol acetyltransferase
- OC
osteocalcin
References
- 1.Prockop D J, Kivirikko K I. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 2.Dunbar C E. Annu Rev Med. 1996;47:11–20. doi: 10.1146/annurev.med.47.1.11. [DOI] [PubMed] [Google Scholar]
- 3.O’Marcaigh A S, Cowan M J. Curr Opin Oncol. 1997;9:126–130. [PubMed] [Google Scholar]
- 4.Berenson R J, Andrews R G, Bensinger W I, Kalamasz D, Knitter G, Buckner C D, Bernstein I D. J Clin Invest. 1988;81:951–955. doi: 10.1172/JCI113409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terstappen L W, Huang S, Safford M, Lansdorp P M, Loken M R. Blood. 1991;77:1218–1227. [PubMed] [Google Scholar]
- 6.Dunbar C E, Cottler-Fox M, O’Shaughnessy J A, Doren S, Carter C, Berenson R, Brown S, Moen R C, Greenblatt J, Stewart F M. Blood. 1995;85:3048–3057. [PubMed] [Google Scholar]
- 7.Prockop D J. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 8.Friedenstein A J, Chailakhyan R K, Gerasimov U V. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 9.Owen M. J Cell Sci. 1988;10:63–76. doi: 10.1242/jcs.1988.supplement_10.5. [DOI] [PubMed] [Google Scholar]
- 10.Aubin J E, Liu F. In: Principles of Bone Biology. Bilezikian J P, Raisz L G, Rodan G A, editors. San Diego: Academic; 1996. pp. 51–68. [Google Scholar]
- 11.Haynesworth S E, Goshima J, Goldberg V M, Caplan A I. Bone. 1992;13:81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 12.Johnstone B, Hering T M, Caplan A I, Goldberg V M, Yoo J U. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 13.Hurwitz D R, Kirchgesser M, Merrill W, Galanopoulos T, McGrath C A, Emami S, Hansen M, Cherington V, Appel J M, Bizinkauskas C B, et al. Hum Gene Ther. 1997;8:137–156. doi: 10.1089/hum.1997.8.2-137. [DOI] [PubMed] [Google Scholar]
- 14.Wakitani S, Saito T, Caplan A I. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 15.Pereira R F, Halford K W, O’Hara M D, Leeper D B, Sokolov B P, Pollard M D, Bagasra O, Prockop D J. Proc Natl Acad Sci USA. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Triffitt J T. In: Principles of Bone Biology. Bilezikian J P, Raisz L G, Rodan G A, editors. San Diego: Academic; 1996. pp. 39–50. [Google Scholar]
- 17.Nakahara H, Bruder S P, Goldberg V M, Caplan A I. Clin Orthop. 1990;259:223–232. [PubMed] [Google Scholar]
- 18.Falla N, van Vlasselaer P, Bierkens J, Borremans B, Schoeters G, van Gorp U. Blood. 1993;82:3580–3591. [PubMed] [Google Scholar]
- 19.Gronthos S, Graves S E, Ohta S, Simmons P J. Blood. 1994;84:4164–4173. [PubMed] [Google Scholar]
- 20.van Vlasselaer P, Falla N, Snoeck H, Mathieu E. Blood. 1994;84:753–763. [PubMed] [Google Scholar]
- 21.Pittenger M F, Mackay A M, Beck S C, Jaiswal R K, Douglas R, Mosca J D, Moorman M A, Simonetti D W, Craig S, Marshak D R. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 22.Bruder S P, Ricalton N S, Boynton R E, Connolly T J, Jaiswal N, Zaia J, Barry F P. J Bone Miner Res. 1998;13:655–663. doi: 10.1359/jbmr.1998.13.4.655. [DOI] [PubMed] [Google Scholar]
- 23.Rahman S, Oberdorf A, Montecino M, Tanhauser S M, Lian J B, Stein G S, Laipis P J, Stein J L. Endocrinology. 1993;133:3050–3053. doi: 10.1210/endo.133.6.8243336. [DOI] [PubMed] [Google Scholar]
- 24.Lian J B, Stein G S, Stein J L, van Wijnen A J. In: Vitamins and Hormones. Litwack G, editor. Vol. 55. San Diego: Academic; 1998. pp. 443–509. [DOI] [PubMed] [Google Scholar]
- 25.Baker A R, Hollingshead P G, Pitts-Meek S, Hansen S, Taylor R, Stewart T A. Mol Cell Biol. 1992;12:5541–5547. doi: 10.1128/mcb.12.12.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kesterson R A, Stanley L, DeMayo F, Finegold M, Pike J W. Mol Endocrinol. 1993;7:462–467. doi: 10.1210/mend.7.3.8483481. [DOI] [PubMed] [Google Scholar]
- 27.Frenkel B, Capparelli C, van Auken M, Bryan J, Stein J L, Stein G S, Lian J B. Endocrinology. 1997;138:2109–2116. doi: 10.1210/endo.138.5.5105. [DOI] [PubMed] [Google Scholar]
- 28.Sims N A, White C P, Sunn K L, Thomas G P, Drummond M L, Morrison N A, Eisman J A, Gardiner E M. Mol Endocrinol. 1997;11:1695–1708. doi: 10.1210/mend.11.11.0008. [DOI] [PubMed] [Google Scholar]
- 29.Ko S C, Cheon J, Kao C, Gotoh A, Shirakawa T, Sikes R A, Karsenty G, Chung L W. Cancer Res. 1996;56:4614–4619. [PubMed] [Google Scholar]
- 30.Desbois C, Hogue D A, Karsenty G. J Biol Chem. 1994;269:1183–1190. [PubMed] [Google Scholar]
- 31.Bourque W T, Gross M, Hall B K. J Histochem Cytochem. 1993;41:1429–1434. doi: 10.1177/41.9.7689084. [DOI] [PubMed] [Google Scholar]
- 32.Cattoretti G, Pileri S, Parravicini C, Becker M H, Poggi S, Bifulco C, Key G, D’Amato L, Sabattini E, Feudale E. J Pathol. 1993;171:83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- 33.Huang W M, Gibson S J, Facer P, Gu J, Polak J M. Histochemistry. 1983;77:275–279. doi: 10.1007/BF00506570. [DOI] [PubMed] [Google Scholar]
- 34.Nilsson S K, Hulspas R, Weier H U, Quesenberry P J. J Histochem Cytochem. 1996;44:1069–1074. doi: 10.1177/44.9.8773573. [DOI] [PubMed] [Google Scholar]
- 35.Bruder S P, Fink D J, Caplan A I. J Cell Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira R F, O’Hara M D, Laptev A V, Halford K W, Pollard M D, Class R, Simon D, Livezey K, Prockop D J. Proc Natl Acad Sci USA. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazarus H M, Haynesworth S E, Gerson S L, Rosenthal N S, Caplan A I. Bone Marrow Transplant. 1995;16:557–564. [PubMed] [Google Scholar]
- 38.Jaiswal N, Haynesworth S E, Caplan A I, Bruder S P. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 39.Keating A, Singer J W, Killen P D, Striker G E, Salo A C, Sanders J, Thomas E D, Thorning D, Fialkow P J. Nature (London) 1982;298:280–283. doi: 10.1038/298280a0. [DOI] [PubMed] [Google Scholar]
- 40.Vile R G, Nelson J A, Castleden S, Chong H, Hart I R. Cancer Res. 1994;54:6228–6234. [PubMed] [Google Scholar]
- 41.Sokol D L, Gewirtz A M. Crit Rev Eukaryotic Gene Expression. 1996;6:29–57. doi: 10.1615/critreveukargeneexpr.v6.i1.30. [DOI] [PubMed] [Google Scholar]
- 42.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 43.Onyia J E, Clapp D W, Long H, Hock J M. J Bone Miner Res. 1998;13:20–30. doi: 10.1359/jbmr.1998.13.1.20. [DOI] [PubMed] [Google Scholar]
- 44.Deryugina E I, Muller-Sieburg C E. Crit Rev Immunol. 1993;13:115–150. [PubMed] [Google Scholar]
- 45.Bearpark A D, Gordon M Y. Bone Marrow Transplant. 1989;4:625–628. [PubMed] [Google Scholar]
- 46.Phinney D G, Kopen G, Isaacson R L, Prockop D J. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 47.Anklesaria P, Kase K, Glowacki J, Holland C A, Sakakeeny M A, Wright J A, FitzGerald T J, Lee C Y, Greenberger J S. Proc Natl Acad Sci USA. 1987;84:7681–7685. doi: 10.1073/pnas.84.21.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nilsson S K, Dooner M S, Weier H U, Frenkel B, Lian J B, Stein G S, Quesenberry P J. J Exp Med. 1999;189:729–734. doi: 10.1084/jem.189.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]