Abstract
The homeobox gene Cdx2, a homologue of the Drosophila gene caudal, has been implicated in the control of cell differentiation in the intestinal epithelium. Recently, we showed that mice in which one allele of the Cdx2 gene had been inactivated by homologous recombination developed multiple intestinal polyp-like lesions that did not express Cdx2 and that contained areas of squamous metaplasia in the form of keratinizing stratified squamous epithelium, similar to that occurring in the mouse esophagus and forestomach. We have now examined colonic lesions from 98 Cdx2+/− mice and report that the lesions are composed of heterotopic stomach and small intestinal mucosa. We conclude that Cdx2 directs endodermal differentiation toward a caudal phenotype and that haploinsufficient levels of expression in the developing distal intestine lead to homeotic transformation to a more rostral endodermal phenotype, such as forestomach epithelium that does not express Cdx2 during normal development. Intercalary growth (epimorphic regeneration), which previously has never been described in mammals, then occurs, resulting in the ordered “filling in” of tissue types at the discontinuity between the gastric and colonic epithelia. This intercalary growth in a restricted space results in the formation of the polypoid lesions observed.
Keywords: development, homeobox
Organized metazoan development depends on the expression of a hierarchy of genes that sequentially provide increasingly detailed positional information. Many of them contain a conserved “homeobox” sequence coding for a DNA binding homeodomain. A group of such genes, specifying the individual characteristics of body segments and known as the homeotic selector genes, are clustered in Drosophila into two gene complexes together called the HOM genes and consisting of the Antennapedia and Bithorax complexes. These are strongly conserved during evolution and occur in mammals as four paralogous groups known as the Hox complexes, again specifying positional information during development.
Homeobox genes are also present outside the Hox cluster, and some of these are linked to form a recently defined ParaHox cluster, which is thought to be an ancient paralogue of an original “ProtoHox” gene cluster (1).
The part played by Hox genes in determining positional values is established by numerous gain-and loss-of-function studies, particularly those involving ectodermal and mesodermal structures. Rather less is known about the anatomical specification of the gut. Hox and ParaHox genes may be involved, because many are expressed both in the endoderm and in the splanchnic mesoderm. For Drosophila, it is thought that regional specification of the splanchnic mesoderm may confer positional clues to the endoderm, and mesodermal influences may also be important in mammals. Little is known, however, about the genes involved in this process.
A Drosophila homeobox gene called caudal (cad) was isolated by Mlodzik and Gehring (2). Like the Antennapedia-type genes, it belongs to the hexapeptide class of homeobox genes (3) but is not a member of the HOM cluster. The posterior parts of Drosophila larvae that lack both maternal and zygotic cad gene products are shortened severely, with variable deletions of many of the posterior segments. Duprey et al. (4) isolated the first mammalian homologue of cad and noted that expression in the adult mouse was confined to the posterior gut endoderm, although it was found subsequently that the gene—called Cdx1—was more widely expressed during embryogenesis (5). Subsequently, two additional cad homologues, known as Cdx2 and Cdx4, have been cloned from murine cDNA or genomic libraries (6, 7). Both are widely expressed during development, but the expression of Cdx2, like that of Cdx1, is confined to the posterior gut endoderm during later development and after birth. The conserved linkage of Cdx2 with Pdx1—a gene expressed in the duodenal and pancreatic endoderm—indicates that it belongs to the ParaHox cluster (1).
It has been shown that, in the gut, Cdx2 modifies the expression of molecules involved in cell–cell and cell–substratum interaction and stimulates markers of enterocyte differentiation (8), thus triggering cells toward the phenotype of differentiated enterocytes. Gene inactivation by recombination with a Cdx2 null mutant construct results in the death of all Cdx2−/− animals, probably because of a failure to implant. The death of homozygous null mutants reflects the strong expression of Cdx2 protein normally seen in the trophectoderm at implantation (9). Cdx2+/− heterozygotes manifest a variety of effects, including an anterior homeotic shift involving the cervical and thoracic spine. In this context, it is interesting to note that Xcad3, the amphibian homologue of Cdx4, regulates the expression of Hox genes downstream of fibroblast growth factor in specifying axial position in the frog (10) and that Cdx1 has a direct effect on Hoxa-7, whereas its absence alters the mesodermal expression of and axial specification by Hox genes in mice (11). Of particular interest, however, is the fact that Cdx2+/− animals develop multiple intestinal polyp-like lesions, most frequently in the proximal colon, that contain areas of stratified squamous epithelium (SSE), similar to that occurring in the forestomach. In this communication, we show that the lesions consist of heterotopic gastric and small intestinal tissue, which we believe is the result of homeosis (12) involving intestinal epithelium and which in turn initiates a process of intercalation. The intercalated tissue eliminates the discontinuity between gastric and colonic mucosa by generation of the intermediate intestinal epithelial phenotypes.
MATERIALS AND METHODS
Cdx2+/− Heterozygotes.
The generation of mice bearing a null mutation of Cdx2 created by homologous recombination has been described (9). Animals were in a mixed 129Sv/C57BL6 genetic background.
Histological Preparation.
Segments of intestine bearing lesions were immersion-fixed in 4% (vol/vol) paraformaldehyde, embedded in paraffin by standard methods, cut into 5-μm sections, and stained with hematoxylin and eosin or by Mowry’s technique to identify intestinal mucins (13).
Parietal-specific H+,K+-ATPase (antiserum obtained from A. Smolka of the Center for Ulcer Research and Education, Los Angeles, and University of California, Los Angeles) was localized in 12-μm cryostat sections. These were incubated with monoclonal antibody (mouse) raised against ATPase isolated from porcine parietal cells and used at 7.5 μg of protein per ml in incubation for 24 h at room temperature. The bound primary antibodies were located by using streptavidin–Texas Red coupled to biotinylated horse anti-mouse IgG. Reacted sections were mounted in buffered glycerol and viewed on a Zeiss fluorescence microscope.
Paraffin sections stained for trefoil factor family 2 peptide (TFF2) were incubated with a mouse IgM monoclonal antibody raised against the 16 C-terminal amino acids of TFF2, followed by visualization by using a goat anti-mouse IgM horseradish peroxidase conjugate (14). Methacarn-fixed paraffin sections were stained with a polyclonal antibody to Cdx2 as described by Beck et al. (15). The specificity of the antibody had been established previously (15).
RESULTS
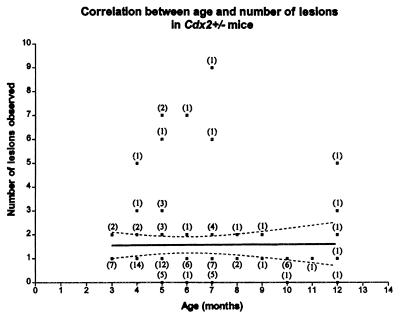
The alimentary tracts from 98 Cdx2+/− mice and 25 littermate wild-type controls aged between 3 and 18 months were examined macroscopically. All areas of abnormal-looking mucosa from heterozygotes and corresponding areas in wild-type controls were removed and sampled histologically. Heterotopic mucosa was identified in 85 (87%) of Cdx2 heterozygotes but in none of the controls. Lesions occurred most frequently in the proximal colon, which is the site of maximal expression of the Cdx2 gene in the adult (16). They were occasionally seen in the small intestine and the distal colon, with decreasing frequency with distance from the proximal colon. Lesions were not observed in the stomach, esophagus, or rectum; they were therefore confined to those parts of the alimentary tract in which some expression of Cdx2 occurs during development (15). The mean number (± SEM) of lesions observed macroscopically was 1.67 ± 0.17, and the frequency and incidence did not rise with age (Fig. 1). These results suggest that fresh lesions do not arise in adult animals.
Figure 1.
The frequency of polyps in 98 mice plotted against their age. The numbers of animals at each monthly time point are indicated in brackets. The slope of the line is not significantly different from zero (P = 0.92).
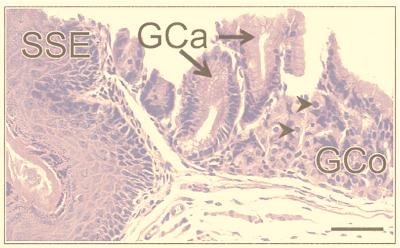
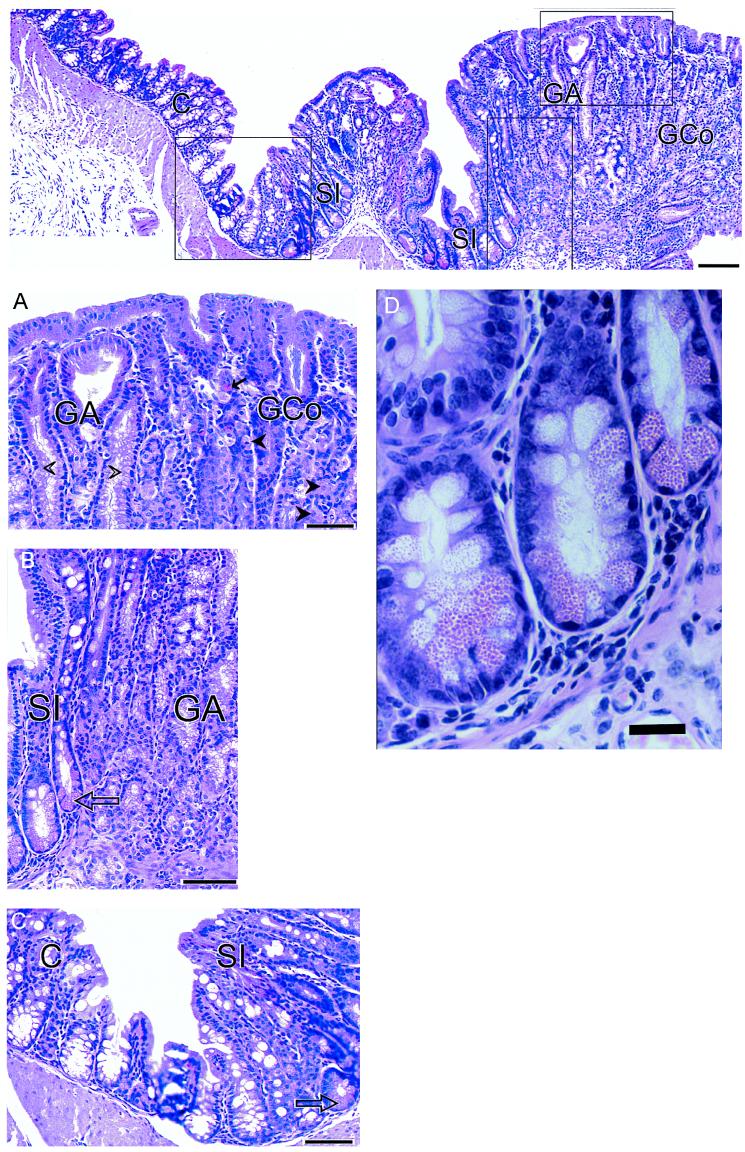
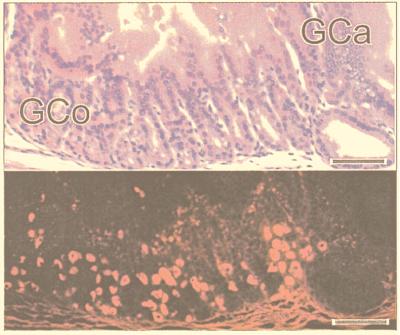
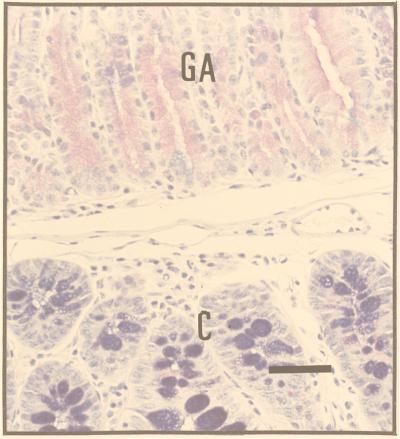
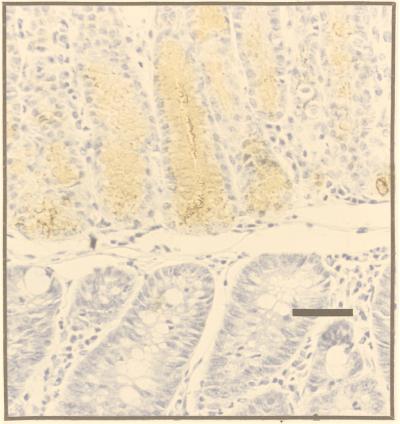
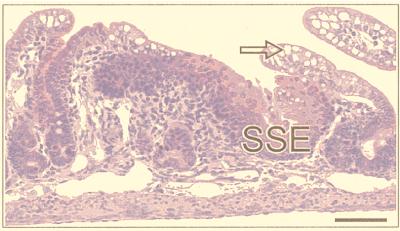
The sectioning procedure we adopted meant that lesions were cut at various planes. The most instructive were those (≈30%) that passed through SSE as a reference point (Fig. 2). The adjacent tissue showed evidence of intercalation (Figs. 2 and 3). Thus, if one took an ectopic area in the proximal colon consisting of SSE, then (passing laterally on either side) the sequence SSE, cardia, corpus, pylorus, small intestine, and normal colon would be encountered regularly, though one of the regions might occasionally be abbreviated or even absent. Between the SSE and the normal colon, gastric and intestinal mucosa were thus interposed to restore normal continuity of intestinal tissue types. Prominent in this intercalated region was tissue typical of the gastric corpus. This tissue was recognizable in hematoxylin- and eosin-stained sections by its glandular structure and by the presence of eosinophilic parietal cells and mucus-neck cells. The identity of the parietal cells was confirmed by immunohistochemistry, which identified the presence of the parietal cell-specific H+,K+-ATPase (the gastric proton pump; ref 17; Fig. 4). Also present were gastric enterochromaffin-like cells (Fig. 3A). These were recognizable by their prominent granules and subepithelial location. Between the SSE and gastric corpus tissue there was often a narrow band of tissue, consisting of one or two tubular glands, lined with neutral-staining mucus cells and having the appearance of gastric cardiac glands (Fig. 2). Another band of tubular glands usually appeared on the side of the parietal cell-containing epithelium away from the SSE; these glands were branched and had a preponderance of neutral-staining mucus cells characteristic of gastric antrum (Figs. 3 A and B and 5). The glands also expressed TFF2, which is characteristic of the deeper glandular elements of the gastric antrum and is cosecreted with mucus and probably involved in its stabilization (ref. 18; Fig. 6). Beyond the glands and before colonic epithelium was encountered, we found small intestine which was typical, except that villous height was reduced. In hematoxylin- and eosin-stained sections, this tissue could be recognized by prominent Paneth cells with typical birefringent granules in the crypts and by goblet cells in the villous epithelium (Fig. 3 C and D). Sections that did not pass through SSE showed the same sequence of tissue between the most rostral tissue type cut and the surrounding colon.
Figure 2.
Portion of a colonic polyp from a Cdx2+/− mouse showing the sequence SSE, gastric cardia (GCa) containing columnar mucus-secreting cells with basal nuclei, and gastric corpus (GCo) containing clearly identifiable parietal (oxyntic) cells (arrows). (Bar = 45 μm.)
Figure 3.
Montage showing the lateral edge of a colonic polyp in a Cdx2+/− mouse. (Top) The sequence—gastric corpus (GCo), gastric antrum (GA), small intestine (SI), and colon (C)—is clearly seen. (Bar = 130 μm.) (A) Transition from GCo to GA. Parietal (oxyntic) cells (closed arrow heads) and enterochromaffin-like cells (arrows) are easily identifiable, as are the columnar mucus-secreting cells of the gastric antrum (open arrow heads). (Bar = 55 μm.) (B) Transition from GA to SI. Goblet cells in the villi and Paneth cells (arrow) situated in the crypts are clearly seen adjacent to the branched mucus-secreting cells of the gastric antrum. (Bar = 55 μm.) (C) Transition from SI to C. Stunted villi with goblet cells and Paneth cells (arrow) in the crypts between villi, lie adjacent to colonic tissue. (Bar = 55 μm.) (D) Portion of B magnified further to show Paneth cells with characteristic eosinophilic granules. (Bar = 20 μm.)
Figure 4.
Portion of a colonic polyp showing the junction of gastric cardia (GCa) and gastric corpus (GCo). (Upper) This section is stained with hematoxylin and eosin. (Lower) An adjacent section was incubated with antiserum to the gastric proton pump to locate oxyntic cells in the gastric corpus. (Bars = 55 μm.)
Figure 5.
This section of a colonic polyp shows characteristics of gastric antrum (GA) and normal colon (C) distal to it. The section was stained by Mowry’s technique (13) to show PAS-staining (neutral) mucopolysaccharides in the polyp (upper portion of section) and alcian-blue-staining (acid) mucopolysaccharides in the normal colon (lower portion of section). (Bar = 50 μm.)
Figure 6.
A section adjacent to Fig. 5 was stained for TFF2. The heterotopic tissue with the histological phenotype of gastric antrum (upper portion of section) stains strongly, whereas the phenotypically normal colonic-type mucosa is negative (lower portion of section). (Bar = 50 μm.)
In 20% of the heterotopias, the adjacent native colonic epithelium showed some degree of epithelial proliferation and cytological atypia, which we attribute to regeneration accompanying local inflammation and ulceration. In about a quarter of these, the cytological appearance suggested some low-grade dysplasia. In our previous publication (9), this appearance was referred to as neoplasia, but, in the light of the present results, this description is not correct; the predominant cells making up the lesions belong to heterotopic, well differentiated, organotypically normal gastrointestinal populations.
To monitor the ontogeny of the lesions, we examined the gut from six newborn (i.e., less than 1 week old) heterozygotes and from pups aged 1 week (n = 2), 2 weeks (n = 1), 3 weeks (n = 1), 4 weeks (n = 2), 5 weeks (n = 2), and 6 weeks (n = 1) in step serial sections. Distinct heterotopia was present in five of the newborn mice, taking the form of SSE in each case (Fig. 7) but without evidence of intercalated tissue between it and the surrounding histologically normal gut. In addition to SSE, surface mucous cells were apparent in one 2-week-old specimen, and in the 3-week-old specimen, intercalated small intestine could be seen. Gastric corpus tissue containing parietal cells was apparent at 4, 5, and 6 weeks of age. Heterotopic gastric tissue was negative for Cdx2 on immunostaining at all stages. The gut from four wild-type littermates was normal, and no distinct lesions were seen in one heterozygote. The appearance of intercalated surface mucous and small intestine phenotypes before the appearance of gastric corpus suggests that the process of intercalation may originate from both the heterotopic areas and the surrounding intestine, but clonal analysis would be required to verify this place of origin.
Figure 7.
Ectopic SSE in the small intestine of a 5-day-old Cdx2+/− mouse. The large endosomal vacuoles in the villous enterocytes (arrow) are typical of the small intestine before weaning. (Bar = 80 μm.)
DISCUSSION
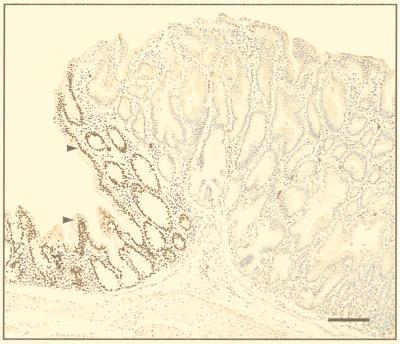
Our model for the role of Cdx2 in establishing positional information in gut development can be summarized as follows. At the beginning of gut development, all the lining epithelial cells are capable of dividing and do so frequently, resulting in normal lengthening of the gut tube. For Cdx2+/− animals, we postulate that Cdx2 protein levels are lower than optimal but, in most cells, still above the level required for normal morphogenesis to take place. The observation that histologically normal regions of the colon stain for Cdx2 supports this supposition (9). Occasionally, because of epigenetic factors or random mutations that alter the genetic background in which the Cdx2 gene acts, there results a heritable situation in which levels of Cdx2 protein in a single cell fall below the functional threshold. Such a situation is most likely to be topographically located where Cdx2 levels are normally high, namely in the proximal colon, but may occur at any point where the Cdx2 gene is active during development. For example, an epigenetic mechanism might be operative if Cdx2, like Hoxa 4, is an autoregulatory homeobox gene (19). Support for this idea comes from the finding that the gene sequence of Cdx2 contains Cdx binding sites within the first intron (20). In a rapidly dividing tissue, such as the gut, it is conceivable that insufficient translation of (lower than normal) message levels might occur between cell divisions in an individual cell. An affected cell will, in the course of further development, give rise to a clone of cells that express levels of Cdx2 that are too low to code for the formation of the proximal colon phenotype. We postulate that the function of Cdx2 during embryogenesis is to code for a factor that determines developmental fate and that clones of cells expressing deficient functional levels of the gene product will differentiate in response to a more rostral protocol specified by a low or absent level of the colon-differentiating signal. This postulation is supported by our observation that the heterotopic stomach tissue does not stain for Cdx2 (9). Subsequently, a process of epimorphic regeneration is initiated between the heterotopic clone and the surrounding colon, leading to the development of intercalated tissue expressing a gradient of positional information between forestomach and colon. Interestingly, the intercalated small intestinal mucosa at the edge of the lesions stains positively for Cdx2 (Fig. 8); its level of Cdx2 protein expression thus corresponds with that found in unaffected small intestine.
Figure 8.
Polyp-like lesion in the proximal colon of a Cdx2+/− mouse. The cells in the main part of the lesion stain negatively with Cdx2 antibody and are typical of the mucous glands of pyloric antrum. The intercalated small intestinal-type mucosa at the edge of the lesion, however, stains positive for Cdx2 (arrowhead). (Bar = 100 μm.)
Southern blotting of dissected tissues from 18 lesions in all cases failed to identify loss of heterozygosity (9). Although we cannot rule out the possibility that loss of Cdx2 expression from the remaining Cdx2 allele could be due to a mutation in the gene or its regulatory sequence, it seems much more likely that the phenotype of the gastric mucosa reflects the absence of adequate levels of Cdx2 protein caused by a mechanism such as the one described above.
Pattern, during normal development, is established initially by the mapping of major domains by diffusible signals supplying information over a morphogenetic field (as in limb development; ref. 21) and subsequently with the onset of more detailed organogenesis by short-range signals resulting from cell-to-cell contact. Either of these mechanisms might be operative in the development of the systematically ordered intercalated tissue observed in the colonic lesions. Because epimorphic regeneration involves local cell proliferation, spatial constraints result in the formation of polyp-like lesions. On occasion, these space constraints resulted in the formation of “inverted polyps” in which ectopic elements protruded beneath and elevated the overlying mucosa. Dilated cystic elements were often present in these structures as well as in the larger pedunculated lesions. Such distortions of the local stromal environment in the gut have been suggested recently as a factor in the development of epithelial neoplasia (22).
Why is there no evidence of intercalary growth when discontinuous portions of the adult alimentary canal are apposed as a result of, for example, surgical intervention in humans where the results of extensive histological examination of the junctional zones are available? We are able to answer this by reference to the highest form of life in which extensive regenerative capacity is easily observed, namely the Amphibia. The ability to regenerate limbs in Xenopus laevis declines as development proceeds. Thus, young tadpoles can completely replace an amputated limb, whereas in postmetamorphic froglets and adults, recognizable limb structures do not regenerate (23). It seems reasonable to conclude that similar mechanisms are operative in mammals, and the plasticity present at early developmental stages is lost during embryonic or fetal life.
The hypothesis that homeotic transformation in mammals may take the form of epithelial heterotopia was put forward by Slack (24), and our observations provide direct evidence that such a process does, in fact, occur. Intercalary growth has been well described in Amphibia (25) and in insect limbs (26), and its nature has been reviewed comprehensively by Lewis (27). It has been put forward as a hypothetical explanation for some of the discontinuities in axial identity resulting from changes of Hox gene expression in null mutants (28). The work presented here, however, provides direct evidence of intercalary growth in a mammalian system as well as providing insight into the mechanisms involved in normal gut development.
Acknowledgments
We thank Siobhan Lavin, Natalie Corlett, and George Elia for help with the histological preparations, Jeremy Jass and Duncan McGregor for helpful comments on the sectioned material, and Jeremy Brockes for valuable discussion. This work was supported by funding from the Anti-Cancer Council of Victoria (to F.B. and K.C.), the Wellcome Trust (to R.J.P.), and the Medical Research Council (to F.B.).
ABBREVIATIONS
- SSE
stratified squamous epithelium
- TFF2
trefoil factor family 2 peptide
References
- 1.Brooke N M, Garcia-Fernandez J, Holland P W. Nature (London) 1998;392:920–922. doi: 10.1038/31933. [DOI] [PubMed] [Google Scholar]
- 2.Mlodzik M, Gehring W. Cell. 1987;48:465–478. doi: 10.1016/0092-8674(87)90197-8. [DOI] [PubMed] [Google Scholar]
- 3.Burglin T R. In: Guidebook to the Homeobox Genes. Duboule D, editor. New York: Oxford Univ. Press; 1994. pp. 27–71. [Google Scholar]
- 4.Duprey P, Chowdhury K, Dressler G R, Balling R, Simon D, Guernet J L, Gruss P. Genes Dev. 1988;2:1647–1654. doi: 10.1101/gad.2.12a.1647. [DOI] [PubMed] [Google Scholar]
- 5.Meyer B L, Gruss P. Development. 1993;117:191–203. doi: 10.1242/dev.117.1.191. [DOI] [PubMed] [Google Scholar]
- 6.James R, Kazenwadel J. J Biol Chem. 1991;266:3246–3251. [PubMed] [Google Scholar]
- 7.Gamer L, Wright C V E. Mech Dev. 1993;43:71–81. doi: 10.1016/0925-4773(93)90024-r. [DOI] [PubMed] [Google Scholar]
- 8.Lorentz O, Duluc I, DeArcangelis A, Simon-Assmann P, Kedinger M, Freund J-N. J Cell Biol. 1997;139:1553–1556. doi: 10.1083/jcb.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chawengsaksophak K, James R, Hammond V E, Köntgen F, Beck F. Nature (London) 1997;386:84–88. doi: 10.1038/386084a0. [DOI] [PubMed] [Google Scholar]
- 10.Isaacs H V, Pownall M E, Slack J M W. EMBO J. 1998;117:3413–3427. doi: 10.1093/emboj/17.12.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramanian V, Meyer B I, Gruss P. Cell. 1995;83:641–653. doi: 10.1016/0092-8674(95)90104-3. [DOI] [PubMed] [Google Scholar]
- 12.Bateson W. Material for the Study of Variation. Cambridge, U.K.: Cambridge Univ. Press; 1894. [Google Scholar]
- 13.Mowry R W. J Histochem Cytochem. 1956;4:407. [Google Scholar]
- 14.Elia G, Chinnery R, Hanby A, Poulsom R, Wright N A. Histochem J. 1994;26:644–647. doi: 10.1007/BF00158289. [DOI] [PubMed] [Google Scholar]
- 15.Beck F, Erler R, James R. Dev Dyn. 1995;204:219–227. doi: 10.1002/aja.1002040302. [DOI] [PubMed] [Google Scholar]
- 16.James R, Kazenwadel J. J Biol Chem. 1994;269:15229–15237. [PubMed] [Google Scholar]
- 17.Daugherty D F, Lucey M R, Yamada T. In: Textbook of Gastroenterology. Yamada T, editor. Philadelphia: Lippincott; 1991. pp. 233–264. [Google Scholar]
- 18.Playford R J. J R Coll Physicians Lond. 1997;31:37–41. [PMC free article] [PubMed] [Google Scholar]
- 19.Wu K, Wolgemuth D J. J Cell Biochem. 1993;52:449–462. doi: 10.1002/jcb.240520409. [DOI] [PubMed] [Google Scholar]
- 20.Suh E, Chen L, Taylor J, Traber P. Mol Cell Biol. 1994;14:7340–7351. doi: 10.1128/mcb.14.11.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeller R, Duboule D. BioEssays. 1997;19:541–546. doi: 10.1002/bies.950190703. [DOI] [PubMed] [Google Scholar]
- 22.Kinzler K W, Vogelstein B. Science. 1998;280:1036–1037. doi: 10.1126/science.280.5366.1036. [DOI] [PubMed] [Google Scholar]
- 23.Sessions S S, Bryant S V. J Exp Zool. 1988;247:39–44. doi: 10.1002/jez.1402470106. [DOI] [PubMed] [Google Scholar]
- 24.Slack J M W. J Theor Biol. 1985;114:463–490. doi: 10.1016/s0022-5193(85)80179-x. [DOI] [PubMed] [Google Scholar]
- 25.Brockes J. Science. 1997;276:81–87. doi: 10.1126/science.276.5309.81. [DOI] [PubMed] [Google Scholar]
- 26.French V, Bryant P J, Bryant S V. Science. 1976;193:969–981. doi: 10.1126/science.948762. [DOI] [PubMed] [Google Scholar]
- 27.Lewis J. J Theor Biol. 1981;88:371–392. doi: 10.1016/0022-5193(81)90082-5. [DOI] [PubMed] [Google Scholar]
- 28.Crawford M. BioEssays. 1995;17:1065–1073. doi: 10.1002/bies.950171211. [DOI] [PubMed] [Google Scholar]