Abstract
The somatomedin hypothesis proposed that insulin-like growth factor I (IGF-I) was a hepatically derived circulating mediator of growth hormone and is a crucial factor for postnatal growth and development. To reassess this hypothesis, we have used the Cre/loxP recombination system to delete the igf1 gene exclusively in the liver. igf1 gene deletion in the liver abrogated expression of igf1 mRNA and caused a dramatic reduction in circulating IGF-I levels. However, growth as determined by body weight, body length, and femoral length did not differ from wild-type littermates. Although our model proves that hepatic IGF-I is indeed the major contributor to circulating IGF-I levels in mice it challenges the concept that circulating IGF-I is crucial for normal postnatal growth. Rather, our model provides direct evidence for the importance of the autocrine/paracrine role of IGF-I.
The insulin-like growth factors (IGFs) are members of the larger family of insulin-related peptides, which include insulin, IGF-I, and IGF-II, and are one of the most well-characterized families of growth factors (1). In the circulation the IGFs are predominantly bound to binding proteins (IGFBPs), of which six have been well characterized (2). The IGFBPs prolong the half-life of the IGFs and play a role in delivering them to the target tissues, where they are capable of modulating IGF’s biological response. At the target tissue the major receptor mediating the effects of both IGF-I and IGF-II is the type I IGF receptor, a transmembrane tyrosine kinase receptor. In contrast to the related insulin receptor, which affects both metabolic and mitogenic responses of the tissue, the IGF-I receptor primarily is responsible for mediating the mitogenic responses of the cell (3).
Both IGF-I and IGF-II play essential roles in growth and development, as demonstrated by null mutation experiments (4–6). IGF-I-null mice are growth retarded, and most die after birth. Those that survive are severely growth retarded, have developmental defects in brain, muscle, bone, and lung, and are infertile. These findings confirmed the importance of IGF-I in prenatal and postnatal growth and development. IGF-II-null mice, on the other hand, were born small [60% of the size of their wild-type (WT) littermates] yet grew normally postnatally, suggesting that IGF-II is primarily responsible for intra-uterine growth. Inactivation of the IGF-I receptor by gene targeting results in peri-natal lethality caused by respiratory failure (4). These mice are also more severely growth retarded, being only 45% of normal birth weight because of the loss of both IGF-I and IGF-II action.
There appears to be strong evidence for both endocrine and autocrine/paracrine roles for IGF-I in stimulating growth and development of most tissues. Many, if not most, cells in culture express the IGF-I receptor and respond to IGF-I at physiological concentrations (3). Because all tissues express IGF-I as well as its receptor, these findings support the concept that, in vivo, tissues also respond to this locally produced IGF-I in an autocrine/paracrine manner. Further support has been found from animal models where the response of the various organs is not always determined by the circulating IGF-I levels. D’Ercole et al. (7, 8) noted that IGF-I was detectable in multiple fetal mouse tissues, and that growth hormone (GH) treatment of hypophysectomized rats increased the peptide levels in many other nonhepatic tissues. Although these effects of GH on tissue levels of IGF-I initially were considered to be the result of changes in circulating IGF-I, Isaksson and colleagues (9) raised the possibility of local autocrine/paracrine production and function of IGF-I. When injected directly into the proximal tibial epiphysis of hypophysectomized rats, both GH and IGF-I stimulated growth, and growth plate igf1 mRNA was stimulated by GH (10).
The goal of the present study was to assess the importance of endocrine vs. autocrine/paracrine roles of IGF-I in growth and development. Because the liver is believed to be the major contributor to circulating endocrine IGF-I, we set out to delete the IGF-I gene specifically in the liver. For this purpose we used the Cre/loxP system (11, 12) whereby mice with loxP-flanked igf1 gene were mated with albumin-Cre transgenic mice expressing the Cre recombinase exclusively in the liver. This technique enabled us to produce mice with total deletion of the igf1 gene in a liver-specific manner, resulting in abrogation of liver igf1 mRNA and a significant reduction in serum IGF-I peptide levels. However growth and development of the liver-specific igf1 gene-deleted animals were normal, suggesting that autocrine/paracrine IGF-I can support normal postnatal growth and development.
MATERIALS AND METHODS
Transgene Construction.
To express the cre transgene in liver the 2.7-kb XhoI–HindIII DNA fragment of the plasmid pBS185 containing the cre-coding region together with the MT-I (A)n poly(A) tail (12) was inserted downstream to the XhoI–HincII site of pGEMAlbSVPA (13–16) vector containing the albumin enhancer-promoter sequence. The correct sequence of the albumin-cre transgene in the expression vector was verified by sequencing.
Transgenic Mouse Production.
The 8.5-kb linearized DNA containing the albumin enhancer-promoter sequence and the albumin-cre transgene was obtained by ApaI digestion of the transgene construct and purified from agarose gel. The transgenic founders were produced by pronuclear injection of the linearized DNA into FVB/N zygotes. The albumin-cre founders were backcrossed onto the FVB/N background for production of transgenic offspring. Characterization of the transgene lines was done by calculating the percentage deletion of the loxP-flanked igf1 allele in liver as well as in other tissues.
Transgenic Mice Intercrossing Strategy.
The albumin-cre transgenic mice were crossed with mice homozygotic for igf1 flanked by loxP sites (L/L) (17) to obtain heterozygous mice carrying one WT allele and one allele of the igf1 gene flanked by loxP sites (W/L). This cross yielded W/L animals that were cre positive (W/L+). The W/L+ mice were crossed with L/L animals to obtain L/L− and L/L+ or with hemizygote mice that have one allele of the igf1 gene flanked by loxP sites and one null allele (N/L) (17) to obtain N/L− and N/L+ mice.
All manipulations were approved by the Animal Care and Use Committee of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD.
Genotyping of Transgenic Mice.
The genotypes of all of the offspring were analyzed by Southern blot analysis using tail DNA (17). DNA was digested overnight by using BamHI or HindIII and hybridized with the riboprobes p5′-cre or pSP-3 (Fig. 1) (17).
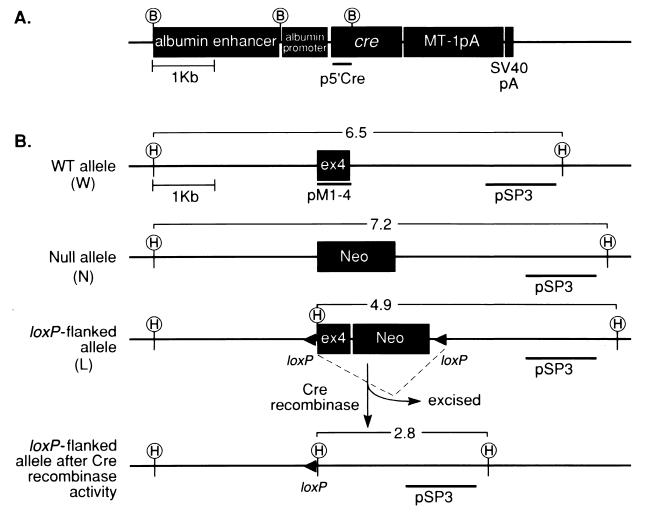
Figure 1.
Plasmid constructs. (A) Schematic representation of the albumin-cre construct for production of liver-specific Cre-expressing transgenic mice. p5′cre, the probe used to detect cre positive mice after genomic DNA digestion with BamHI (B) as described (17). MT-1pA, metallothionein poly(A) tail; SV40pA, polyadenylation signal. (B) Schematic representation of the igf1 exon 4 (ex4) locus in the WT allele (W), null (N), and the loxP-flanked allele (L). pSP3, the probe used to detect different igf1 genotypes after DNA digestion with HindIII (H) as described (17); pMI-4, the cDNA probe used to detect igf1 mRNA.
The deletion index [(intensity of the band representing the deleted allele/intensity of the band representing the loxP-flanked allele plus the intensity of the band representing the deleted allele) × 100] was calculated in different tissues by using BamHI DNA digest and pSP-3 riboprobe.
Solution Hybridization/RNase Protection Assay.
Tissues were homogenized with a polytron homogenizer (Brinkmann) in RNazol B reagent (Tel-Test, Friendswood, TX), and total RNA was isolated according to the manufacturer’s instruction. Total RNA (50 μg) was hybridized with 32P-labeled pMI4 riboprobe and 18S rRNA (Ambion, Austin, TX) overnight at 45°C. RNase protection assays were carried out as described (18), and protected bands were denatured, separated on 8% polyacrylamide gel, and exposed to X-Omat AR film overnight. The protected bands corresponding to igf1 mRNA and 18S rRNA were analyzed by the PhosphorImager 400E (Agfa).
Serum IGF-I and GH Concentration.
Serum concentration of IGF-1 was determined by using an RIA kit based on acid-ethanol extraction (rat IGF-1 DSL-2900, Diagnostic Systems Laboratories, Webster, TX) according to the manufacturer’s instructions. Serum concentration of GH in single mice samples was determined by using an RIA kit (rat growth hormone assay system, Amersham Life Science). Sera also were analyzed by HPLC by using a Protein-Pak 125 (10 μm) 7.8- × 300-mm column and a mobile phase containing 0.2 μ acetic acid/0.1 μ trimethylamine (pH 2-3).
Growth and Development.
The growth rate of mice was determined by measuring the body weight from 1 to 6 weeks after birth. Developmental parameters such as body length (nose to anus), bone length (femur acetabulum to trochanter), and tissue wet weight were measured at 6 weeks. Animals were grouped according to their genotypes as determined by Southern blot analysis.
Statistical Analysis.
Statistical analysis was performed by using t test. Values shown are mean ± SD. For growth curves, significance was determined at a P value of < 0.01 because of the size of the groups separated by gender.
RESULTS
Generation and Characterization of Albumin-cre Transgenic Mice.
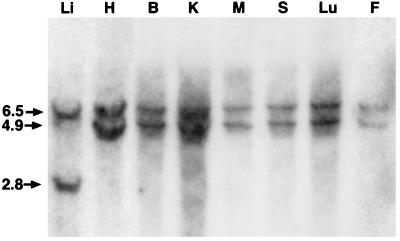
For production of the liver-specific cre transgenic animals we chose the albumin enhancer/promoter combination (Fig. 1A), which is uniquely expressed in hepatocytes (13, 14). The albumin promoter is expressed in liver at low levels in fetal development (day 19) with promoter activity increasing gradually until adult levels are reached at 1–2 weeks postnatally (19–21). We produced a total of seven independent lines of albumin-cre transgenic mice by pronuclear injection of the linearized cre DNA construct (Fig. 1A). Southern blot analysis of tail DNA from the offspring of these founders indicated that all of them transmitted up to 20 copies of the albumin-cre gene (data not shown). To evaluate the efficiency of Cre-mediated recombination each albumin-cre founder line was crossed with mice homozygous for the loxP-flanked allele of exon 4 of the igf1 gene (L/L) (Fig. 1B) (17). Genomic DNA from various organs of albumin-cre transgenic/loxP-flanked igf1 mice (W/L+) was analyzed by Southern blot to determine the percentage deletion of the loxP-flanked igf1 allele. The liver-restricted expression of Cre with a deletion index of 100 ± 7.9% (mean ± SEM) was found in one line (Fig. 2), and this albumin-cre mouse line therefore was used for subsequent experiments. The loxP-flanked igf1 gene deletion in the Cre-positive mice was detectable as early as 10 days after birth (data not shown). In an attempt to obtain more dramatic reductions in liver IGF-I production we also crossed the cre-transgenic animals with hemizygous mice that have only one copy of the loxP- flanked igf1 gene and a null allele (N/L) (Fig. 1B) (4).
Figure 2.
Liver-restricted Cre-mediated recombination. Mice carrying the albumin-cre transgene were crossed with homozygous mice carrying loxP-flanked igf1 allele (L/L). Heterozygous mice carrying one WT (W) allele of igf1 and one loxP-flanked allele that also were positive for Cre recombinase (W/L+) were analyzed. Genomic DNA was prepared from various tissues (liver, Li; heart, H; brain, B; kidney, K; muscle, M; spleen, S; lung, Lu; fat, F) digested with HindIII and subjected to Southern blot analysis. Detection with the pSP3 probe shows a 6.5-kb fragment of WT allele and a 4.9-kb fragment of loxP-flanked allele. In the presence of Cre recombinase the pSP3 probe detects a 2.8-kb fragment of the truncated allele.
Liver IGF-I Expression in Albumin-cre Transgenic igf1/loxP Mice.
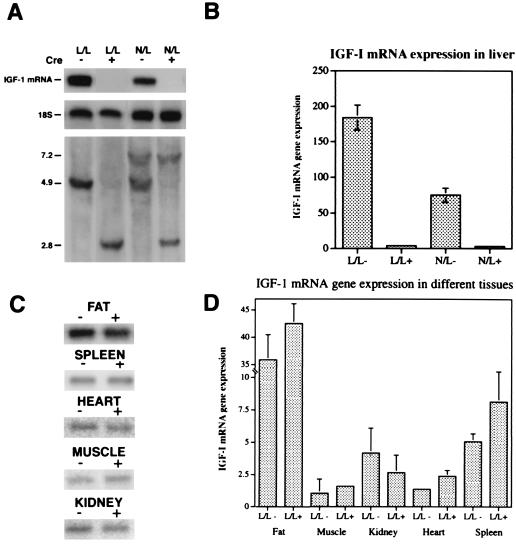
The heterozygous WT/loxP-flanked igf1 animals carrying the cre transgene (W/L+) were backcrossed with L/L mice to generate L/L− and L/L+ animals or with hemizygous mice (N/L) to generate N/L− and N/L+ mice. IGF-I expression in liver as well as in other tissues was studied in 3-, 4-, and 6-week-old mice by using RNase protection assay with the pMI-4 cDNA probe corresponding to exon 4 of this growth factor. In the presence of Cre recombinase there was abrogation of igf1 mRNA in liver of 3-, 4-, and 6-week-old mice that are hemizygous (N/L+) or homozygous (L/L+) for the loxP-flanked igf1 allele, respectively, compared with the same genotypes lacking the Cre enzyme (Fig. 3A Top; Fig. 3B shows representative data from 6-week-old mice only). Liver DNA analysis demonstrated that the appearance of the igf1-truncated allele directly correlates the reduction in hepatic igf1 mRNA expression (Fig. 3A, Bottom). However, IGF-I expression in other tissues including fat, muscle, kidney, spleen, and heart was not affected (Fig. 3 C and D). There seemed to be a modest increase in splenic and fat IGF-I gene expression, which could be reflective of a compensatory effect, but this increase was not significant.
Figure 3.
igf1 mRNA in liver is abolished in mice expressing Cre recombinase. (A) A representative RNase protection assay (18) using the pMI-4 riboprobe shows that the level of liver igf1 mRNA is abolished in 6-week-old mice positive for Cre (+) and homozygous for the loxP-flanked igf1 allele (L/L+) as well as hemizygous null, loxP-flanked igf1 mice (N/L+) (Top). This reduction correlates with the liver igf1 genotype (Bottom). (B) Quantification of liver igf1 mRNA in mice with various genotypes (L/L−, n = 12; L/L+, n = 15; N/L−, n = 10; N/L+, n = 9). (C) A representative RNase protection assay using the pMI-4 riboprobe showing that igf1 mRNA levels in fat, muscle, kidney, heart, and spleen are not affected by the absence of liver igf1 mRNA in homozygous mice for the loxP-flanked igf1 allele (L/L) in the presence or absence of Cre recombinase. (D) Quantification of fat (L/L−, n = 6; L/L+, n = 10), muscle (L/L−, n = 3; L/L+, n = 5), kidney (L/L−, n = 5; L/L+, n = 9), heart (L/L−, n = 8; L/L+, n = 13), and spleen (L/L−, n = 8; L/L+ n = 14) igf1 mRNA in homozygous mice for the loxP-flanked igf1 allele (L/L) in the presence or absence of Cre recombinase. The protected bands corresponding to igf1 mRNA were corrected to the 18S rRNA by PhosphorImmaging (relative arbitrary units). Statistical analysis of igf1 mRNA gene expression in different tissues was performed by using t test. Values shown are mean ± SD.
Serum IGF-I and GH Levels in Albumin-cre Transgenic igf1/loxP Mice.
Serum concentrations of IGF-I are low in the fetal and early postnatal periods compared with adult levels, increasing markedly during puberty (1). It is believed that the adult liver, which exhibits a several order of magnitude higher level of postnatal igf1 gene expression than any other tissue, is the source for circulating IGF-I (22–25). Sera of 6-week-old mice were examined for the presence of immunoreactive IGF-I (Table 1). Measurement of circulating IGF-I concentrations by RIA indicated that the serum IGF-I levels were markedly reduced in the liver-specific knockout (KO) mice (L/L+ 299 ± 98 ng/ml and N/L+ 295 ± 76 ng/ml) as compared with WT littermates (901 ± 71 ng/ml) and to the same genotype lacking the Cre enzyme (L/L− 660 ± 187 ng/ml and N/L− 523 ± 173 ng/ml). The sensitivity, or minimum detection limit, of the assay is 150 ng/ml. Serum IGF-I levels analyzed by HPLC showed that normal mice have 260 ng/ml of the peptide compared to 60 ng/ml in the conditional KO. This reduction in IGF-I levels is by 75%. The low, but detectable, levels of serum IGF-I seen in the liver-specific KO mice most likely are caused by the contribution of extra-hepatic tissues to circulating IGF-I. Therefore, our mouse model shows directly that the liver is the major contributor to circulating IGF-I. However, nonhepatic tissues also may contribute to the circulating IGF-I levels, which provide an integrated index of IGF-I production throughout the body. IGF-I levels at 26 days of age also were reduced (≈50%) in igf1 gene-deleted animals (data not shown), demonstrating that this effect was detectable at the early stages of peri-pubertal growth spurt. It is well established that the neuroendocrine regulation of GH secretion involves two factors: a stimulator, which is the hypothalamic GH-releasing-hormone, and an inhibitor, which is the serum IGF-I. Because there are low levels of serum IGF-I in the liver-specific IGF-I-KO mice we expected to see elevated amounts of serum GH caused by the lack of IGF-I-mediated inhibition of GH secretion. Indeed, measurement of circulating GH concentrations from single samples in individual mice by RIA indicated that the serum GH levels were markedly increased in the liver-specific KO mice (2.8 ± 0.5 ng/ml in the L/L− mice vs. 17.8 ± 11 ng/ml in the L/L+ mice P = 0.01). IGF-I gene expression in nonhepatic tissues is also GH dependent. However, previous studies have shown that to demonstrate this GH effect hypophysectomized animals with reduced IGF-I gene expression are required. Therefore, not surprisingly in the present study no increase in the IGF-I gene expression in nonhepatic tissues was seen despite the significantly elevated GH levels.
Table 1.
Serum levels of IGF-I
| Genotype | Number of animals | IGF-I levels in serum, ng/ml |
|---|---|---|
| WL− | 6 | 901 ± 36 |
| WL+ | 6 | 651 ± 56* |
| LL− | 13 | 660 ± 187 |
| LL+ | 14 | 299 ± 98* |
| WN− | 6 | 501 ± 69 |
| WN+ | 6 | 505 ± 90 |
| NL− | 9 | 523 ± 113 |
| NL+ | 7 | 295 ± 76* |
All mice were littermates generated from the cross of WL+ with NL− animals. WL− animals represent a normal mouse because they have two copies of the IGF-I gene and lack the Cre recombinase. The sensitivity, or minimum detection limit, of the assay is 150 ng/ml. W represents the wild-type, N the null, and L the loxP-flanked allele, ± indicate the presence or absence of Cre recombinase.
Significantly different (P < 0.01) from its Cre control.
Postnatal Growth of Mice Exhibiting Liver-Specific Deletion of igf1.
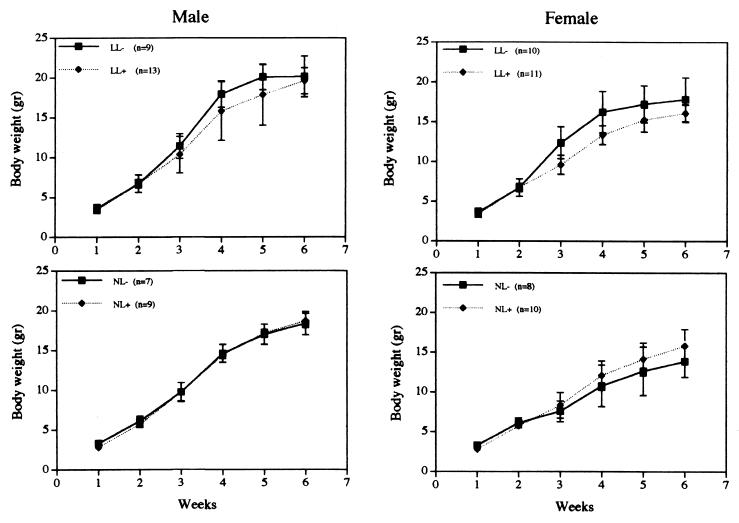
Total body weight and body length (nose to anus) are used as phenotypic criteria for growth and are considered to reflect proliferative events during organogenesis and development (26, 27). We studied the growth of the albumin-cre transgenic/loxP-flanked igf1 mice (L/L− vs. L/L+ and N/L− vs. N/L+) by measuring the following parameters: tissue wet weight, body length, and bone (femur) length as well as total body weight (Table 2). Liver, spleen, kidney, and heart were chosen as representative organs, and their wet weights were similar between groups (Table 1). Moreover, igf1 mRNA levels in kidney, fat, muscle, spleen, and heart of the liver-specific igf1 gene-deleted mice were similar to the control mice (Fig. 3 C and D). Body length measured as the distance between nose to anus was similar among the groups, regardless of Cre recombinase expression. Additionally, femur length, measured from acetabulum to trochanter, was also indistinguishable between groups. Comparison of body weight of L/L− vs. L/L+ or N/L− vs. N/L+ mice revealed no significant differences during the first 6 weeks of postnatal development regardless of Cre recombinase expression (Fig. 4). Although there was a tendency of both sexes in the L/L+ vs. L/L− mice to show a deficit in body weight at 3 and 4 weeks of age this deficit in body weight was not significant (P > 0.01). Additionally, this tendency was not observed in the N/L+ vs. N/L− animals. Moreover, the growth of L/L− vs. L/L+ and N/L− vs. N/L+ animals was similar to their WT littermates and to hemizygous mice carrying one WT allele of the igf1 gene, respectively (P > 0.01, data not shown). The liver-specific KO mice are viable for at least 3–4 months. Both sexes are fertile, give rise to litters of normal size (8–10 pups), and nurse normally for 3 weeks (before weaning). Thus in comparison to the generalized IGF-I-deficient mice, where severe retardation in growth rate and organ weights was present, the effect of liver-specific deletion of the igf1 gene had little or no effect on these parameters.
Table 2.
Growth parameters in mice with various genotypes
| Genotype | Body length cm | Femur length mm | Liver, mg | Kidney, mg | Spleen, mg | Heart, mg |
|---|---|---|---|---|---|---|
| L/L− | 7.9 ± 0.8 | 13.6 ± 0.5 | 991 ± 246 | 128 ± 31 | 67 ± 16* | 105 ± 28 |
| L/L+ | 7.9 ± 0.6 | 13.1 ± 0.6 | 1040 ± 194 | 103 ± 31 | 45 ± 15* | 95 ± 34 |
| N/L− | 7.7 ± 0.6 | 13.3 ± 0.6 | 693 ± 132 | 111 ± 32 | 46 ± 5 | 106 ± 36 |
| N/L+ | 7.8 ± 0.7 | 13.0 ± 0.5 | 981 ± 212 | 148 ± 73 | 57 ± 10 | 120 ± 1 |
L, loxP-flanked allele; N, null allele.
Significantly different (P < 0.01).
Figure 4.
Growth of mice with various genotypes was analyzed by measuring total body weight at weekly intervals. Mice were divided into two major groups according to their gene dosage in extra-hepatic tissues: mice that have two copies (Upper) and one copy (Lower) of the igf 1 gene. Statistical analysis done on a weekly basis showed no significant differences (P > 0.01) in both sexes during the first 6 weeks.
DISCUSSION
For almost two decades the dogma has been that hepatic IGF-I production is the major source of circulating or “endocrine” IGF-I and is crucial for postnatal growth and development, as determined indirectly by the large increases in plasma IGF-I that paralleled the growth spurts. Liver igf1 mRNA increases between 10- and 100-fold between early postnatal days and adulthood and is associated with the large postnatal increases in circulating IGF-I levels. On the other hand, it has been suggested that IGF-I production by extra-hepatic tissues plays a role in growth and development during fetal neonatal and pubertal stages. IGFs have specialized functions in differentiated tissues, including reproductive, cardiovascular, and neurologic tissues, which produce IGF-I as well as express the IGF-I receptor. In addition, recent studies have shown that adipose tissue expresses high levels of igf1 mRNA in a GH-dependent manner, almost as high as that expressed by liver (28, 29). Bone cells synthesize and secrete significant amounts of IGF, which is regulated by both paracrine and endocrine factors. Moreover the presence of IGF-I receptors on osteoblasts is strong evidence for an autocrine/paracrine role for skeletal IGF-I production (30, 31).
To reassess the importance of the endocrine role for IGF-I, we used a tissue-specific gene deletion strategy. The conventional igf1 gene KO results in animals with severe developmental defects, which preclude analysis of igf1 gene function during postnatal stages of development. Moreover the unrestricted igf1 and igf1 receptor gene-deletion models made it difficult to attribute the abnormal phenotypes to a loss of gene function in a particular tissue. The Cre/loxP system enabled us to delete exon 4 of the igf1 gene specifically in the liver, which resulted in abrogation of liver igf1 mRNA and a 75% reduction in circulating levels of IGF-I in the igf1 gene-deleted animals. Surprisingly, postnatal growth was similar, and at 6 weeks body weight, body length, and femoral length were indistinguishable between the groups. Organ wet weight of liver, kidney, spleen, and heart were similar between the various groups. However, there seemed to be liver enlargement in the conditional KO mice, which could be secondary to the high levels of circulating GH. Splenic wet weight decreased in the homozygous liver-specific KO mice (LL+), most likely because of the chronically low levels of IGF-I; however, this phenomenon was not observed in the hemizygous liver-specific KO mice (NL+). Kidney and heart showed a similar tendency (which was not significant) in the homozygous liver-specific KO mice (LL+), which was not observed in the hemizygous liver-specific KO mice (NL+). In addition, these mice with liver igf1 gene deletion were fertile and gave rise to litters of normal size. Thus we can conclude from this study that hepatic IGF-I production is a major source of circulating IGF-I peptide levels, but is not essential for postnatal growth and development. Other tissues such as kidney, spleen, fat, muscle, and bone, which have a high relative mass, also may contribute to circulating IGF-I levels; however, in the present experiments the source of IGF-I cannot be determined. These results have major implications on the generally accepted theory implicating liver IGF-I as the critical endocrine form of IGF-I controlling postnatal and peri-pubertal growth. It previously has been demonstrated that administration of recombinant human IGF-I (rhIGF-I) into sex-linked dwarf mutant chickens had no effect on growth (32). In addition, spontaneously diabetic BB rats administered rhIGF-I did not exhibit changes in epiphyseal width, osteoblast surfaces, or osteocalacin concentration (33). In ovariectomized rats administration of rhIGF-I only partially restored femoral calcium but had no effect on osteoblasts or osteoclasts. However, direct administration of rhIGF-I into the arterial supply of the right hind limb in ambulatory rats increased trabecular bone formation and cortical bone formation (34). These studies show that circulating IGF-I may have little or no effect on growth, whereas local production of this factor is probably responsible for the growth-promoting properties of IGF-I. Our studies strongly suggest that local production of IGF-I, which was not affected by the absence of liver igf1 mRNA (Fig. 3D), is most likely critical for postnatal growth and development and the abnormal phenotypes observed in the total KO mice are the result of a lack of the autocrine/paracrine action of IGF-I. Further studies using these and other similar models will be required to determine which tissues are most important for postnatal growth and development and which tissues are contributors to the circulating IGF-I. Furthermore, the role of circulating IGF-I during this stage of development needs to be elucidated.
Acknowledgments
We thank Dr. Argiris Efstratiadis (Columbia University, New York) for providing the IGF-I null mice, Teresa Larson for the transgene injections, Dr. Peter Rotwein (Oregon Health Sciences University, Portland, OR), for providing the pMI-4 probe, Dr. Zaret (Brown University, Providence, RI) for the albumin promoter, Dr. Oksana Gavrilova and Haya Ben-Hur (National Institutes of Health, Bethesda, MD) for technical assistance, and Dr. Fairouz Makhlof (National Institutes of Health) for statistical analysis.
ABBREVIATIONS
- IGF
insulin-like growth factor
- GH
growth hormone
- KO
knockout
- WT
wild type
Note Added in Proof
Serum levels of IGF-I measured by using an HPLC technique showed that IGF-I levels in 13 knockout mice were 60 ± 26 ng/ml compared to 260 ± 33 ng/ml in controls, reaffirming that the liver is the major source of circulating IGF-I.
References
- 1.Daughaday W H, Rotwein P. Endocr Rev. 1989;10:68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- 2.Rajaram S, Baylink D J, Mohan S. Endocr Rev. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- 3.LeRoith D, Werner H, Beitner-Johnson D, Roberts C T., Jr Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 4.Liu J P, Baker J, Perkins A S, Robertson E J, Efstratiadis A. Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 5.Baker J, Liu J P, Robertson E J, Efstratiadis A. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 6.Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart T A. Genes Dev. 1993;7:2609–2617. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- 7.D’Ercole A J, Applewhite G T, Underwood L E. Dev Biol. 1980;75:315–328. doi: 10.1016/0012-1606(80)90166-9. [DOI] [PubMed] [Google Scholar]
- 8.D’Ercole A J, Stiles A D, Underwood L E. Proc Natl Acad Sci USA. 1984;81:935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isaksson O G, Lindahl A, Nilsson A, Isgaard J. Endocr Rev. 1987;8:426–438. doi: 10.1210/edrv-8-4-426. [DOI] [PubMed] [Google Scholar]
- 10.Isaksson O G, Jansson J O, Gause I A. Science. 1982;216:1237–1239. doi: 10.1126/science.7079756. [DOI] [PubMed] [Google Scholar]
- 11.Sauer B, Henderson N. Proc Natl Acad Sci USA. 1988;85:5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauer B. Methods Enzymol. 1993;225:890–900. doi: 10.1016/0076-6879(93)25056-8. [DOI] [PubMed] [Google Scholar]
- 13.Shiota G, Rhoads D B, Wang T C, Nakamura T, Schmidt E V. Proc Natl Acad Sci USA. 1992;89:373–377. doi: 10.1073/pnas.89.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiota G, Wang T C, Nakamura T, Schmidt E V. Hepatology. 1994;19:962–972. [PubMed] [Google Scholar]
- 15.Zaret K S, DiPersio C M, Jackson D A, Montigny W J, Weinstat D L. Proc Natl Acad Sci USA. 1988;85:9076–9080. doi: 10.1073/pnas.85.23.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaret K S, Liu J K, DiPersio C M. Proc Natl Acad Sci USA. 1990;87:5469–5473. doi: 10.1073/pnas.87.14.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J L, Grinberg A, Westphal H, Sauer B, Accili D, Karas M, LeRoith D. Mol Endocrinol. 1998;12:1452–1462. doi: 10.1210/mend.12.9.0162. [DOI] [PubMed] [Google Scholar]
- 18.Werner H, Woloschak M, Adamo M, Shen-Orr Z, Roberts C T, Jr, LeRoith D. Proc Natl Acad Sci USA. 1989;86:7451–7455. doi: 10.1073/pnas.86.19.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tilghman S M, Belayew A. Proc Natl Acad Sci USA. 1982;79:5254–5257. doi: 10.1073/pnas.79.17.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami T, Yasuda Y, Mita S, Maeda S, Shimada K, Fujimoto T, Araki S. Cell Differ. 1987;22:1–9. doi: 10.1016/0045-6039(87)90408-8. [DOI] [PubMed] [Google Scholar]
- 21.Sellem C H, Frain M, Erdos T, Sala-Trepat J M. Dev Biol. 1984;102:51–60. doi: 10.1016/0012-1606(84)90174-x. [DOI] [PubMed] [Google Scholar]
- 22.Ayer-le Lievre C, Stahlbom P A, Sara V R. Development (Cambridge, UK) 1991;111:105–115. doi: 10.1242/dev.111.1.105. [DOI] [PubMed] [Google Scholar]
- 23.Beck F, Samani N J, Penschow J D, Thorley B, Tregear G W, Coghlan J P. Development (Cambridge, UK) 1987;101:175–184. doi: 10.1242/dev.101.1.175. [DOI] [PubMed] [Google Scholar]
- 24.Hoyt E C, Van Wyk J J, Lund P K. Mol Endocrinol. 1988;2:1077–1086. doi: 10.1210/mend-2-11-1077. [DOI] [PubMed] [Google Scholar]
- 25.Lund P K, Moats-Staats B M, Hynes M A, Simmons J G, Jansen M, D’Ercole A J, Van Wyk J J. J Biol Chem. 1986;261:14539–14544. [PubMed] [Google Scholar]
- 26.Kohler E, Merker H J, Ehmke W, Wojnorowicz F. Naunyn Schmiedebergs Arch Pharmacol. 1972;272:169–181. doi: 10.1007/BF00508767. [DOI] [PubMed] [Google Scholar]
- 27.Winick M, Noble A. Dev Biol. 1965;12:451–466. doi: 10.1016/0012-1606(65)90009-6. [DOI] [PubMed] [Google Scholar]
- 28.Brameld J M, Atkinson J L, Saunders J C, Pell J M, Buttery P J, Gilmour R S. J Anim Sci. 1996;74:1832–1841. doi: 10.2527/1996.7481832x. [DOI] [PubMed] [Google Scholar]
- 29.Peter M A, Winterhalter K H, Boni-Schnetzler M, Froesch E R, Zapf J. Endocrinology. 1993;133:2624–2631. doi: 10.1210/endo.133.6.7694843. [DOI] [PubMed] [Google Scholar]
- 30.Slootweg M C, Hoogerbrugge C M, de Poorter T L, Duursma S A, van Buul-Offers S C. J Endocrinol. 1990;125:271–277. doi: 10.1677/joe.0.1250271. [DOI] [PubMed] [Google Scholar]
- 31.Canalis E, McCarthy T, Centrella M. J Clin Invest. 1988;81:277–281. doi: 10.1172/JCI113318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tixier-Boichard M, Huybrechts L M, Decuypere E, Kuhn E R, Monvoisin J L, Coquerelle G, Charrier J, Simon J. J Endocrinol. 1992;133:101–110. doi: 10.1677/joe.0.1330101. [DOI] [PubMed] [Google Scholar]
- 33.Verhaeghe J, Suiker A M, Visser W J, Van Herck E, Van Bree R, Bouillon R. J Endocrinol. 1992;134:485–492. doi: 10.1677/joe.0.1340485. [DOI] [PubMed] [Google Scholar]
- 34.Spencer E M, Liu C C, Si E C, Howard G A. Bone. 1991;12:21–26. doi: 10.1016/8756-3282(91)90050-s. [DOI] [PubMed] [Google Scholar]