Abstract
Interaction between the epithelium and the mesenchyme is an essential feature of organogenesis, including hair follicle formation. The dermal papilla (DP), a dense aggregate of specialized dermis-derived stromal cells located at the bottom of the follicle, is a major component of hair that signals the follicular epithelial cells to prolong the hair growth process. However, little is known about DP-specific gene activation with regard to hair induction. In this study we demonstrate that a short fragment (839 bp) of the human versican (a core protein of one of the matrix chondroitin sulfate proteoglycans) promoter is sufficient to activate lacZ reporter gene expression in the DP of postnatal transgenic mice and also in the condensed mesenchyme (the origin of the DP) beneath the hair placode during hair follicle embryogenesis. Using the same versican promoter with green fluorescent protein (GFP), large numbers of fresh pelage DP cells were isolated from newborn transgenic skin by high-speed cell sorting. These GFP-positive DP cells showed abundant versican mRNA, confirming that the reporter molecules reflected endogenous versican gene expression. These sorted GFP-positive cells showed DP-like morphology in culture, but both GFP and versican expression was lost during primary culture. In vivo hair growth assays showed that GFP-positive cells could induce hair when grafted with epithelial cells, whereas GFP-negative cells grafted with epithelium or GFP-positive cells alone did not. These results suggest that versican may play an essential role both in mesenchymal condensation and in hair induction.
Epithelial—mesenchymal interaction during development is essential for the induction of organogenesis for many tissues (e.g., kidney, gut, respiratory organ, cutaneous appendage). Among these, the hair follicle provides an excellent model for studying epithelial—mesenchymal interactions because (i) it is located on the outermost layer of the body, allowing easy access and observation; (ii) it has distinct epithelial and mesenchymal components; and (iii) a definitive functional assay for in vivo hair induction already exists (1).
The dermal papilla (DP) is located at the bottom of the hair follicle and is the major mesenchymal component. The DP originates from condensed mesenchymal cells that lie beneath the epithelial hair germ cells (placode) in embryonic skin. These specialized mesenchymal cells are believed to be the source of the dermal-derived signaling molecule(s) involved in hair follicle development and embryogenesis and, later, in postnatal hair cycling (2). Hair follicle development during embryogenesis requires a series of reciprocal interactions between the epithelium and the underlying mesenchymal cells. Initially, the dermal mesenchyme signals the epithelium to form the epidermal placode. In response, the epithelium sends a message to the underlying mesenchyme to initiate mesenchymal condensation. The condensed mesenchyme then sends a message back to the epithelium, promoting hair elongation (3). Recent studies have identified fibroblast growth factors (FGFs), bone morphogenetic proteins (BMPs), and sonic hedgehog (Shh) as epithelial-derived signaling molecules in the early stages of hair follicle development (4). However, little is known about target gene(s) in the mesenchyme that have a direct role in the dermal condensation process.
A recent study localized the large, aggregating chondroitin sulfate proteoglycan, versican, to the DP of adult murine hair follicles. This was shown by immunohistochemistry by using antibodies directed against a core protein moiety of versican (5). Versican (also called PG-M) was first identified in the condensed mesenchyme of the chicken embryonic limb bud (6) and is proposed to be a multifunctional molecule involved in cell adhesion and intercellular signaling, although its actual biological role is still not clear (7). Cloning of the human versican gene revealed the existence of multiple isoforms (8, 9); further studies showed that these isoforms derive from a single gene (10, 11). The promoter region of versican was characterized by S1 nuclease mapping, and the basic promoter activity of this region was detected by in vitro transfection assays in HeLa cells (12).
These observations prompted us to investigate whether the versican promoter shows a DP-specific expression pattern during hair follicle development in vivo and whether the versican expression is involved in the hair-induction ability of DP cells. We have generated two different transgenic mouse lines, one using lacZ and the other using green fluorescent protein (GFP) as the reporter gene, driven by the promoter for the human versican core protein. LacZ makes it possible to localize the pattern of human versican core protein expression histologically; GFP allows us to isolate fluorescent DP cells by high-speed cell sorting for functional analysis. We have defined and isolated an 839-bp region of the human versican promoter that exhibits DP-specific expression in both embryonic and postnatal skin. We demonstrate that versican-expressing DP cells have hair-induction ability, as assessed by an in vivo hair growth assay.
MATERIALS AND METHODS
Generation of Transgenic Mice.
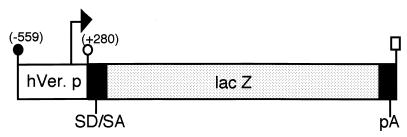
The 839-bp fragment of the functional human versican promoter (−559 to +280) (12) was obtained from human genomic DNA. This fragment contains the functional promoter region (−559 to +1) and the first untranslated exon that is not interrupted by an intron. The fragment was inserted in front of the lacZ (β-galactosidase) reporter gene in the PNASSβ vector (CLONTECH). Transgene DNA for pronuclear injection was excised as an EcoRI–XbaI, 4,732-bp fragment (Fig. 1). The linearized construct was injected into fertilized oocytes of DBA2 × C57BL6 (DBF1) mice, and the eggs were implanted into pseudopregnant foster mothers. The offspring (F0) were tested for chromosomal integration of the transgene by Southern hybridization or PCR. All transgenic analyses were performed on F1–F3 hemizygous offspring. For the GFP reporter transgenic mouse, lacZ was replaced by the EGFP (enhanced GFP) gene fragment (CLONTECH) in the same transgene cassette.
Figure 1.
Construction of versican–lacZ transgene. The number in parentheses indicates the human versican sequence position from the major transcription site as +1 (arrow). Synthetic splice donor and acceptor sites are labeled SD/SA; polyadenylation signal sequence is labeled pA. ●, EcoRI; ○, XhoI; rectangular box, XbaI.
β-Galactosidase Histochemistry.
Collected transgenic mouse embryos [from embryonic day (E)13.5, E14.5, E15.5, and E17.5] were fixed with 0.5% glutaraldehyde in PBS for 30 min to 12 hr, depending on the embryonic stage. Embryos at E15.5 and E17.5 were cut in half for easy substrate penetration into the skin. The histochemical staining procedure for β-galactosidase activity was followed as described previously by using 5-bromo-4-chloro-3-indolyl β-d-galactoside as a substrate (13). Sections were either mounted directly or counterstained with eosin after dewaxing. For postnatal skin tissue, 5-mm strips of back skin were fixed for 15 min and subjected to the same procedure. Sections then were counterstained with eosin.
In Situ Hybridization.
For in situ hybridization, transgenic embryo (E13.5) or newborn skin was fixed in freshly prepared 4% paraformaldehyde and processed through a standard paraffin-embedding protocol under RNase-free conditions. Digoxigenin-labeled in situ hybridization was performed on 8-μm paraffin sections, as described previously (14). cDNA for lacZ and mouse versican [nucleotides 243–880, corresponding to a portion of the hyaluronan-binding domain that detects all versican isoforms (8)] were amplified by reverse transcription–PCR (RT-PCR). Digoxigenin-labeled antisense and sense RNA probes were prepared by in vitro transcription with T7 RNA polymerase, using these cDNAs as templates.
Cell Sorting.
Dissected skins from newborn versican–GFP transgenic mice (1–3 days old) were floated on a 0.25% trypsin solution (GIBCO/BRL) for 16–20 hr at 4°C, after which time the epidermis was discarded. The separated dermis was minced and incubated with 0.25% collagenase for 1 hr at 37°C with gentle stirring to dissociate cells. Microscopic observation revealed that this treatment dissociated most dermal cells, preformed follicles, and follicle-associated DP cells. Debris and remaining preformed follicles that were not dissociated were removed by passing the cell suspension through a 75-μm filter followed by low-speed centrifugation to avoid clogging the cell sorter. The resultant cell suspensions were mostly single cells, which allowed sorting for GFP-positive selection. Cell sorting was performed with a Moflo high-speed cell sorter (Cytomation, Ft. Collins, CO). GFP-positive and GFP-negative cells were pooled in collection tubes with 20% FCS solution. These isolated GFP-positive cells were defined as “sorted DP-derived cells.”
Sorted GFP-Positive Cell Culture.
Sorted DP-derived cells were plated at 2 × 106 cells per 100-mm dish and cultured either in DMEM (Life Technologies, Grand Island, NY) with 10% FCS or complete Chang’s medium (Irvine Scientific). Cells were passaged every 4 days after trypsin treatment. Some cells were cultured on chamber slides (Nunc) for microscopic observation. Fluorescent and phase-contrast images were taken by a spot-cooled color digital camera (Diagnostic Instruments, Sterling Heights, MI), and merged images were created by using the Adobe photoshop software program.
RT-PCR.
Total RNAs were prepared from approximately 5 × 106 sorted or cultured DP-derived cells in Trizol solution (Life Technologies), and first-strand cDNAs were synthesized by using the Advantage RT-for-PCR Kit (CLONTECH). Semiquantitative RT-PCR amplifications were performed by using the following settings: 94°C for 30 sec, 55°C for 30 sec, and 72°C for 1 min; 25 cycles. The primers were specific to the sequences for mouse versican sequences (8), 5′-GACGACTGTCTTGGTGG-3′ and 5′-ATATCCAAACAAGCCTG-3′; GFP, 5′-TGCAGTGCTTCAGCCGCTAC-3′ and 5′-CTCGTTGGGGTCTTTGCTCA-3′; and glyceraldehyde-3-phosphate dehydrogenase, 5′-TGAAGGTCGGAGTCAACGGA-3′ and 5′-GATGGCATGGACTGTGGTCA-3′.
Induction of Hair Growth in Vivo.
The method for inducing hair growth in vivo was performed as described previously, except that 3T3 fibroblast cells were not added because they showed no significant contribution to hair inductivity in initial experiments (15). Epithelial cells (keratinocytes) were freshly prepared from either newborn CD-1 or C3H strain mouse skins by trypsin treatment and release from epidermal sheets by gentle stirring. Either sorted GFP-positive cell suspension (5 × 106), sorted negative cell suspension (5 × 106), or unsorted cell suspension (1 × 107) was combined with keratinocytes (4 × 106), resuspended in 100 μl of medium, and transferred to a grafting chamber, which was implanted onto the dorsal skin of nude mice (nu/nu). Sorted positive cells without epithelial cells also were grafted. The chamber was removed after 1 week, and hair follicle formation was assessed 3 weeks later and thereafter. For histological observation, grafting sites were dissected 3 weeks after the graft. For the grafting using cultured cells, the cultured GFP-positive cells were harvested with trypsin after first passage (4 days) and re-sorted for GFP-positive and GFP-negative selection. The same number of resorted GFP-positive and GFP-negative cultured cells (5 × 106) was grafted with keratinocytes. All animal procedures had the approval of the Massachusetts General Hospital Animal Care and Use Committee.
RESULTS
Transgenic Versican Expression Coincides with Endogenous Versican mRNA Expression in Transgenic Embryos.
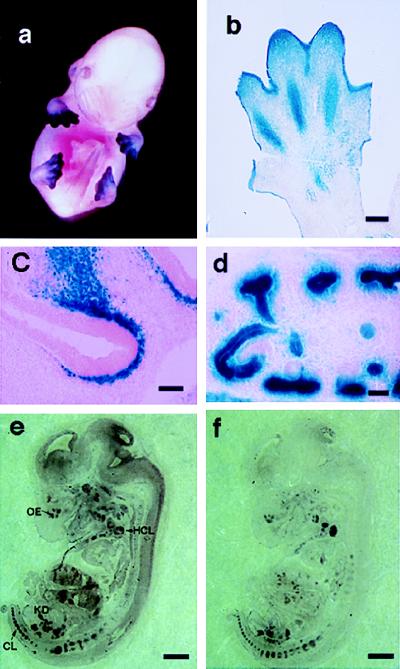
Four independent lines of versican-LacZ transgenic mice that showed easily detectable LacZ staining in their skin were obtained. Transgenic line A4681, which showed the strongest LacZ staining, was chosen for further detailed analysis, but similar expression patterns also were obtained from the other lines as well. Intense LacZ staining was observed in developing fore and hind limbs at the E13.5 embryonic stage of the A4681 line (Fig. 2a) in the region of mesenchymal condensation (Fig. 2b). This region coincides exactly with the area of prominent endogenous versican expression previously identified immunohistochemically (6). Transient expression in the ectoderm also was observed in limb regions (Fig. 2b) but was restricted to the tips of limbs. No skin epidermal expression was observed elsewhere in the body. In addition to the limb bud region, β-galactosidase histochemistry revealed LacZ-positive cells in the mesenchyme adjacent to the olfactory epithelium (Fig. 2c) and in the kidney glomeruli (Fig. 2d). The perichondrocytes surrounding cartilage, the fore- and hindbrain, facial mesenchyme, blood vessels, and muscle cells also exhibited LacZ staining (data not shown). These expression patterns are consistent with previous observations of expression that were obtained by using immunohistochemistry with versican-specific antibodies in rat (16) and human (17). The in situ hybridization study showed that the localization of both LacZ and mouse versican mRNA expression was well correlated in kidney, olfactory epithelium, and vertebral cartilage (Fig. 2 e and f), for example. Sense control probes for lacZ showed only background signals (data not shown). We conclude that the selected 839 bp of promoter sequence (−559 to +280) is sufficient to direct tissue-specific versican expression in vivo.
Figure 2.
Analysis of versican–lacZ transgenic mouse embryos. (a) Whole-mount β-galactosidase staining of E13.5 embryo. (b) Paraffin section of E13.5 limb transgenic mouse after β-galactosidase staining. (c) LacZ in the mesenchyme adjacent to the olfactory epithelium at E14.5. (d) LacZ staining in the developing kidney at E14.5. (e and f) Mouse versican and lacZ mRNA expression in transgenic mice. (e) LacZ antisense probe. (f) Mouse versican antisense probe. OE, olfactory epithelium; HCL, humerus cartilage; KD, kidney; CL, cartilage. [Bar = 100 μm (b), 50 μm (c and d), and 500 μm (e and f).]
Versican Promoter Drives Condensed Mesenchyme-Specific Expression in Embryonic Transgenic Skin.
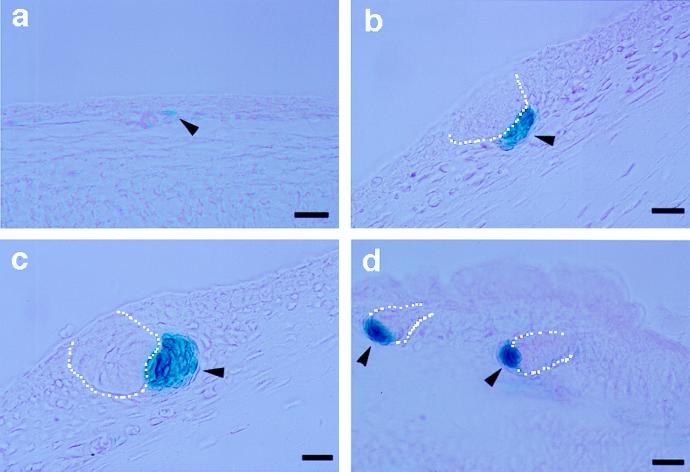
LacZ expression in developing skin was examined by β-galactosidase histochemistry on sagittal sections of E13.5, E14.5, E15.5, and E17.5 transgenic mouse embryos. At E13.5, ectodermal staining was observed only in the hind and fore limbs (see Fig. 2a). Occasional traces of LacZ staining were found in single mesenchymal cells, which may correlate with the earliest stage of condensation (Fig. 3a). Interestingly, at E14.5, condensed mesenchymal cells immediately beneath the ectodermal placodes were clearly LacZ positive, contrasting strongly with the surrounding negative mesenchymal cells (Fig. 3b). These positive sites numbered four to five per sagittal section of whole embryo. At E15.5, LacZ staining of condensed mesenchyme under hair plugs appeared more intense than at E14.5 (Fig. 3c) and showed an increased number of LacZ-positive cells. By E17.5, the number and intensity of LacZ-positive cells were increased dramatically, yet still virtually restricted to the condensed mesenchyme located at the proximal tips of down-growing hair germs (Fig. 3d). Similar to the pelage follicle, the condensed mesenchyme of whisker follicles also exhibited LacZ staining at E13.5 and later (data not shown).
Figure 3.
Versican–LacZ expression in hair follicle embryogenesis of transgenic mice. Paraffin sections of prestained embryonic skin for β-galactosidase activity. (a) Embryonic skin at E13.5. (b) Pelage hair germ at E14.5. (c) Pelage hair germ at E15.5. (d) Hair plug at E17.5. Arrowhead, condensed mesenchyme; dotted line, hair placode. [Bar = 50 μm (a and d) and 25 μm (b and c).]
Versican Promoter Is Active in the Hair Dermal Papilla of Transgenic Skin During the Anagen Phase of the Hair Cycling.
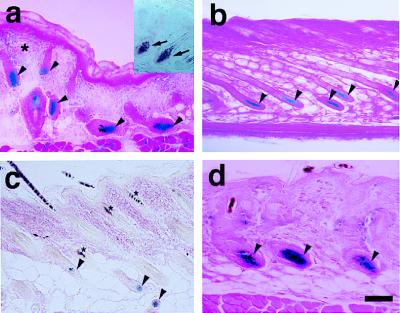
LacZ staining was examined on skin sections from newborn transgenic mice through the second hair cycle. In the newborn, strong staining was confined to the DP cells of preformed hair follicles at the anagen (growth) phase. At this stage some diffuse staining also was observed in the dermis, especially in the upper dermis and surrounding hair follicles (Fig. 4a). In situ hybridization for the endogenous mouse versican probe revealed DP-specific mRNA expression at this stage (Fig. 4a). Interestingly, at mid- to late anagen, transient epithelial LacZ staining was observed in the inner root sheath of the hair follicle (data not shown). However, in the late-anagen stage, LacZ staining again was restricted entirely to DP cells (Fig. 4b). In the catagen (transitional phase)-to-telogen (resting phase) hair follicles, no LacZ staining was observed in club (resting) hair. Presumptive second-germ DP showed trace LacZ staining (Fig. 4c). Strong DP-specific staining again was observed in the second-anagen hair cycle phase (Fig. 4d). These LacZ expression patterns in embryonic skin suggest that versican promoter activity is associated with the growth phase of hair cycling.
Figure 4.
Versican–LacZ expression in postnatal skin of transgenic mice. (a) Newborn skin. LacZ staining in DP (arrowheads); faint LacZ in the upper dermis (asterisk). Mouse versican in situ hybridization (inner box); specific versican mRNA signal in DP (arrow). (b) Late-anagen hair follicle; all DP cells are still LacZ positive but the volume of DP decreases (arrowheads). (c) Catagen-to-telogen hair follicle—club hair shows no LacZ staining and faint LacZ activity in the second-germ DP (arrowhead). Asterisks show melanin pigmentation in the hair shaft. (d) Anagen hair follicle in the second hair cycle. Strong LacZ staining reappears in the DP (arrowhead). (Bar = 100 μm.)
Generation of a Versican–GFP Transgenic Mouse and Its Use for the Isolation of Fresh Pelage Dermal Papilla Cells.
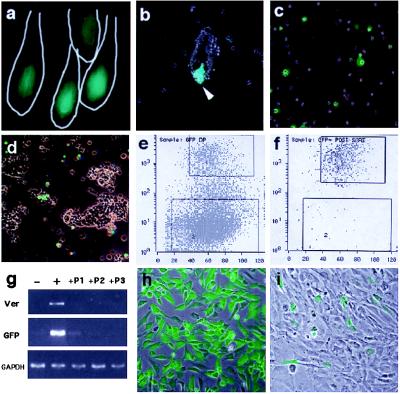
A second transgenic mouse line was generated in which the same versican promoter fragment (−559 to +280) was fused with GFP. The new transgenic line exhibited strong, DP-specific GFP fluorescence in newborn skin (Fig. 5a) in the same pattern as that seen in versican–LacZ transgenic mouse skin. Partially dissociated dermal cell suspensions revealed further that strong fluorescence originated from DP cells located at the bottom of preformed follicles (Fig. 5b). After complete dissociation of the dermis by collagenase, these follicle-associated, GFP-positive DP cells were released into the cell suspension (Fig. 5c). Most of the preformed follicles that were found in the pellet had lost their fluorescent signal (Fig. 5d), indicating that the majority of the GFP-positive DP cells were released into the suspension. Cell suspensions were sorted according to GFP-positive and GFP-negative fluorescence (which should represent cells with either an active or inactive versican promoter) by using a high-speed cell sorter. An intense GFP-fluorescent cell subpopulation (10–15% of the entire cell suspension) was observed in cells prepared from transgenic mice (Fig. 5e), whereas dermal cells from nontransgenic littermates showed no fluorescence (data not shown). Postsorting analysis showed that sorted positive cells were approximately 98% pure (Fig. 5f). RT-PCR analysis showed an abundance of both GFP and versican mRNA in sorted GFP-positive cells relative to sorted negative cells, in which both GFP and versican mRNA were not detected. This confirms that versican promoter-driven GFP expression correlated with endogenous versican expression (Fig. 5g, lanes − and +).
Figure 5.
Isolation of fresh pelage dermal papilla from versican–GFP transgenic mouse. (a) Fluorescence microscopy of frozen section from newborn versican–GFP transgenic mouse skin. Line indicates hair follicle outline. (b) Partially dissociated dermal cell suspensions. GFP-positive DP at the bottom of undissociated follicles. (c) Complete dissociated cell suspension. DP-derived cells are now seen as single cells. (d) Undissociated follicle pellet removed from cell suspension. No DP associated with the remaining preformed follicle, indicating most of the DP cells were released into the cell suspension. (e–f) Cell-sorting chart of the dermal cells from versican–GFP transgenic mouse skin (e) and postsorting analysis of sorted GFP-positive cell pools (f). Upper windows indicate cells collected as GFP-positive and lower windows indicate those collected as GFP-negative cells. (g) RT-PCR analysis of sorted cells and subsequent culture mRNA expression. −, Negative sorted fresh cells; +, positive sorted fresh cells; +P1, +P2, +P3, passaged culture from GFP-positive sorted cells. Ver, mouse versican primers. (h and i) Cultured DP from positive sorted cells. (h) Primary culture day 1. (i) Primary culture day 4. (b–d, h, and i) Merged images from fluorescence and phase-contrast microscopy.
Loss of Versican Expression with Cell Passage.
Most of the sorted GFP-positive cells survived in primary culture (Fig. 5h). These cultured GFP positives were spindle-shaped, small, aggregating, multi-layer-forming cells (Fig. 5 h and i) with a short doubling time in Chang’s medium (about 2 days) and a longer doubling time in DMEM + FCS medium (about 4 days). This morphology and behavior is consistent with that of human scalp DP cells reported previously (18). However, even after the first passage (4 days), the relative number of GFP-fluorescent cells was decreased significantly (Fig. 5i), with approximately less than 20% of the initial GFP-positive cells remaining. This loss coincided with a decrease in versican mRNA (Fig. 5g).
Versican Expression Coincided with in Vivo Dermal Condensation and Hair-Inductive Ability.
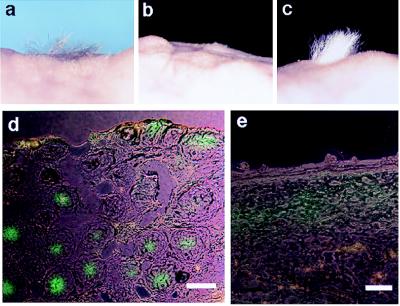
To examine whether versican expression correlates with hair-inductive ability, freshly sorted DP-derived cells mixed with keratinocytes were grafted to the back skin of nude mice. Only GFP-positive cells resulted in newly formed hair follicle; sorted negative cells did not (Table 1 and Fig. 6a and b). Full development of hair shaft elongation was maintained for 4 months after grafting (Fig. 6c). Sorted GFP-positive cells that were cultured for 4 days, at which time most cells had lost the fluorescent signal, were resorted. The resorted GFP-positive subpopulation also was able to induce hair follicle formation whereas GFP-negative cultured cells from a second sorting did not (Table 1), confirming that versican expression correlated with hair-inductive ability. Histologic sections after 3 weeks from sites at which the GFP-positive cells were grafted with keratinocytes clearly showed GFP-positive fluorescence within the condensed DP (Fig. 6d). Grafting with GFP-positive sorted cells alone in the absence of epithelial cells did not result in hair follicle formation (Table 1). Sections from these grafting sites exhibited diffuse GFP fluorescence in the upper dermis but with no sign of condensation (Fig. 6e). Thus, the epidermal component is required for dermal condensation and hair induction. GFP fluorescence was observed in condensed DP cells in the tissue weeks after grafting, implying that the loss of GFP fluorescence in culture on passage reflects the loss of versican promoter activity, rather than merely the fading of GFP protein.
Table 1.
Hair-forming potency of GFP-positive and GFP-negative cells by an in vivo cell-grafting assay
| Dermal component | Epidermal component | Success rate |
|---|---|---|
| Sorted GFP + cells | Keratinocytes | 7 /7 (4) 100% |
| Sorted GFP − cells | Keratinocytes | 0/5 (3) 0% |
| Sorted GFP + cells | None | 0/3 (2) 0% |
| Unsorted dermal cells | Keratinocytes | 3/3 (2) 100% |
| Re-sorted GFP + cultured cells | Keratinocytes | 3/3 (2) 100% |
| Re-sorted GFP − cultured cells | Keratinocytes | 0/4 (2) 0% |
Success rate is number of haired mice/number of mice grafted; numbers in parentheses are the number of experiments performed separately.
Figure 6.
Hair-induction assay on nude mouse skin. (a) Grafting after 4 weeks with sorted GFP-positive cells + C3H epithelial cells. (b) Grafting with sorted negative cells + C3H epithelial cells. (c) Grafting after 4 months with sorted GFP-positive cells + CD-1 epithelial cells. (d and e) Frozen sections of the graft site after 3 weeks. (d) Grafting of sorted positive cells with epithelial cells. GFP fluorescence was seen at the condensed DP in the deeper dermis. (e) Grafting with sorted positive cells only. Note only diffused fluorescence was observed in the upper dermis and with no sign of condensation. (Bar = 100 μm.)
DISCUSSION
In this report we demonstrate that a short fragment of the versican promoter is sufficient to drive mesenchymal condensation and DP-specific gene expression during hair follicle development. This versican gene expression correlates with the in vivo hair-inductive ability of isolated DP-derived cells. Yu et al. (19) have reported that the message of one of the serine protease inhibitors, named nexin-1, was specifically localized in adult rat dermal papilla by in situ hybridization. Whether the expression of nexin-1 is correlated with versican promoter activation in isolated DP-derived cells needs to be examined.
Involvement of glycosaminoglycans or, more specifically, chondroitin sulfate proteoglycans (CSPG) in hair follicle development and/or hair growth has been implicated because of their DP-specific localization in anagen hair follicle, as shown by immunohistochemistry by using CSPG-specific antibodies in several species including human (20–22). However, because several proteoglycans contain the same glycosaminoglycan side chains, it is important to identify the actual gene product. Although the functional mechanism of versican in hair follicle development and hair growth is still unclear, several researchers have suggested that versican functions as an inhibitor of cell—cell or cell–extracellular matrix adhesion (7, 23, 24). Because DP cells are densely packed in postnatal skin, versican could selectively prevent the incorporation of dermal fibroblasts (non-DP-destined cells) into the DP. Our results suggest that continuous expression of versican may be required for the aggregation of DP cells, although more information is needed on how the condensed dermal papilla cells can escape from the antiadhesion property of versican. Possibly, selectively blocking dermal fibroblasts from aggregating with DP cells permissively allows DP cells to aggregate with each other.
Condensed mesenchyme-specific expression of the versican promoter under the hair epidermal placode in embryonic skin is particularly interesting. We observed no LacZ-positive “free” fibroblasts in the surrounding dermis close to the already condensed mesenchymal cells. This suggests that dermal condensation results from the proliferation of a small number (possibly even one) of mesenchyme cells that first associate with the epithelial placode. Continuous versican expression in condensed mesenchymal cells may be required to exclude additional surrounding dermal fibroblasts from the condensation, maintaining the purity of the induced DP cell population. Although the versican immunoreactivity was not detected in early embryonic mouse (5), this may be due to the higher sensitivity of the histochemical detection of LacZ based on the β-galactosidase enzymatic reaction. In any case, this property of the versican promoter may be useful for targeting condensed, mesenchyme-specific gene expression in the study of epithelial–mesenchymal interactions in general as well as in the study of hair follicle development. Because the 280 bp of 5′-untranslated region after the transcriptional initiation site is included in a transgene construct that exhibits the condensing mesenchyme-specific expression, response elements for DP-specific expression may be confined to the remaining 559 bp of the promoter sequence. Cursory examination of the sequence reveals potential AP-2 (12), LEF-1 (25), and Pax (26) transcription factor-binding sequences. Actual involvement of transcriptional regulation of versican by these factors will require further analysis of the versican promoter.
Passaging DP-derived primary cell cultures rapidly turned off versican expression. Previous reports showed that cultured dermal cells, presumably including pelage DP cells, passaged from primary to four times failed to support hair growth in a cell-grafting assay whereas cultured vibrissal follicle dermal papilla did induce hair even after several passages (1). The present data showed that versican-expressing pelage DP cells have hair inductivity even after primary culture if versican-positive cells are concentrated by resorting. This could suggest that there is an insufficient number of versican-expressing pelage DP cells in ordinary dermal culture. Alternatively, the cell densities of the primary cell cultures may affect the versican expression and hair inductivity of DP cells. Recently, Inamatsu et al. (27) reported that coculturing rat vibrissa DP cells with keratinocytes or adding keratinocyte-conditioned medium maintains their hair-inductive ability over many passages. This implies that DP cells probably require a factor(s) originating from epithelial cells for versican expression and, possibly, for hair-inductive ability. This hypothesis is also supported by the fact that dermal condensation and hair follicle formation occurred only when epithelial cells were grafted with concentrated DP cells. Using these sorted DP-derived cells (with GFP as a reporter) to search for the condition or factors that stimulate versican expression could lead to detection of an essential signaling molecule(s).
Whether versican is essential for normal hair follicle development remains to be elucidated. A recent genetic mapping study of the cardiac-deficient mutant mouse hdf, which was generated by random gene insertion (28), suggests that a possible site for the gene disruption in this mouse is CSPG2 (versican) (29). Because of the early mortality of the hdf mouse (at E10.5 as a result of a heart defect), the actual requirement of versican expression for normal hair follicle development during embryogenesis may be difficult to evaluate by using the versican null mutant mouse. One feasible approach is to see whether forced expression of versican protein moiety in late-passage sorted cells, which have lost GFP fluorescence, can restore their hair-inductive ability in the grafting assay.
Acknowledgments
We express our gratitude to Dr. Janice Brissette for critical reading and comments on the manuscript. These studies were supported by the Massachusetts General Hospital/Harvard Cutaneous Biology Research Center and by Shiseido Co., Ltd., Japan.
ABBREVIATIONS
- DP
dermal papilla
- GFP
green fluorescent protein
- En
embryonic day n
References
- 1.Weinberg W C, Goodman L V, George C, Morgan D L, Ledbetter S, Yuspa S H, Lichti U. J Invest Dermatol. 1993;100:229–236. doi: 10.1111/1523-1747.ep12468971. [DOI] [PubMed] [Google Scholar]
- 2.Hardy M H. Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- 3.Messenger A G. J Invest Dermatol. 1993;101:4S–9S. doi: 10.1111/1523-1747.ep12362437. [DOI] [PubMed] [Google Scholar]
- 4.Oro A E, Scott M P. Cell. 1998;95:575–578. doi: 10.1016/s0092-8674(00)81624-4. [DOI] [PubMed] [Google Scholar]
- 5.du Cros D L, LeBaron R G, Couchman J R. J Invest Dermatol. 1995;105:426–431. doi: 10.1111/1523-1747.ep12321131. [DOI] [PubMed] [Google Scholar]
- 6.Kimata K, Oike Y, Tani K, Shinomura T, Yamagata M, Uritani M, Suzuki S. J Biol Chem. 1986;261:13517–13525. [PubMed] [Google Scholar]
- 7.Lebaron R G. Perspect Dev Neurobiol. 1996;3:261–271. [PubMed] [Google Scholar]
- 8.Ito K, Shinomura T, Zako M, Ujita M, Kimata K. J Biol Chem. 1995;270:958–965. doi: 10.1074/jbc.270.2.958. [DOI] [PubMed] [Google Scholar]
- 9.Zako M, Shinomura T, Ujita M, Ito K, Kimata K. J Biol Chem. 1995;270:3914–3918. doi: 10.1074/jbc.270.8.3914. [DOI] [PubMed] [Google Scholar]
- 10.Naso M F, Morgan J L, Buchberg A M, Siracusa L D, Iozzo R V. Genomics. 1995;29:297–300. doi: 10.1006/geno.1995.1251. [DOI] [PubMed] [Google Scholar]
- 11.Iozzo R V, Naso M F, Cannizzaro L A, Wasmuth J J, McPherson J D. Genomics. 1992;14:845–851. doi: 10.1016/s0888-7543(05)80103-x. [DOI] [PubMed] [Google Scholar]
- 12.Naso M F, Zimmermann D R, Iozzo R V. J Biol Chem. 1994;269:32999–33008. [PubMed] [Google Scholar]
- 13.Kawabe T T, Rea T J, Flenniken A M, Williams B R, Groppi V E, Buhl A E. Development (Cambridge, UK) 1991;111:877–879. doi: 10.1242/dev.111.4.877. [DOI] [PubMed] [Google Scholar]
- 14.Kishimoto J, Cox H, Keverne E B, Emson P C. Mol Brain Res. 1994;23:33–39. doi: 10.1016/0169-328x(94)90208-9. [DOI] [PubMed] [Google Scholar]
- 15.Kamimura J, Lee D, Baden H P, Brissette J, Dotto G P. J Invest Dermatol. 1997;109:534–540. doi: 10.1111/1523-1747.ep12336704. [DOI] [PubMed] [Google Scholar]
- 16.Bignami A, Perides G, Rahemtulla F. J Neurosci Res. 1993;34:97–106. doi: 10.1002/jnr.490340110. [DOI] [PubMed] [Google Scholar]
- 17.Bode-Lesniewska B, Dours-Zimmermann M T, Odermatt B F, Briner J, Heitz P U, Zimmermann D R. J Histochem Cytochem. 1996;44:303–312. doi: 10.1177/44.4.8601689. [DOI] [PubMed] [Google Scholar]
- 18.Warren R, Chestnut M H, Wong T K, Otte T E, Lammers K M, Meili M L. J Invest Dermatol. 1992;98:693–699. doi: 10.1111/1523-1747.ep12499909. [DOI] [PubMed] [Google Scholar]
- 19.Yu D W, Yang T, Sonoda T, Gaffney K, Jensen P J, Dooley T, Ledbetter S, Freedberg I M, Lavker R, Sun T T. J Cell Sci. 1995;108:3867–3874. doi: 10.1242/jcs.108.12.3867. [DOI] [PubMed] [Google Scholar]
- 20.Couchman J R, King J L, McCarthy K J. J Invest Dermatol. 1990;94:65–70. doi: 10.1111/1523-1747.ep12873363. [DOI] [PubMed] [Google Scholar]
- 21.Westgate G E, Craggs R I, Gibson W T. J Invest Dermatol. 1991;97:417–420. doi: 10.1111/1523-1747.ep12481002. [DOI] [PubMed] [Google Scholar]
- 22.Couchman J R, McCarthy K J, Woods A. Ann N Y Acad Sci. 1991;642:243–251. doi: 10.1111/j.1749-6632.1991.tb24391.x. [DOI] [PubMed] [Google Scholar]
- 23.Landolt R M, Vaughan L, Winterhalter K H, Zimmermann D R. Development (Cambridge, UK) 1995;121:2303–2312. doi: 10.1242/dev.121.8.2303. [DOI] [PubMed] [Google Scholar]
- 24.Yamagata M, Saga S, Kato M, Bernfield M, Kimata K. J Cell Sci. 1993;106:55–65. doi: 10.1242/jcs.106.1.55. [DOI] [PubMed] [Google Scholar]
- 25.Zhou P, Byrne C, Jacobs J, Fuchs E. Genes Dev. 1995;9:700–713. doi: 10.1101/gad.9.6.700. [DOI] [PubMed] [Google Scholar]
- 26.Dahl E, Koseki H, Balling R. BioEssays. 1997;19:755–765. doi: 10.1002/bies.950190905. [DOI] [PubMed] [Google Scholar]
- 27.Inamatsu M, Matsuzaki T, Iwanari H, Yoshizato K. J Invest Dermatol. 1998;111:767–775. doi: 10.1046/j.1523-1747.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- 28.Yamamura H, Zhang M, Markwald R R, Mjaatvedt C H. Dev Biol. 1997;186:58–72. doi: 10.1006/dbio.1997.8559. [DOI] [PubMed] [Google Scholar]
- 29.Mjaatvedt C H, Yamamura H, Capehart A A, Turner D, Markwald R R. Dev Biol. 1998;202:56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]