Abstract
Genetic studies in mice have previously demonstrated an intrinsic requirement for the vascular endothelial growth factor (VEGF) receptor Flk-1 in the early development of both the hematopoietic and endothelial cell lineages. In this study, embryonic stem (ES) cells homozygous for a targeted null mutation in flk-1 (flk-1 (−/−)) were examined for their hematopoietic potential in vitro during embryoid body (EB) formation or when cultured on the stromal cell line OP9. Surprisingly, in EB cultures flk-1 (−/−) ES cells were able to differentiate into all myeloid-erythroid lineages, albeit at half the frequency of heterozygous lines. In contrast, although flk-1 (−/−) ES cells formed mesodermal-like colonies on OP9 monolayers, they failed to generate hematopoietic clusters even in the presence of exogenous cytokines. However, flk-1 (−/−) OP9 cultures did contain myeloid precursors, albeit at greatly reduced percentages. This defect was rescued by first allowing flk-1 (−/−) ES cells to differentiate into EBs and then passaging these cells onto OP9 stroma. Thus, the requirement for Flk-1 in early hematopoietic development can be abrogated by alterations in the microenvironment. This finding is consistent with a role for Flk-1 in regulating the migration of early mesodermally derived precursors into a microenvironment that is permissive for hematopoiesis.
Keywords: embryonic stem cells, embryoid body, endothelial cell, OP9 cells, hemangioblast
The vertebrate hematopoietic system originates from distinct anatomical sites at different stages of embryogenesis before it ultimately migrates to its adult residence, the bone marrow. In mammals, hematopoiesis is first evident in the extraembryonic yolk sac and is restricted to primitive nucleated cells of the erythroid lineage. Later in development, the fetal liver becomes the predominant site of hematopoiesis and includes progenitor cells for both the myeloid and lymphoid cell lineages, as well as pluripotent hematopoietic stem cells capable of long-term repopulation of the entire hematopoietic system. Recent evidence suggests that fetal liver and ultimately adult bone marrow hematopoietic stem cells originate from an intra-embryonic source, the aorta-gonads-mesonephros (AGM) or its anatomical predecessor, the para-aortic-splanchnopleure (1, 2). Thus, cell migration is an intrinsic aspect of normal hematopoietic development.
Three receptor tyrosine kinases, whose genes—PDGFRα, flk-1, and c-kit—are closely linked on chromosome 4 in humans and chromosome 5 in mice, are critical for differentiation of mesoderm. Loss of function mutations in these genes lead to abnormal development of specific mesodermal lineages: somites (PDGFRα; refs. 3 and 4), hematopoietic cells (c-kit; ref. 5), and hematopoietic and endothelial cells (flk-1; ref. 6). In chimeras between flk-1 (−/−) embryonic stem (ES) cells and normal embryos, flk-1 (−/−) cells are unable to give rise to either cell lineage (7), suggesting an intrinsic and direct requirement for Flk-1 in the development of both cell lineages, perhaps from a common bipotential hemangioblast. Furthermore, flk-1 (−/−) embryonic cells fail to migrate to the posterior primitive streak (7), where hematopoiesis is thought to arise early in embryogenesis.
The Flk-1 receptor is markedly up-regulated as ES cells differentiate to form embryoid bodies (EBs) (ref. 7; W.L.S., unpublished observations) or EB-derived blast cell colonies (8, 9), and when ES cells are plated on OP9 stromal cells (10). Each culture system gives rise to Flk-1-expressing cells that differentiate into both hematopoietic and endothelial cells. Together with the phenotypic analysis of flk-1 (−/−) embryos, these observations suggest that Flk-1 plays an essential role in the early development of both hematopoietic and endothelial cells from primitive mesoderm. To address these questions further, we investigated the developmental potential of flk-1 (−/−) ES cells in vitro as they differentiate into EBs and when grown on OP9 stromal cells.
MATERIALS AND METHODS
Cell Lines.
Parental wild-type R1 ES cells (11), two homozygous flk-1 (−/−) ES cell lines (5547 and 5548), and two heterozygous flk-1 (+/−) ES cell lines (5539 and 5540; ref. 7) were maintained as previously described. The OP9 stromal cell line (a gift from T. Nakano; ref. 12) and ES cell cocultures with OP9 were cultured as previously described (13).
Differentiation of ES Cells.
ES cell lines were tested for their ability to support hematopoietic differentiation in four in vitro culture systems. In each differentiation system, two clones of each genotype were used, and experiments were performed in a minimum of triplicate cultures. First, EB differentiation was performed as previously described (14). Because of differences in plating efficiency, 1,000 wild-type and flk-1 (+/−) cells and 500 flk-1 (−/−) cells were seeded in methylcellulose media. Approximately 100–150 EBs were formed from each ES cell line. Day 7 EBs were disaggregated by collagenase and replated into fresh methylcellulose media containing erythropoietin (Epo) (2 units/ml) or Epo/steel factor (SLF) (50 ng/ml) to count erythroid colonies. Second, ES cells were allowed to differentiate into attached EBs as previously described (15). In half the cultures, 5 ng/ml recombinant mouse vascular endothelial growth factor (mVEGF) was added. Day 3 EBs were then transferred to tissue culture plates and fed every other day with fresh medium with or without mVEGF. In some experiments (Table 1), day 3 suspension EBs were plated in tissue culture plates and counted. On day 11, cultures were stained with benzidine, and the number of benzidine-positive (hemoglobinized) blood islands were counted. Third, ES cells were differentiated on OP9 stroma as previously described (13). Mesoderm colonies and hematopoietic clusters were counted on days 5 and 10, respectively. In some experiments, IL-3 (1 ng/ml), IL-6 (1 ng/ml), IL-11 (1 ng/ml), Epo (2 units/ml), SLF (10 ng/ml), Flt3 ligand (FL; 10 units/ml) were added to OP9 cultures. IL-3, IL-6, and IL-11 were purchased from PharMingen. Epo was purchased from Boehringer Mannheim. SLF and FL were gifts kindly provided by Immunex and DNAX, respectively. Fourth, “switch cultures” were performed by passaging 5 × 104 cells from trypsinized attached EB cultures at various time points onto confluent OP9 stroma and culturing for 5 days, treating with trypsin, and replating for an additional 5 days. Colony assays were performed with 105 cells from attached EBs, OP9 cultures, and switch cultures and replating in a complete methylcellulose medium (M3434, Stem Cell Technologies, Vancouver) and counting 10–14 days later. β-Galactosidase (β-gal) activity was analyzed by staining with 5-bromo-4-chloro-3-indolyl β-galactoside (X-Gal) as previously described (13).
Table 1.
Comparison of blood island formation in EBs of flk-1 (+/−) and flk-1 (−/−) ES clones
| Clone | Benzidine-positive blood islands per 10 EBs plated |
|---|---|
| 5539 (+/−) | 6.13 ± 0.98 |
| 5540 (+/−) | 5.77 ± 0.71 |
| 5547 (−/−) | 4.12 ± 0.36 |
| 5548 (−/−) | 4.48 ± 0.78 |
Cultures were fixed and stained 11 days after dispase treatment. The difference in number of blood islands between the flk-1 (+/−) and (−/−) is statistically significant (P < 0.02). The differences in number of blood islands between clones of the same genotype are not statistically significant [flk-1 (+/−), P > 0.2; flk-1 (−/−), P > 0.3].
Immunofluoresence.
Immunofluorescence was performed on attached EBs and ES–OP9 cocultures by growing cells on gelatinized coverslips in six-well plates. On day 8 after treatment with dispase (Collaborative Research), cells were stained with the fluorescent β-gal substrate fluorescein di-β-d-galactopyranoside (FDG; Molecular Probes kit I-2904). Endogenous murine β-gal activity was blocked in medium containing 300 mM chloroquine diphosphate for 30 min at 37°C, under 5% CO2/95% air. The medium was then replaced with fresh medium containing 33 mM FDG and returned to 37°C, 5% CO2/95% air, for 30 min. The cultures were washed twice in cold PBS containing 2% FBS (SM) and stained with phycoerythrin-conjugated anti-CD31 mAb (PharMingen) for 30 min at 4°C. Cultures were washed twice with SM, and the coverslips were inverted onto slides, which were analyzed by using a fluorescent microscope.
Semiquantitative Reverse Transcription–PCR.
Total RNA was isolated from each culture by acid-phenol extraction (Trizol; GIBCO/BRL), and reverse transcription–PCR was performed on 1 μg of total RNA by using the RNA PCR kit (Perkin–Elmer Cetus). Primer pairs for hprt, Brachyury, βH1-globin, βmajor-globin, epoR, c-kit, scl, flk-1, lacZ, and vegf and the conditions of the PCRs have been described previously (6, 14). Control cDNA reaction mixtures that did not contain reverse transcriptase were unable to amplify specific products.
RESULTS
Hematopoietic Differentiation in flk-1 (−/−) ES Cells.
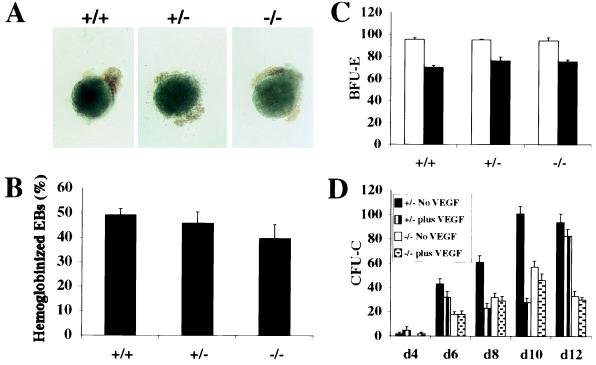
Wild-type, flk-1 (+/−), and flk-1 (−/−) ES cell clones (as in all experiments, two clones of each genotype) were examined for their ability to differentiate into hematopoietic cells during EB formation. Between 40% and 50% of the EBs that developed from ES cells of all three genotypes contained hemoglobinized cells by day 10 of methylcellulose culture (Fig. 1 A and B). To investigate the type of erythropoiesis in these EBs, wild-type, flk-1 (+/−), and flk-1 (−/−) EBs were disaggregated on day 7 and replated in methylcellulose containing Epo, or Epo and SLF. Large nucleated erythrocytes and small enucleated erythrocytes were observed in Epo and Epo/SLF cultures, respectively (data not shown). Day-7 EBs from each ES clone contained similar numbers of precursors for each type of erythroid colony (Fig. 1C). Thus, there were no significant differences in the ability of wild-type, flk-1 (+/−), and flk-1 (−/−) ES cells to differentiate into primitive and definitive erythroid progenitors during EB formation and to continue to differentiate into erythrocytes.
Figure 1.
Hematopoietic development in flk-1 mutant EBs. (A) Day-10 EBs derived from wild-type, flk-1 (+/−), and flk-1 (−/−) ES cells, showing hemoglobinized EBs for all ES cell clones. (B) Proportion of hemoglobinized EBs scored on day 10 after plating in methylcellulose. (C) The numbers of erythroid colonies in secondary methylcellulose culture of flk-1 (+/+), flk-1 (+/−), and flk-1 (−/−) EB cells. Numbers of erythroid colonies were determined by replating disaggregated day 7 EB cells in methylcellulose culture containing Epo (open column) and Epo/SLF (closed column). BFU-E, erythroid burst-forming units. (D) A time course of myeloid–erythroid colony assays of EB-derived hematopoietic progenitors. The number of cell colony-forming units (CFU-Cs; the y-axis) is calculated per 5 × 105 EB input cells. d4, etc., day 4, etc. The black and vertically striped bars represent flk-1 (+/−) cultures without or with exogenously added mVEGF, respectively. The white and speckled bars represent flk-1 (−/−) cultures without or with exogenously added mVEGF, respectively. Both genotypes generated approximately the same proportions of BFU-E and granulocyte–erythrocyte–macrophage–megakaryocyte, granulocyte–macrophage, macrophage, and granulocyte colony-forming units.
We next extended our analysis to examine the role of Flk-1 in the generation of myeloid progenitors. flk-1 (+/−) and flk-1 (−/−) ES cells were treated with dispase and cultured in suspension with or without recombinant mVEGF for 3 days, then allowed to attach to tissue culture plates and grow as attached or flat EBs. In flat cultures, the visceral endoderm layer forms beneath the mesoderm layer, including the blood islands, and thus renders the EB more accessible to observation and experimental manipulation, including the addition of exogenous factors. Culture medium was replaced every other day with and without mVEGF. Colony assays were performed with single-cell suspensions prepared at various time points and replated in methylcellulose containing cytokines. As shown in Fig. 1D, flk-1 (−/−) EBs gave rise to approximately half the numbers of CFU-C as flk-1 (+/−) EBs, although they formed the same types of colonies (data not shown). To understand further the cellular basis for this decrease in hematopoietic progenitors, the ratio of blood islands to EBs was determined. As summarized in Table 1, flk-1 (−/−) EBs generated significantly fewer blood islands than flk-1 (+/−) EBs. Thus, we conclude that the percentage of CFU-C was directly related to the number of blood islands generated in EB cultures.
Interestingly, the addition of exogenous mVEGF to flat cultures led to a reduction in the number of CFU-C colonies from flk-1 (+/−) ES cells. Beginning on day 6 of EB cultures, the decrease in total myeloid colonies ranged from 12% to 72%; however, there was no obvious skewing of colony type (data not shown). As expected, the addition of mVEGF to EB cultures did not result in any significant changes in number or type of colony-forming precursors in flk-1 (−/−) EBs (Fig. 1D). These results are consistent with a previous study which demonstrated that the addition of VEGF to Flk-1+ embryonic avian cells resulted in a decrease in hematopoietic colonies with a concomitant increase in endothelial colonies (16). In our culture conditions, mesodermal colonies were evident but we did not observe the formation of endothelial colonies.
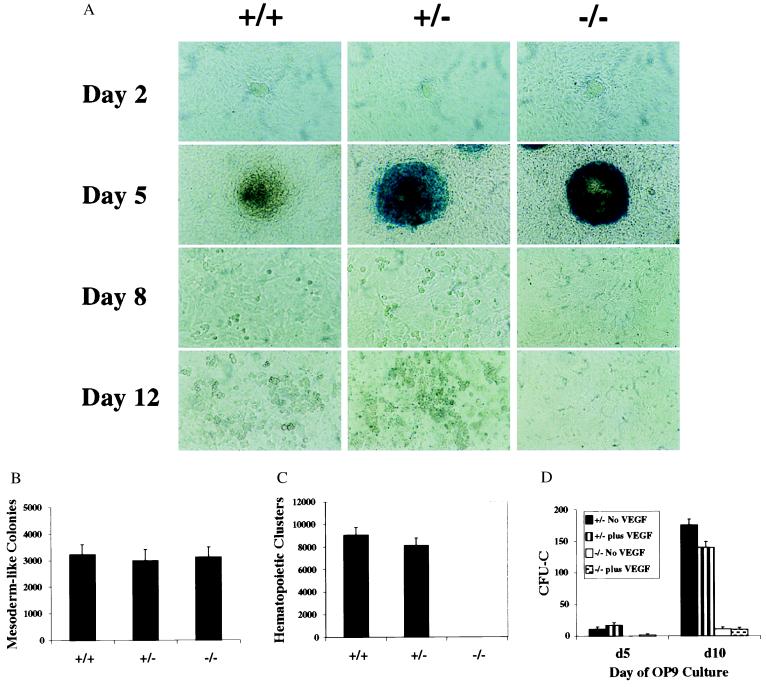
To investigate further the role of Flk-1 in hematopoietic differentiation, flk-1 (−/−) ES cells were tested in an alternative in vitro hematopoietic system involving the use of the OP9 stromal cell line. On OP9 cells, ES cells differentiate into mesodermal precursors, some of which differentiate further into hematopoietic cells (12). Mesodermal colonies were defined by cell and colony morphology and Wright–Giemsa staining and expression of mesoderm markers (Fig. 4 and data not shown). In wild-type cultures, both large nucleated and small enucleated erythrocytes were identified by Wright–Giemsa staining on day 7 and day 12 of induction, respectively (data not shown). The targeted flk-1-null allele contains a bacterial lacZ gene inserted under the transcriptional control of the endogenous flk-1 promoter (6), facilitating analysis of flk-1 expression. In flk-1 (+/−) cultures, a high level of β-gal activity, reflecting flk-1 expression, was observed in the mesodermal colonies around day 5 of coculture, consistent with Flk-1 expression previously described (10). lacZ expression diminished as hematopoietic differentiation progressed (Fig. 2A, flk-1 +/− day 8) and was absent in hematopoietic clusters. Although flk-1 (−/−) ES cells formed mesodermal colonies by day 5 in OP9 cultures as efficiently as did flk-1 (+/−) ES cells, the flk-1-deficient mesodermal cells did not differentiate further into hematopoietic clusters (Fig. 2 A–C). To determine whether hematopoietic factors could substitute for Flk-1 signaling, a mixture of various hematopoietic growth factors was added to OP9 cultures. Wild-type and flk-1 (+/−) ES clones generated approximately twice as many hematopoietic clusters in response to growth factors compared with cultures without added factors. In contrast, flk-1 (−/−) ES cells failed to give rise to hematopoietic clusters under these conditions (data not shown). Together, these data suggest that Flk-1 signaling is critical for ES cell hematopoiesis on OP9 stroma.
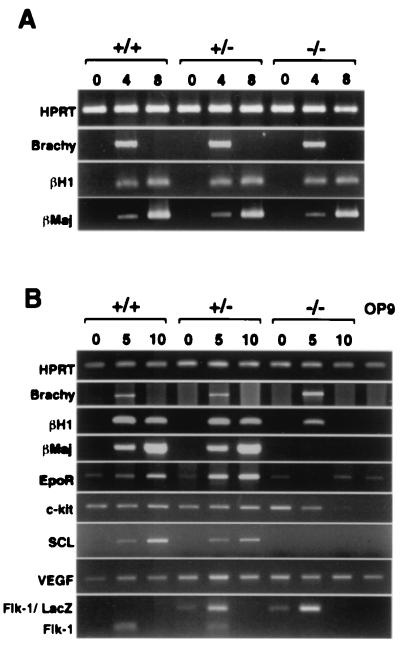
Figure 4.
Expression of various differentiation marker genes was examined by reverse transcription–PCR of EB cultures (A) and ES-OP9 cocultures (B). Number of days in culture is given at the top. Amplified hypoxanthine phosphoribosyltransferase (HPRT) is shown as a positive control. (A) No differences in gene expression between genotypes were observed in EB cultures. (B) Embryonic (βH1) and adult (βmajor) globin, and Epo receptor (EpoR) transcripts were diminished in flk-1 (−/−)-derived cells compared with flk-1 +/−-derived cells in the OP9 system. c-kit was down-regulated by day 5, whereas scl was not expressed in flk-1 (−/−) cultures. vegf was constitutively expressed in these cultures, whereas flk-1 and flk-1/lacZ expression was induced upon ES differentiation.
Figure 2.
Impaired hematopoiesis by flk-1-null cells in OP9 cocultures. (A) Photomicrographs of wild-type, flk-1 (+/−), and flk-1 (−/−) ES cells 2, 5, 8, and 12 days after hematopoietic induction by coculturing with OP9 cells. (×20.) Each culture was fixed with glutaraldehyde and stained for β-gal activity on the day as indicated. Undifferentiated colonies (day 2), differentiated mesodermal colonies (day 5), and hematopoietic clusters (day 8/day 12) are observed in wild-type and flk-1 (+/−) cultures. Strong β-gal expression was detected in mesodermal colonies in both flk-1 (+/−) and flk-1 −/− cultures. No hematopoietic clusters are observed in flk-1 (−/−) cultures. (B and C) The numbers of mesodermal colonies (B) and hematopoietic clusters (C) of wild-type, flk-1 (+/−), and flk-1 (−/−) ES cells on OP9 stromal cells. Mesodermal colonies and hematopoietic clusters were counted on days 5 and day 10, respectively. No hematopoietic clusters are scored from flk-1 (−/−) ES cells. (D) A time course of myeloid–erythroid colony assays of ES-OP9-derived hematopoietic progenitors. The number of CFU-Cs (the y-axis) is calculated per 5 × 105 day 5 or 10 OP9 input cells. The black and vertically striped bars represent flk-1 (+/−) cultures without or with exogenously added mVEGF, respectively. The white and speckled bars represent flk-1 (−/−) cultures without or with exogenously added mVEGF, respectively. No obvious differences in colony types were apparent between flk-1 (+/−) and flk-1 (−/−) cultures.
To determine whether flk-1 (−/−) OP9 cultures contained hematopoietic precursors, colony assays were performed with single-cell suspensions of day 5 and 10 OP9 cultures. As shown in Fig. 2D, flk-1-deficient ES cells gave rise to hematopoietic precursors at about 1/15th the frequency of flk-1 heterozygous ES cells. Addition of mVEGF to the ES-OP9 cocultures resulted in only a modest increase in CFU-C precursors from day 5 flk-1 (+/−) but a 20% decrease (P < 0.05) from day 10 flk-1 (+/−) cultures. Thus, although not absolutely required for mesodermal commitment to hematopoietic precursors in the ES-OP9 coculture microenvironment, Flk-1 does appear to play an important role in hematopoietic commitment.
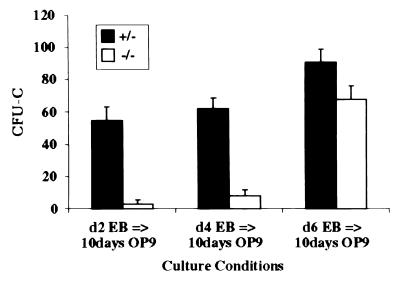
To determine whether Flk-1 signaling in ES cells plated on OP9 cells is critical for generating hematopoietic progenitors or expanding these progenitors, single-cell suspensions were prepared from EB cultures at different times and plated onto OP9 stroma. The cells were grown for 5 days and replated again on OP9 stroma for an additional 5 days, then replated in methylcellulose containing hematopoietic cytokines. The results from these “switch cultures” are shown in Fig. 3. When day 2 and 4 EB cultures were replated on OP9 stroma, the percentage difference between flk-1 (+/−) and flk-1 (−/−) CFU-Cs closely resembled that in conventional OP9 CFU-C cultures (Fig. 3), whereas when day 6 EBs were replated on OP9 stroma, the CFU-Cs more closely resembled EB-derived CFU-Cs. As shown in Fig. 1D, day 6 of EB development marks a dramatic increase in CFU-C progenitors. Thus, the kinetics of the increase in CFU-Cs generated from the switch cultures parallels the kinetics of the generation of hematopoietic progenitors in EBs (Fig. 1D). Furthermore, flk-1-null hematopoietic clusters were observed in switch cultures. Therefore, the block in OP9-induced differentiation of flk-1-null ES cells into hematopoietic cells appears to be at the transition of mesodermal cells into hematopoietic progenitors.
Figure 3.
A time course of myeloid–erythroid colony assays derived from switch cultures. ES cells differentiated as EBs for 2, 4, or 6 days, then were passaged onto OP9 stromal cells for 5 days, then replated onto OP9 cells for an additional 5 days. Colony assays were performed on the day 10 OP9 cultures. The number of CFU-Cs (the y-axis) is calculated per 5 × 105 day 10 OP9 input cells. The black and white bars represent flk-1 (+/−) and flk-1 (−/−) cultures, respectively. No obvious differences in colony types were apparent between flk-1 (+/−) and flk-1 (−/−) cultures.
Gene Expression in flk-1 (−/−) Cultures.
To investigate further the capacity of flk-1 (−/−) ES cells to differentiate into hematopoietic cells, we next compared the levels of RNA transcripts corresponding to genes associated with hematopoiesis. There were no detectable differences in the levels of RNA expression of Brachyury, embryonic (βH1) and adult (βmajor) globin in EBs derived from wild-type, flk-1 (+/−), and flk-1 (−/−) ES cells (Fig. 4A), consistent with the hematopoiesis clearly evident in these cultures. In contrast, although day 5 flk-1 (−/−) ES cells expressed the mesodermal gene Brachyury in OP9 cocultures, embryonic and adult globins and Epo receptor (EpoR) transcripts were greatly diminished in flk-1 (−/−) cells compared with flk-1 (+/−) cells (Fig. 4B). Transcripts for c-kit were down-regulated, and transcripts for the transcription factor SCL were not observed in flk-1 (−/−) cultures, whereas transcripts for both genes were up-regulated in wild-type and flk-1 (+/−) ES cells. Consistent with our functional data, the vegf gene was constitutively expressed in both OP9 and ES cells. These observations are consistent with recent studies demonstrating that SCL acts downstream of Flk-1 to specify hematopoietic mesoderm in Xenopus (17) and zebrafish (18, 19) embryos and that scl-null embryoid bodies express normal levels of flk-1 transcripts but not transcripts for globin genes (20).
Endothelial Potential of flk-1 (−/−) ES Cells.
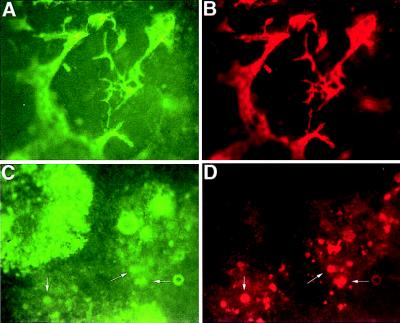
The data presented here demonstrate that Flk-1 is not required for the differentiation of hematopoietic cells within EBs, even though Flk-1 is necessary for the development of a primitive vasculature in EBs (7). Therefore, we next asked whether flk-1 (−/−) EBs might contain cells committed to the endothelial lineage but unable to organize into an endothelial network. To determine whether flk-1 (−/−) EBs contained committed endothelial precursors, attached EBs were stained with the fluorescent substrate (FDG) of β-gal and with antibodies to CD31 (PECAM-1), an adhesion and signaling molecule expressed in early endothelial precursors (21, 22). The flk-1 (+/−) EBs developed a vascular endothelium expressing both flk-1 and CD31, and they exhibited a morphology characteristic of endothelial cells (Fig. 5 A and B, respectively). In contrast, although a significant proportion of cells expressed lacZ in flk-1 (−/−) attached EBs, very few CD31-expressing cells were generated (Fig. 5 C and D). In addition, none of the CD31-positive cells exhibited the characteristic morphology of endothelial cells. These results suggest that Flk-1 is necessary for differentiation of the endothelial lineage and that, at least in EBs, hematopoietic cells can develop in the absence of mature endothelial cells, an organized vessel system, and the Flk-1 receptor.
Figure 5.
β-gal and CD31 expression in flk-1 (+/−) and flk-1 (−/−) EBs. Photomicrographs of day 8 after dispase attached EB cultures stained for β-gal activity with FDG (A and C) and CD31 expression stained with phycoerythrin-conjugated anti-CD31 (B and D). (×4.) flk-1 +/− EBs form a vascular endothelial network expressing β-gal (A) and CD31 (B). Although flk-1 −/− ES cells differentiate into β-gal-positive cells (C), few (arrows) also express CD31 (D), and none exhibit an endothelial-like morphology.
DISCUSSION
The experiments described here were designed to develop in vitro systems that would recapitulate the in vivo requirement for flk-1 in the development of both the hematopoietic and endothelial cell lineages. As observed in vivo (6), commitment to the endothelial lineage is terminated at an early stage of endothelial development in differentiation cultures of flk-1 (−/−) ES cells. Surprisingly, the hematopoietic requirement for flk-1 was conditional, depending on in vitro culture conditions. flk-1 (−/−) ES cells plated on the OP9 stromal cell line were partially blocked at the mesodermal stage of development and gave rise to a much lower percentage of hematopoietic precursors. In contrast, the same flk-1 (−/−) ES cells were unimpaired in their ability to give rise to hematopoietic cells in EBs. The finding that Flk-1 signaling is necessary for endothelial commitment but is conditionally required for hematopoietic commitment suggests that Flk-1 plays an instructive role in endothelial differentiation but a permissive role in hematopoietic differentiation. These observations are consistent with the mosaic analysis of flk-1 mutant ES cells (7) and with recent experiments in Xenopus suggesting a primary role for Flk-1 signaling in cell migration (23). Flk-1-expressing endothelial precursors migrate from the lateral plate mesoderm toward the dorsal aorta, a concentrated source of the Flk-1 ligand, VEGF. Furthermore, ectopic VEGF expression induced ectopic endothelial migration. Together, these data from both mice and frogs suggest that VEGF expression directs the migration of Flk-1+ mesodermal precursors (or hemangioblasts) to appropriate environments in the developing embryo, where other signaling pathways, such as those controlled by the Kit and Tie family receptor tyrosine kinases, become activated and regulate future hematopoietic or endothelial expansion, respectively. In addition, high concentrations of VEGF appear to direct commitment of these hemangioblasts into angioblasts. Thus, our data support a model that the hemangioblast (or at least a subset of hemangioblasts) is a bipotent cell capable of giving rise to both hematopoietic and endothelial lineages rather than an endothelial cell that is able to generate hematopoietic cells (or hemogenic endothelial cells). Finally, our results suggest that the differentiation of ES cells via EB formation or on OP9 stromal cells creates different microenvironments. Thus, complementary differentiation approaches should be used to analyze the in vitro developmental potential of mutant ES cell clones.
During the final preparation of this manuscript, Schuh et al. (24) also reported that flk-1 (−/−) ES cells can differentiate in vitro to give rise to hematopoietic cells in EBs. Interestingly, these authors observed transcripts corresponding to the endothelial genes CD31, tie-1, and tie-2 (tek). We also observed, by immunofluorescence, expression of CD31 in flk-1 (−/−) EB cultures, but we failed to observe the characteristic vascular network associated with Flk-1-expressing cells (Fig. 5) or the endothelial morphology of individual CD31+ cells (data not shown). It therefore seems likely that the expression of these genes in the flk-1 (−/−) cultures reflects either the normal expression of these genes in early hematopoietic cells (25) or the appearance of angioblasts that are unable to differentiate further in the absence of Flk-1. The development of culture conditions, described here and by Schuh et al. (24), in which cells conditionally depend on Flk-1 to initiate hematopoietic and endothelial programs of differentiation will greatly facilitate further analysis of mesodermal specification.
Acknowledgments
We thank C. Ito for critical reading of the manuscript. M.H. was funded by a grant form the Mayor of Kumamoto Foundation. W.L.S. is a Leukemia Society of America Special Fellow. This work was supported by grants from Bristol Myers Squibb, the Medical Research Council, and a Terry Fox Program Grant from the National Cancer Institute of Canada.
ABBREVIATIONS
- ES cells
embryonic stem cells
- EB
embryoid body
- Epo
erythropoietin
- SLF
steel factor
- VEGF
vascular endothelial growth factor
- mVEGF
mouse VEGF
- β-gal
β-galactosidase
- FDG
fluorescein di-β-d-galactopyranoside
- CFU-C
cell colony-forming unit
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Medvinsky A L, Samoylina N L, Muller A M, Dzierzak E A. Nature (London) 1993;364:64–67. doi: 10.1038/364064a0. [DOI] [PubMed] [Google Scholar]
- 2.Godin I E, Garcia-Porrero J A, Coutinho A, Dieterlen-Lievre F, Marcos M A R. Nature (London) 1993;364:67–70. doi: 10.1038/364067a0. [DOI] [PubMed] [Google Scholar]
- 3.Morrison-Graham K, Schatteman G C, Bork T, Bowen-Pope D F, Weston J A. Development (Cambridge, UK) 1992;115:133–142. doi: 10.1242/dev.115.1.133. [DOI] [PubMed] [Google Scholar]
- 4.Orr-Urtreger A, Bedford M T, Do M S, Eisenbach L, Lonai P. Development (Cambridge, UK) 1992;115:289–303. doi: 10.1242/dev.115.1.289. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein A, Forrester L, Reith A D, Dubreuil P, Rottapel R. Semin Hematol. 1991;28:138–142. [PubMed] [Google Scholar]
- 6.Shalaby F, Rossant J, Yamaguchi T P, Gertsenstein M, Wu X F, Breitman M L, Schuh A C. Nature (London) 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 7.Shalaby F, Ho J, Stanford W L, Fisher K-D, Schuh A C, Schwartz L, Bernstein A, Rossant J. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 8.Kabrun N, Buhring H J, Choi K, Ullrich A, Risau W, Keller G. Development (Cambridge, UK) 1997;124:2039–2048. doi: 10.1242/dev.124.10.2039. [DOI] [PubMed] [Google Scholar]
- 9.Choi K, Kennedy M, Kazarov A, Papadimitriou J C, Keller G. Development (Cambridge, UK) 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 10.Kataoka H, Takakura N, Nishikawa S, Tsuchida K, Kodama H, Kunisada T, Risau W, Kita T, Nishikawa S I. Dev Growth Differ. 1997;39:729–740. doi: 10.1046/j.1440-169x.1997.t01-5-00009.x. [DOI] [PubMed] [Google Scholar]
- 11.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J C. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano T, Kodama H, Honjo T. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 13.Stanford W L, Caruana G, Vallis K A, Inamdar M, Hidaka M, Bautch V L, Bernstein A. Blood. 1998;92:4622–4631. [PubMed] [Google Scholar]
- 14.Keller G, Kennedy M, Papayannopoulou T, Wiles M V. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bautch V L, Stanford W L, Rapoport R, Russell S, Byrum R S, Futch T A. Dev Dyn. 1996;205:1–12. doi: 10.1002/(SICI)1097-0177(199601)205:1<1::AID-AJA1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Eichmann A, Corbel C, Nataf V, Vaigot P, Breant C, Le Douarin N M. Proc Natl Acad Sci USA. 1997;94:5141–5146. doi: 10.1073/pnas.94.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mead P E, Kelley C M, Hahn P S, Piedad O, Zon L I. Development (Cambridge, UK) 1998;125:2611–2260. doi: 10.1242/dev.125.14.2611. [DOI] [PubMed] [Google Scholar]
- 18.Liao W, Bisgrove B W, Sawyer H, Hug B, Bell B, Peters K, Grunwald D J, Stainier D Y. Development (Cambridge, UK) 1997;124:381–389. doi: 10.1242/dev.124.2.381. [DOI] [PubMed] [Google Scholar]
- 19.Liao E C, Paw B H, Oates A C, Pratt S J, Postlethwait J H, Zon L I. Genes Dev. 1998;12:621–626. doi: 10.1101/gad.12.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elefanty A G, Robb L, Birner R, Begley C G. Blood. 1997;90:1435–1447. [PubMed] [Google Scholar]
- 21.Tanaka Y, Albelda S M, Horgan K J, van Seventer G A, Shimizu Y, Newman W, Hallam J, Newman P J, Buck C A, Shaw S. J Exp Med. 1992;176:245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller W A, Weigl S A, Deng X, Phillips D M. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleaver O, Krieg P A. Development (Cambridge, UK) 1998;125:3905–3914. doi: 10.1242/dev.125.19.3905. [DOI] [PubMed] [Google Scholar]
- 24.Schuh A C, Faloon P, Hu Q-L, Bhimani M, Choi K. Proc Natl Acad Sci USA. 1999;96:2159–2164. doi: 10.1073/pnas.96.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Porrero J A, Manaia A, Jimeno J, Lasky L L, Dieterlen-Lievre F, Godin I E. Dev Comp Immunol. 1998;22:303–319. doi: 10.1016/s0145-305x(98)00006-8. [DOI] [PubMed] [Google Scholar]