Abstract
Natriuretic peptides (NPs), mainly produced in heart [atrial (ANP) and B-type (BNP)], brain (CNP), and kidney (urodilatin), decrease blood pressure and increase salt excretion. These functions are mediated by natriuretic peptide receptors A and B (NPRA and NPRB) having cytoplasmic guanylyl cyclase domains that are stimulated when the receptors bind ligand. A more abundantly expressed receptor (NPRC or C-type) has a short cytoplasmic domain without guanylyl cyclase activity. NPRC is thought to act as a clearance receptor, although it may have additional functions. To test how NPRC affects the cardiovascular and renal systems, we inactivated its gene (Npr3) in mice by homologous recombination. The half life of [125I]ANP in the circulation of homozygotes lacking NPRC is two-thirds longer than in the wild type, although plasma levels of ANP and BNP in heterozygotes and homozygotes are close to the wild type. Heterozygotes and homozygotes have a progressively reduced ability to concentrate urine, exhibit mild diuresis, and tend to be blood volume depleted. Blood pressure in the homozygotes is 8 mmHg (1 mmHg = 133 Pa) below normal. These results are consistent with the sole cardiovascular/renal function of NPRC being to clear natriuretic peptides, thereby modulating local effects of the natriuretic peptide system. Unexpectedly, Npr3 −/− homozygotes have skeletal deformities associated with a considerable increase in bone turnover. The phenotype is consistent with the bone function of NPRC being to clear locally synthesized CNP and modulate its effects. We conclude that NPRC modulates the availability of the natriuretic peptides at their target organs, thereby allowing the activity of the natriuretic peptide system to be tailored to specific local needs.
Keywords: gene targeting, gene “knock out”, guanylyl cyclase activity, urine osmolality, bone metabolism
The natriuretic peptides (NPs) play important roles in cardiovascular homeostasis. Three isoforms, atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), and C-type natriuretic peptide (CNP), constitute the natriuretic peptide family (reviewed in ref. 1). ANP and BNP are mainly produced in the cardiac atria and ventricles, respectively; both are present in the circulation; and they directly influence blood pressure and body fluid homeostasis (reviewed in ref. 2). CNP is most strongly expressed in the brain but also is produced in vascular endothelial cells and in other tissues; its normal level in the circulation is very low; and it may have a paracrine/autocrine role. The biological functions of the natriuretic peptides are mediated by two receptors, natriuretic peptide receptor A (NPRA) [also known as guanylyl cyclase (GC) A] (3) and NPRB (GC-B) (4), which have cytoplasmic GC domains that are stimulated when the receptors bind ligand. NPRA responds to ANP and, to a 10-fold lesser degree, to BNP; NPRB responds primarily to CNP. NPRA is strongly expressed in the vasculature, kidneys, and adrenal glands, and its stimulation mediates vasorelaxant and natriuretic functions and decreases aldosterone synthesis. NPRB is strongly expressed in the brain, including the pituitary gland, and may have a role in neuroendocrine regulation. A third natriuretic peptide receptor (NPRC) has only a short cytoplasmic domain with no GC activity; it is generally thought to act as a clearance receptor and remove natriuretic peptides from the circulation (5), although several reports have suggested roles in addition to this clearance function (reviewed in ref. 6). NPRC interacts with all three natriuretic peptides in the order ANP > CNP > BNP (7). NPRC is the most widely and abundantly expressed natriuretic peptide receptor with a tissue distribution that includes many but not all tissues that express a guanylyl cyclase receptor; for example, kidney glomeruli (8) strongly express both NPRA and NPRC whereas Leydig cells in the testis strongly express NPRA but not NPRC (9). To gain a better understanding of the relationship between the receptors and their ligands and to test how NPRC affects the cardiovascular and renal systems, we have inactivated its gene (Npr3) in mice by homologous recombination. We find strong evidence that NPRC, in addition to being involved in the systemic clearance of circulating natriuretic peptides, plays an important role in controlling local effects of the natriuretic peptide system. Its complete absence produces unexpected skeletal abnormalities.
MATERIALS AND METHODS
Gene Targeting.
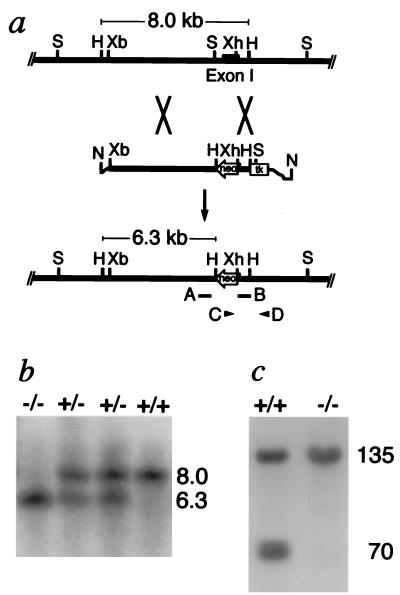
Portions of Npr3 (the mouse gene coding for NPRC) were cloned from mouse strain 129 genomic DNA fragments by using a probe based on the mouse Npr3 exon 1 sequence (D. G. Lowe, personal communication). The 5′ region of homology in the targeting construct (Fig. 1a) was a 5.9-kilobase (kb) XbaI-SpeI fragment from upstream of exon 1. The 3′ homology region was a 0.7-kb XhoI-HindIII fragment that includes parts of exon 1 and intron 1. Four electroporations of strain 129 embryonic stem cells were carried out as described (10). Candidate targeted clones were identified by PCR amplification using primer C (5′-ACGCGTCACCTTAATATGCG-3′) and primer D (5′-TCGGCTCCTTCCTCTATCTA-3′) (Fig. 1a). Targeting was confirmed by the presence of a 6.3-kb hybridizing band in addition to an 8-kb endogenous band in Southern blots of DNA digested with HindIII and hybridized to probe A (a 0.7-kb BglII-SpeI fragment) (Fig. 1b). Chimeric mice carrying the nonfunctional allele were generated and mated to C57BL/6 females to yield F1 heterozygotes, which were intercrossed to obtain Npr3 +/+, +/−, and −/− F2 animals for use in the present studies. Animals were handled under University of North Carolina-approved protocols.
Figure 1.
Targeted inactivation of the Npr3 gene. (a) The targeting strategy. (Top line) The region of the Npr3 gene that includes exon 1 (black bar). (Middle line) The targeting construct. (Bottom line) The targeted locus in which the gene is disrupted and from which 215 amino acids of the ligand-binding domain have been deleted. neo, neomycin resistance gene; tk, Herpes simplex thymidine kinase gene. Restriction sites are H, HindIII; N, NotI; S, SpeI; Xb, XbaI; Xh, XhoI. The positions of two probes, A and B, and of two primers, C and D, are indicated. (b) Southern blots of tail DNA from wild-type (+/+), heterozygous (+/−), and homozygous mutant (−/−) mice digested with HindIII and hybridized to probe A. The sizes of the hybridizing bands in kb are shown. (c) An autoradiogram showing the presence or absence of the 70-kDa band corresponding to NPRC after SDS gel electrophoresis under reducing conditions of 4-azidobenzoyl [125I]ANP photoaffinity-labeled lung plasma membranes from +/+ and −/− mice. The 135-kDa radio-labeled band corresponding to NPRA is indicated.
Photoaffinity Labeling of ANP Receptors.
Plasma membranes (200 pg) were incubated with 4-azidobenzoyl [125I]ANP as described (11). After photolysis, samples were washed twice and were subjected to SDS/PAGE. The receptor bands were localized by autoradiography.
ANP Clearance Measurements.
[125I]ANP(rat; 1–28) was rapidly injected (0.2 μCi/mouse) into the jugular vein; blood was collected from the carotid artery as published (12) 0.5, 1.5, 2.5, 4, 8, and 16 min after administration of the labeled peptide. Plasma (25 μl) separated from the blood samples (60 μl) was precipitated with 125 μl of ice-cold 10% trichloracetic acid. 125I-radioactivity in the pellets was determined by γ-scintillation. The residual counts at 16 min represent trichloroacetic acid-soluble radioactivity trapped in the pellet and were subtracted from the other values before calculations. The data were normalized to 106 cpm injected per animal. The P value of +/+ versus −/− was calculated by analysis of covariance.
Radioimmunoassay for ANP and BNP.
The ANP assay was with a published protocol (13) using a rabbit anti-ANP antiserum (Phoenix Laboratories, Belmont, CA). The BNP assay was developed for this study. Synthetic mouse BNP conjugated to bovine thyroglobulin was used to immunize sheep. The resulting anti-mBNP antiserum cross-reacts 0.01% with rat BNP and 0% with rat ANP[1–28], CNP-22, arginine vasopressin, and angiotensin II.
Blood Pressure Measurements.
Blood pressures, measured in conscious young adult male mice aged from 3 to 4 months by a noninvasive computerized tail-cuff method (14), were the means of at least six measurement sessions on each of 5 days. Animals were fed regular chow.
Biochemical Examination of Peripheral Blood and Urine.
Blood samples were drawn from the retroorbital sinus from aged-matched anesthetized (Avertin, 2.5%, 0.3 ml/25 g body wt) male mice of the three genotypes and were analyzed with an Ektachem DT60II Analyzer (Johnson & Johnson, Rochester, NY). Hematological determinations were with a Cobas Micro Hematology Analyzer (Roche Diagnostics).
For urine studies, mice were maintained on a 12-hour light/dark cycle in metabolic cages with free access to water and food. On day 3, 24-hour water intake, urine excretion, and urinary electrolytes were measured. Urinary cGMP was measured as described (15). All values were normalized to a body weight of 30 g. To test ability to dilute urine, each mouse was gavaged with a volume of water equal to 4% of its body weight. Body weight and ensuing urine osmolalities were measured at 60 and 120 min. To test ability to concentrate urine, animals were placed in cages without food or water for 12 hours, and urine osmolalities were measured.
X-Ray and Histological Examination.
Radiographs of mice were taken with soft x-rays. For histology, mice aged 10 days and 2 months were killed, were fixed in 4% paraformaldehyde in PBS, and were decalcified in 10% EDTA, and samples were embedded in paraffin. Five-micrometer sections were stained for tartrate-resistant acid phosphatase as published (16) and with hematoxylin. Some sections of bone were rehydrated and stained with 0.1% Sirus Red (Sigma-Aldrich, Milwaukee, WI) in saturated aqueous picric acid (pH 2.0) for 30 min. After a brief wash with 0.01 M HCl, sections were dehydrated, were mounted in synthetic resin, and were photographed by using polarized light (17).
In Situ Hybridization.
A 522-bp fragment from exon 1 of Npr3 was amplified by PCR with the primers 5′-GCGTAGCGTGGAGGGCAAT-3′ and 5′-CTGCCTTGGATGTAGCGCACTAT-3′ and was inserted into pBluescript II KS(+/−) plasmid (Strategene) for transcription of either a sense or antisense 35S-labeled riboprobe. Frozen vertebra bones from 10-day-old mice were cut into 10-μm sections. Hybridization was overnight at 50°C, and washes were at 65°C. Slides were dipped in emulsion, were exposed for 2 weeks, and were counterstained with hematoxylin and eosin.
Bone Marrow Cell Culture.
Bone marrow stromal cell cultures (18) were established with bone marrow from long bones of 2-month-old −/− and wild-type mice. After 8 days in culture, cells were evaluated for alkaline phosphatase (ALP) activity by using a commercial kit (Sigma-Aldrich). ALP-positive colonies were counted under low power magnification on 20 randomly chosen fields, and the numbers of cells in 100 consecutive colonies were counted. Osteoclast formation in vitro was assayed as described (19) after staining cultures for tartrate-resistant acid phosphatase. Positively staining cells with three or more nuclei (osteoclasts) were counted under high power magnification on 20 randomly chosen visual fields. The number of nuclei in 100 consecutive cells was counted.
Bone Resorption.
To assess bone resorption, urinary excretion of pyridonoline and deoxypyridonoline was determined by hydrolyzing urine samples with HCl followed by HPLC analysis as described (20). Values were normalized by the urinary creatinine concentration.
Statistics.
Except when indicated, analysis of variance and pairwise comparisons were by the Bonferroni method. In some comparisons, the data were analyzed by P-stat, a correlation/permutation test in which the null distribution is approximated by Monte Carlo sampling. Information about P-stat and a program for its execution are available from its originator, W. R. Engels (Genetics Department, University of Wisconsin, Madison, WI 53706; http://www.wisc.edu/genetics/CATG/pstat).
RESULTS AND DISCUSSION
Generation of Mutants.
We used homologous recombination in embryonic stem cells to generate animals in which the Npr3 gene was disrupted and partially deleted (Fig. 1 a and b). The resulting heterozygotes (+/−) survived and reproduced normally. The homozygous (−/−) mutants survived to birth, but about half died before weaning; about one-third of the survivors reproduced at least once. Ligand-binding analysis on lung membrane extracts demonstrated the complete absence of functional NPRC in Npr3 −/− animals but the continuing presence of NPRA (Fig. 1c). The amounts of the two guanylyl cyclase receptors (NPRA and NPRB) assessed by semiquantitative binding studies with isolated lung and kidney membrane preparations from Npr3 +/+, +/−, and −/− mice were indistinguishable in the three genotypes (data not shown).
Clearance of ANP.
The effect of absence of NPRC on clearance of natriuretic peptides from the circulation was assessed by measuring the disappearance of injected [125I]ANP. The results (Fig. 2a and Table 1) show that ANP half-life in Npr3 −/− mice is two-thirds longer than in wild-type mice (P < 0.0001), demonstrating that NPRC plays a significant role in its clearance from the circulation. This conclusion agrees with earlier studies in rats in which clearance of [125I]ANP was reduced when NPRC was blockaded with a truncated ANP (12). When ANP clearance decreases, its steady state plasma level should increase unless ANP production changes. However, in our Npr3 +/− and −/− mice, the steady state levels of ANP and BNP were not higher than in the wild type (Table 1); in fact, they tended to be lower than normal, although the difference did not reach significance. Two important inferences can be drawn from these measurements: first, that some factor(s) in the +/− and −/− mice have probably induced a homeostatic decrease in the cardiac secretion of the two peptides; and second, that any changes in the Npr3 +/− and −/− animals that we observe are not caused by increased levels of ANP or BNP in the circulation.
Figure 2.
Clearance of ANP and water handling. (a) Decline in trichloroacetic acid precipitable radioactivity after injection of [125I] rat ANP (1–28) into wild-type (n = 5, filled circles) and homozygous mutant mice (n = 4, open circles). The plotted points (log cpm) are means ± SE of logarithms of trichloroacetic acid-precipitable counts normalized as described in Materials and Methods. Where not shown, error bars are smaller than the symbols. ∗, P versus wild type < 0.0001. (b) Ability to dilute and concentrate urine assessed by loading and depriving water; wild-type (n = 7, filled circles), heterozygous (n = 6, open triangles), and homozygous mutants (n = 4, open circles). Urinary osmolalities (Uosm) at time 0, and 1, and 2 hours after 4% body weight water loading by gavage, and after 12 hours of water deprivation, are shown as means ± SE in mOsm/kgH2O. †, P versus wild type < 0.05; ‡, P versus wild type < 0.02.
Table 1.
Physiological and biochemical data
|
Npr3 genotype
|
|||
|---|---|---|---|
| +/+ | +/− | −/− | |
| ANP half-life, min | 1.44 ± 0.05 | N.D. | 2.40 ± 0.08* |
| Plasma ANP, pg/ml | 114.5 ± 5.9 | 89.5 ± 13.4 | 88.5 ± 9.0† |
| Plasma BNP, pg/ml | 21.6 ± 3.8 | 23.4 ± 4.9 | 22.1 ± 1.9† |
| BP, mmHg | 118.7 ± 1.9 | 118.0 ± 1.8 | 110.4 ± 2.3‡ |
| Hct, % | 49.9 ± 1.0 | 51.8 ± 0.9§ | 53.6 ± 0.9§ |
| HGB, g/dl | 15.6 ± 0.2 | 16.2 ± 0.2§ | 16.7 ± 0.2§ |
| RBC, 106/mm3 | 9.7 ± 0.1 | 10.1 ± 0.3§ | 10.4 ± 0.1§ |
| Water intake, ml/day | 3.0 ± 0.2 | 3.2 ± 0.4 | 3.9 ± 0.3 |
| Urine output, ml/day | 1.1 ± 0.1 | 1.4 ± 0.1¶ | 2.0 ± 0.1¶ |
| Urine cGMP, nmol/day | 3.2 ± 0.3 | 6.5 ± 0.6‡ | 10.9 ± 1.4* |
Data are means ± standard error using at least four males. N.D., not determined; BP, blood pressure; Hct, hematocrit; HGB, hemoglobin; RBC, red blood cells.
P versus +/+ <0.0001.
P versus +/+ > 0.8.
P versus +/+ <0.05.
P < 0.01 by a correlation/permutation test (P-stat, see Materials and Methods) for effect of genotype on variable.
P < 0.0001, also by P-stat.
Circulatory and Renal Effects.
Blood pressures in the homozygous mutants were significantly lower by 8 mmHg (1 mmHg = 133 Pa) than in the wild type; blood pressures in the heterozygotes were not significantly lower (Table 1). No significant effects of genotype were observed on plasma Na+, K+, Ca2+, Cl−, creatinine, and urea nitrogen concentrations or on daily urinary excretion of Na+, K+, Cl−, creatinine, or protein (data not shown). However, a substantial and progressive increase in the +/− and −/− mice relative to the wild type was seen in their total daily urine output, and their water intakes tended to increase (Table 1), suggesting alterations in renal function. Visual inspection of the Npr3 −/− mice shortly after birth suggested that they were dehydrated. Hematocrits, hemoglobin levels, and red blood cell counts of adults (Table 1) also increased progressively and highly significantly in the +/− and −/− mice relative to the wild type, indicating that a reduced amount of NPRC in the heterozygotes or its absence in the homozygotes decreases intravascular volume. As indicated above, this would be expected to induce a decrease in the cardiac secretion of ANP and BNP in the Npr3 +/− and −/− mice.
Because both NPRA and NPRC are strongly expressed in renal glomeruli (8), if NPRC is acting in the kidney as a clearance receptor, reduced amounts or absence of glomerular NPRC should expose the glomerular NPRA to progressively greater local concentrations of natriuretic peptides, thereby enhancing its guanylyl cyclase activity and increasing cGMP in the glomerular filtrate and in urine. To test this expectation, we determined the total daily excretion of cGMP in the urine of Npr3 +/+, +/−, and −/− mice. We found that a markedly increased cGMP excretion does indeed occur in the Npr3 +/− and −/− mice (Table 1), being respectively >200 and >300% normal. Because the levels of ANP and BNP in the circulation of the +/− and −/− mice were not greater than in wild-type mice, this result clearly demonstrates that decreasing NPRC substantially increases the renal effects of systemically delivered and/or locally synthesized natriuretic peptides. This in turn would be expected to alter the ability of the mice to concentrate their urine. We tested this expectation by comparing the urine osmolalities of the +/+, +/−, and −/− mice (Fig. 2b) and found that all genotypes can excrete dilute urine after water loading but that the +/− and −/− mice were progressively and significantly less able than wild-type mice to concentrate urine.
Interpretation of Renal and Hypotensive Effects.
In the kidney, ANP and BNP are derived from the circulation whereas urodilatin is derived from cells of distal tubules. NPRA is expressed strongly in the renal vasculature and glomerular podocytes and in medullary structures of the kidney. NPRC is expressed in endothelial and vascular smooth muscle cells throughout the circulation and is strongly expressed in glomerular podocytes, to a lesser degree in glomerular mesangial cells, and minimally in medullary interstitial cells (2, 21–23). In interpreting the effects of a decreased level or absence of NPRC, it is important to recollect that the levels of the natriuretic peptides in the systemic circulation and of the guanylyl cyclase receptors in the kidney are essentially normal in the Npr3 +/− and −/− mice. We therefore infer that the observed renal effects of decreasing NPRC are not caused by changes in the systemic levels of the natriuretic peptides. Rather, they are caused by local increases in the concentration of the peptides and possibly also by the effects of increased cGMP in the glomerular filtrate (24). We presume, but at present cannot prove, that the concentration of natriuretic peptides in the circulation in and downstream of the glomeruli will be less decreased than normally (i.e., increased relative to normal). As a result, the known effects of ANP on the structures exposed to glomerular and post-glomerular blood flow are likely to occur: namely, an increase in filtered volume and a decrease in water reabsorption (2, 25). In contrast, the effects of urodilatin produced in the distal tubules are unlikely to be affected because NPRC is not normally present in significant amounts in the distal nephron.
Our finding that the Npr3 +/− and −/− mice maintain their ability to dilute urine indicates that their defective ability to concentrate urine is unlikely to be caused by impairment of salt transport in the thick ascending limb. Anatomical inspection of the Npr3 +/− and −/− mice also indicates that the deficiency in urine concentration is not caused by a shortening of the renal papilla in these genotypes. Accordingly, the simplest interpretation of our findings is that they are the effect of higher than normal post-glomerular concentrations of natriuretic peptides in the blood and of cGMP in the ultrafiltrate.
We interpret the hypotensive effect of the absence of NPRC as being caused by a decreased blood volume resulting from the observed diuresis or from a locally increased concentration of the natriuretic peptides in endothelial and vascular smooth muscle cells in the systemic vasculature. [The latter would likely lead to vasorelaxation and/or to a shift of fluid from the intravascular to the interstitial compartment, with a resulting decrease in plasma volume (2).]
We conclude that NPRC in the kidney and other tissues normally decreases in a “dose-dependent” manner the local concentration of systemically delivered or locally synthesized natriuretic peptides, thereby modulating and helping to compartmentalize their physiological effects. We suggest that human genetic variations (if they exist) that modestly decrease but do not abolish NPRC formation could have beneficial cardiovascular effects under some circumstances whereas genetic variations that increase NPRC formation could be detrimental. For example, hypertensive tendencies might be ameliorated by decreases in intravascular volume that accompany decreases in NPRC or might be exaggerated by increases in NPRC expression. Likewise, a genetic decrease (or increase) in NPRC might be protective (or detrimental) in situations that lead to cardiac hypertrophy because absence of NPRA in mice causes exaggerated and pathological cardiac hypertrophy (26). A search in humans for genetic variations affecting the expression of the gene coding for NPRC therefore appears to be worthwhile.
Skeletal Effects.
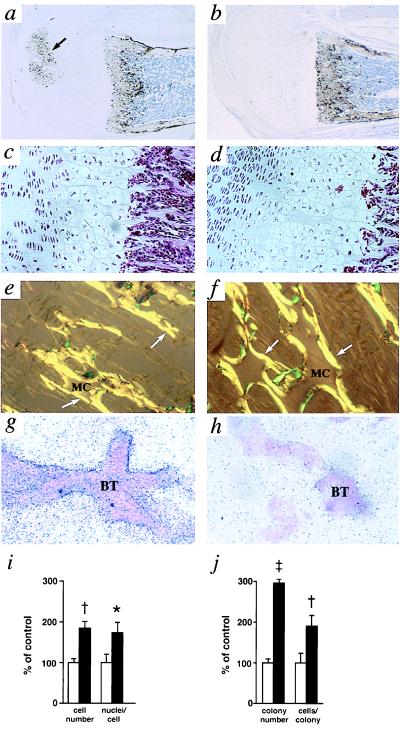
Unexpectedly, the −/− mice completely lacking NPRC exhibit striking skeletal abnormalities, recognizable 1 week after birth, including hunched backs, dome-shaped skulls, and elongated tails that, in some mice, were initially wavy (Fig. 3a). They had elongated femurs, tibias, metatarsal, and digital bones, longer vertebral bodies, increased body length, and decreased weight (Fig. 3 b–f). The thoracic cages of the −/− mice were smaller than the wild type and were constricted. Development of secondary ossification centers in long bones was delayed in the −/− mice (Fig. 4 a and b). In the cartilage growth plates of 10-day-old −/− mice, cellular expansion was apparent in the zone of hypertrophic chondrocytes although not in the zones with resting or proliferating chondrocytes (Fig. 4 c and d). This difference was no longer apparent in 3-month-old mice. The amino acid compositions of the organic bone matrix collagen in the wild-type and −/− mice were virtually indistinguishable (data not shown). The bony trabeculae in young −/− mice were thicker and longer than in the wild type (Fig. 4 e and f). In situ hybridization with an exon 1 probe showed strong expression of NPRC mRNA in the osteoblastic cells lining the bony trabeculae of developing wild-type bone (Fig. 4g). No signal was seen in the same cells of the −/− mice (Fig. 4h). The number and size of osteoclasts in cultures of bone marrow cells from 2-month-old −/− mice were nearly twice that of the wild type (Fig. 4i). The number of osteoblastic precursors from the −/− mice was ≈3× that of the wild type, and they proliferated almost twice as rapidly (Fig. 4j). These findings indicate a more active bone metabolism in the mutants.
Figure 3.
Skeletal phenotypes of Npr3 +/+ and −/− mice. (a) Wild-type (left) and homozygous mutant mice (right) at 2 months of age. (b–e) Soft x-ray analysis of phalanges [wild type (b) and homozygous mutant (c)] and lower bodies [wild type (d) and homozygous mutant (e)] of mice at 3 months of age. (f) Body and bone dimensions of homozygous mutants at 3 months of age as percent difference from the wild type. ∗, P versus wild type < 0.001.
Figure 4.
(a and b) Histochemical localization of osteoclasts by tartrate-resistant acid phosphatase staining (dark brown) in developing femurs of 10-day-old Npr3 +/+ (a) and −/− mice (b); original magnification 40×. The arrow indicates a secondary ossification center. (c and d) Cartilagenous growth plates of femurs from 10-day-old +/+ (c) and −/− (d) mice; hematoxylin and eosin stain; original magnification 200×. (e and f) Picro-Sirus Red staining of bony trabeculae in femurs from 10-day-old Npr3 +/+ (e) and −/− mice (f); original magnification 400×. The amounts of unresorbed mineralized cartilage (MC) and newly deposited bone matrix (arrows) are both increased in the mutant. (g and h) In situ hybridization for mouse NPRC mRNA in trabecular bones of 10-day-old wild-type (g) and homozygous mutant mice (h); original magnification 400×. The signal is specifically localized to the osteoblastic cells lining the bony trabeculae (BT). (i) In vitro osteoclast formation assessed by osteoclast number and by the average number of nuclei per osteoclast. Open bars, wild type; filled bars, homozygous mutant. (j) In vitro osteoblast formation assessed by number of ALP-positive colonies and the average cell number per colony in bone marrow stromal cell cultures from wild-type (open bars) and homozygous mutant mice (filled bars). ∗, P versus +/+ < 0.05; †, P versus +/+ < 0.01; ‡, P versus +/+ < 0.001.
Bone Metabolism.
We therefore compared the levels of several relevant blood and urine components in 2-month-old +/+, +/−, and −/− mice. The data (Table 2) strongly indicate that the homozygous −/− mice, but not the +/− mice, have substantially increased bone turnover. Their plasma ALP, an indicator of bone formation, is 150% of the wild-type level, and urinary pyridonoline and deoxypyridonoline, indicators of bone resorption, are all significantly increased (>200% that of the wild type).
Table 2.
Bone-related variables
|
Npr3 genotype
|
|||
|---|---|---|---|
| +/+ | +/− | −/− | |
| Plasma ALP* | 70 ± 3 | 67 ± 7 | 106 ± 10§ |
| Urine Pyr/Cr† | 430 ± 120 | 620 ± 80 | 1040 ± 200§ |
| Urine deoxy-Pyr/Cr‡ | 510 ± 90 | 570 ± 70 | 1090 ± 80¶ |
Data are means ± standard error for five males.
Plasma alkaline phosphatase (units/liter).
Urine pyridonoline (mmol)/urine creatinine (mmol).
Urine deoxypyridonoline (mmol)/urine creatinine (mmol).
P versus +/+ < 0.01.
P versus +/+ < 0.001.
Interpretation of Bone Effects.
Various components of the natriuretic peptide system are known to be present in cells or tissues involved in bone formation. For example, CNP and NPRB (the guanylyl cyclase receptor essentially specific for CNP) and the corresponding mRNAs have been demonstrated in cultured fetal mouse tibia (27), and mRNA for NPRC is readily detected in osteoblasts (ref. 28 and our present data). A simple hypothesis consistent with previous and our present observations is that NPRC in growing bone modulates the autocrine/paracrine effects of locally produced natriuretic peptides (mainly CNP). In favor of this hypothesis are observations that CNP, much more than ANP, increases cGMP production in the fetal mouse tibia organ cultures and causes an increase in longitudinal bone length, that this bone growth stimulation is mimicked by 8-bromo-cGMP, and that it is inhibited by HS-142-1, a nonpeptide GC-coupled natriuretic peptide receptor antagonist (27). The growth stimulation in the organ cultures was accompanied by an increase in the height of the proliferative and hypertrophic chondrocyte zones in the growth plate that also was seen in our Npr3 −/− mice (Fig. 4d). Additionally 1,25 dihydroxyvitamin D3, a key regulator of mineral metabolism, stabilizes NPRC mRNA (28), indicating a role for NPRC in this regulation.
Other Mutant Animals.
Spontaneous and N-ethyl-N-nitrosourea-induced mutations in mice causing skeletal abnormalities identical to those described here have recently been identified in the Npr3 gene (J. Jaubert, personal communication). Similar abnormalities also have been seen in transgenic mice grossly over-expressing BNP (29), which could be due partly to cross-stimulation of NPRB by the high levels (200 times normal) of circulating BNP and partly to chronic blockade of NPRC by this BNP.
Conclusions.
We do not expect the effects of changes in NPRC levels to be restricted to those that we have described here in the kidney and bone. Effects can be expected in any tissues or organs in which at least one of the natriuretic peptides, an active receptor, and the clearance receptor are all present in significant amounts. The interplay of these elements is complex and is likely affected by the precise anatomical location of the cells synthesizing each component of the system, which may differ in different organs and will need to be more carefully delineated. In several different situations, expression of NPRC is controlled differently from the expression of the two biologically active natriuretic peptide receptors and of the natriuretic peptides themselves. For example, chronic high salt intake leads to a decrease in expression of NPRC in kidney glomeruli and papilla without affecting the expression of the biologically active receptors (30). In the heart, progressive hypertrophy induced by an aortovenocaval fistula in the rat is accompanied by a striking gradual disappearance of NPRC transcripts whereas expression of ANP, BNP, and NPRA increases (31). In bone, 1,25 dihydroxyvitamin D3 affects the expression of CNP and NPRC but not that of NPRB (28). We conclude from these and our present results that NPRC provides a biologically effective variable allowing the physiological effects of the natriuretic peptide system to be tailored to local needs because the several elements of the system (ligands, active receptors, and clearance receptor) can be changed independently. The potential flexibility of the natriuretic peptide system and its possible compartmentalization are thereby substantially enhanced.
Acknowledgments
We thank C. F. Best, T. M. Coffman, M. F. Goy, S. Hiller, H.-S. Kim, J. Knowles, T. Maack, N. Maeda, F. Matsukawa, Y. Miyamoto, S. Pornprasertsuk, H. Sagawa, K. Sakaguchi, Y. Tse, K. Uzawa, H. Ueno, J. Vanhorne, and J. Vorobiov for help and discussions. cGMP determinations were by the University of North Carolina Center for Gastrointestinal Biology and Disease (National Institutes of Health Center Grant DK34987). Our work was supported by the Heart and Stroke Foundation of Ontario (Grant NA-3479), the W.M. Keck Foundation, and the National Institutes of Health (Grants HL49277, HL62145, and GM20069).
ABBREVIATIONS
- ALP
alkaline phosphatase
- ANP
atrial natriuretic peptide
- BNP
B-type natriuretic peptide
- GC
guanylyl cyclase
- NPRA
natriuretic peptide receptor A
- kb
kilobase
References
- 1.Flynn T G. In: Contemporary Endocrinology: Natriuretic Peptides in Health and Disease. Samson W K, Levin E R, editors. Totowa, NJ: Humana; 1997. pp. 1–19. [Google Scholar]
- 2.Maack T. Kidney Int. 1996;49:1732–1737. doi: 10.1038/ki.1996.257. [DOI] [PubMed] [Google Scholar]
- 3.Chinkers M, Garbers D L, Chang M S, Lowe D G, Chin H M, Goeddel D V, Schulz S. Nature (London) 1989;338:78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- 4.Schulz S, Singh S, Bellet R A, Singh G, Tubb D J, Chin H, Garbers D L. Cell. 1989;58:1155–1162. doi: 10.1016/0092-8674(89)90513-8. [DOI] [PubMed] [Google Scholar]
- 5.Maack T, Suzuki M, Almeida F A, Nussenzveig D, Scarborough R M, McEnroe G A, Lewicki J A. Science. 1987;238:675–678. doi: 10.1126/science.2823385. [DOI] [PubMed] [Google Scholar]
- 6.Trachte G J. In: Contemporary Endocrinology: Natriuretic Peptides in Health and Disease. Samson W K, Levin E R, editors. Totowa, NJ: Humana; 1997. pp. 259–274. [Google Scholar]
- 7.Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, Shirakami G, Arai H, Saito Y, Kambayashi Y, Inouye K, et al. Endocrinology. 1992;130:229–239. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- 8.Martin E R, Lewicki J A, Scarborough R M, Ballermann B J. Am J Physiol. 1989;257:F649–F657. doi: 10.1152/ajprenal.1989.257.4.F649. [DOI] [PubMed] [Google Scholar]
- 9.Khurana M L, Pandey K N. Endocrinology. 1993;133:2141–2149. doi: 10.1210/endo.133.5.8404664. [DOI] [PubMed] [Google Scholar]
- 10.Koller B H, Hagemann L J, Doetschman T, Hagaman J H, Huang S, Williams P J, First N L, Maeda N, Smithies O. Proc Natl Acad Sci USA. 1989;86:8927–8931. doi: 10.1073/pnas.86.22.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey K N, Inagami T, Misono K S. Biochemistry. 1986;25:8467–8472. doi: 10.1021/bi00374a022. [DOI] [PubMed] [Google Scholar]
- 12.Almeida F A, Suzuki M, Scarborough R M, Lewicki J A, Maack T. Am J Physiol. 1989;256:R469–R475. doi: 10.1152/ajpregu.1989.256.2.R469. [DOI] [PubMed] [Google Scholar]
- 13.Sarda I R, de Bold M L, de Bold A J. Clin Biochem. 1989;22:11–15. doi: 10.1016/s0009-9120(89)80063-3. [DOI] [PubMed] [Google Scholar]
- 14.Krege J H, Hodgin J B, Hagaman J R, Smithies O. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 15.Goy M F. J Biol Chem. 1990;265:20220–20227. [PubMed] [Google Scholar]
- 16.Clark S A, Ambrose W W, Anderson T R, Terrell R S, Toverud S U. J Bone Miner Res. 1989;4:399–405. doi: 10.1002/jbmr.5650040315. [DOI] [PubMed] [Google Scholar]
- 17.Junqueira L C, Junqueira L C, Brentani R R. Anal Biochem. 1979;94:96–99. doi: 10.1016/0003-2697(79)90795-4. [DOI] [PubMed] [Google Scholar]
- 18.Krebsbach P H, Kuznetsov S A, Satomura K, Emmons R V, Rowe D W, Robey P G. Transplantation. 1997;63:1059–1069. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi N, Yamana H, Yoshiki S, Roodman G D, Mundy G R, Jones S J, Boyde A, Suda T. Endocrinology. 1988;122:1373–1382. doi: 10.1210/endo-122-4-1373. [DOI] [PubMed] [Google Scholar]
- 20.Yamauchi M, Katz E P. Connect Tissue Res. 1993;29:81–98. doi: 10.3109/03008209309014236. [DOI] [PubMed] [Google Scholar]
- 21.Chansel D, Pham P, Nivez M-P, Ardaillou R. Am J Physiol. 1990;259:F619–F627. doi: 10.1152/ajprenal.1990.259.4.F619. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Feng L, Mizuno T, Hirose S, Kawasaki K, Yaoita E, Kihara I, Wilson C B. Am J Physiol. 1994;267:F318–F324. doi: 10.1152/ajprenal.1994.267.2.F318. [DOI] [PubMed] [Google Scholar]
- 23.Fontoura B M, Nussenzveig D R, Pelton K M, Maack T. Am J Physiol. 1990;258:C692–C699. doi: 10.1152/ajpcell.1990.258.4.C692. [DOI] [PubMed] [Google Scholar]
- 24.Neant F, Bailly C. Kidney Int. 1993;44:741–746. doi: 10.1038/ki.1993.308. [DOI] [PubMed] [Google Scholar]
- 25.Nonoguchi H, Sands J M, Knepper M A. J Clin Invest. 1988;82:1383–1390. doi: 10.1172/JCI113742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver P M, Fox J E, Kim R, Rockman H A, Kim H-S, Reddick R L, Pandey K N, Milgram S L, Smithies O, Maeda N. Proc Natl Acad Sci USA. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasoda A, Ogawa Y, Suda M, Tamura N, Mori K, Sakuma Y, Chusho H, Shiota K, Tanaka K, Nakao K. J Biol Chem. 1998;273:11695–11700. doi: 10.1074/jbc.273.19.11695. [DOI] [PubMed] [Google Scholar]
- 28.Yanaka N, Akatsuka H, Kawai E, Omori K. Am J Physiol. 1998;275:E965–E973. doi: 10.1152/ajpendo.1998.275.6.E965. [DOI] [PubMed] [Google Scholar]
- 29.Suda M, Ogawa Y, Tanaka K, Tamura N, Yasoda A, Takigawa T, Uehira M, Nishimoto H, Itoh H, Saito Y, et al. Proc Natl Acad Sci USA. 1998;95:2337–2342. doi: 10.1073/pnas.95.5.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michel H, Bäcker A, Kramer H J. Clin Sci. 1992;83:139–142. doi: 10.1042/cs0830139. [DOI] [PubMed] [Google Scholar]
- 31.Brown L A, Nunez D J, Wilkins M R. J Clin Invest. 1993;92:2702–2712. doi: 10.1172/JCI116887. [DOI] [PMC free article] [PubMed] [Google Scholar]