Full text
PDF
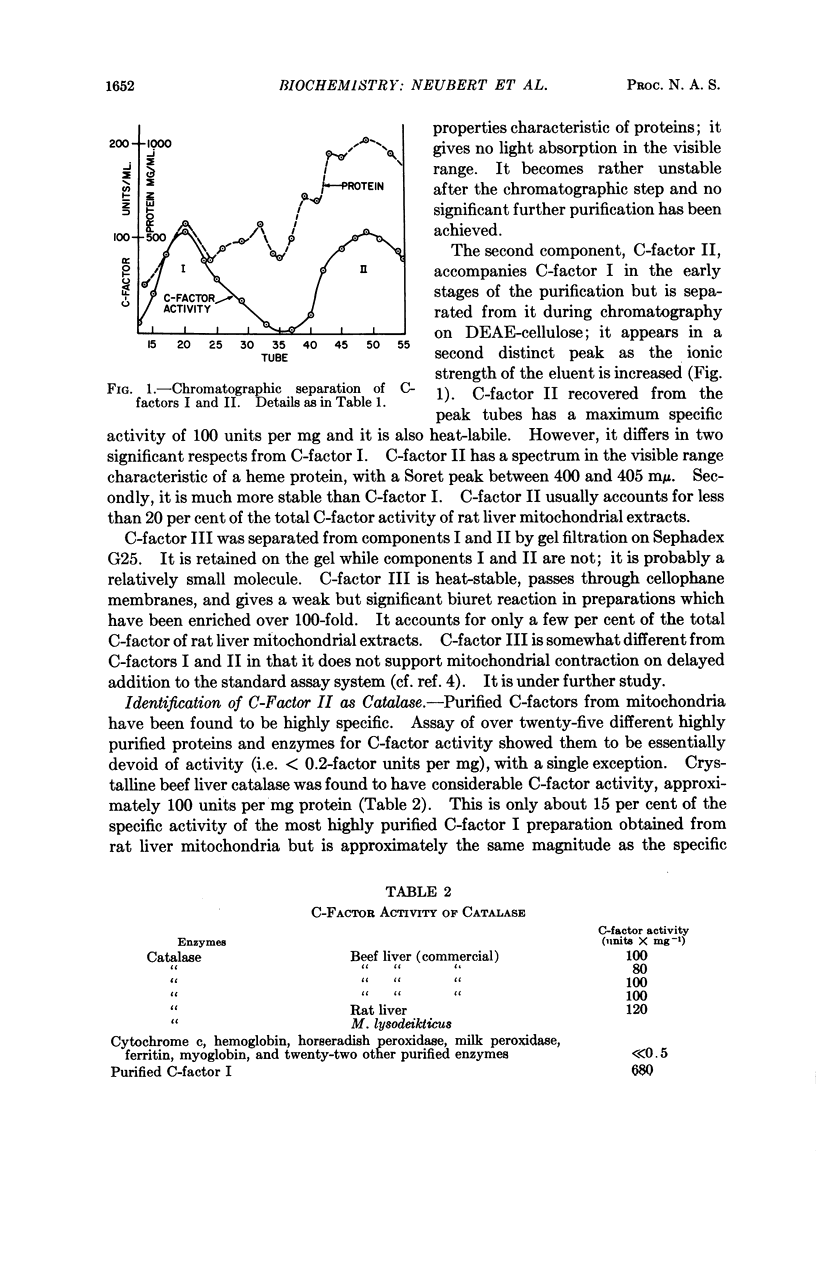
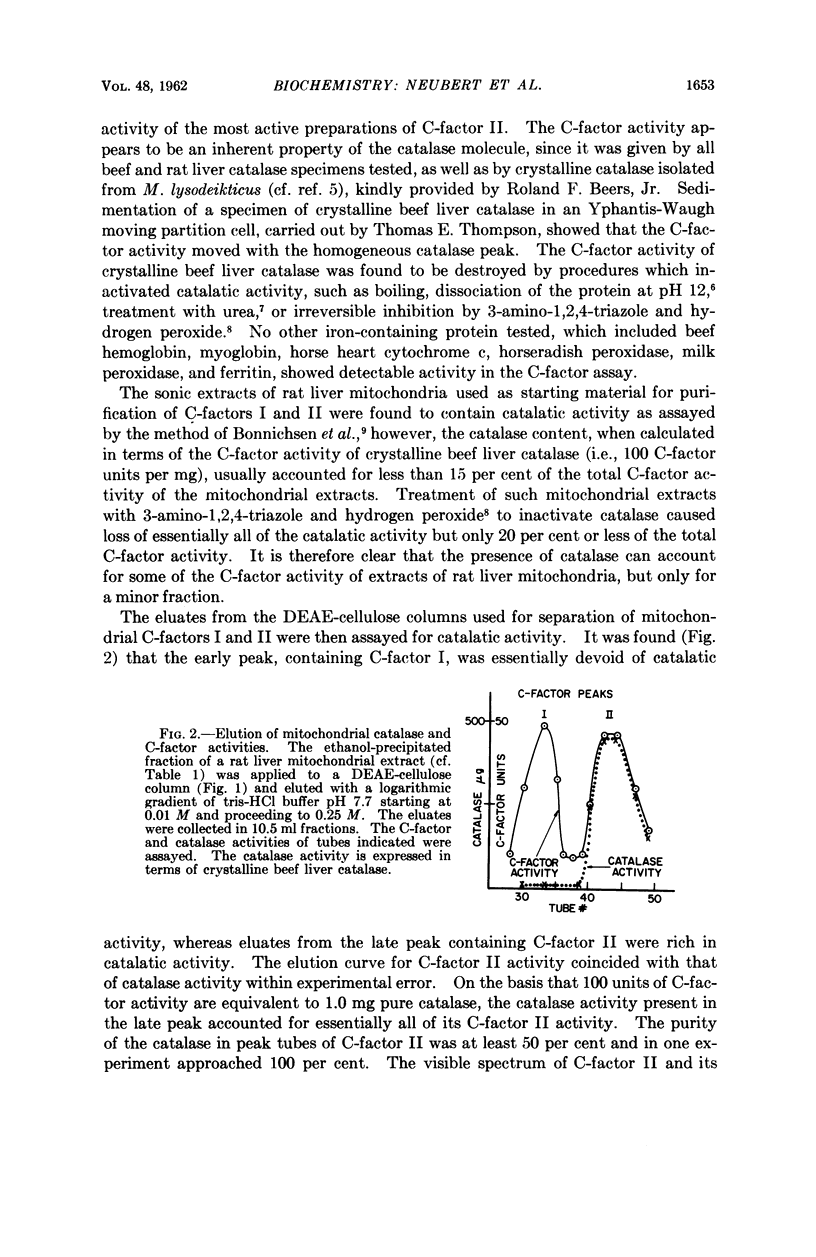
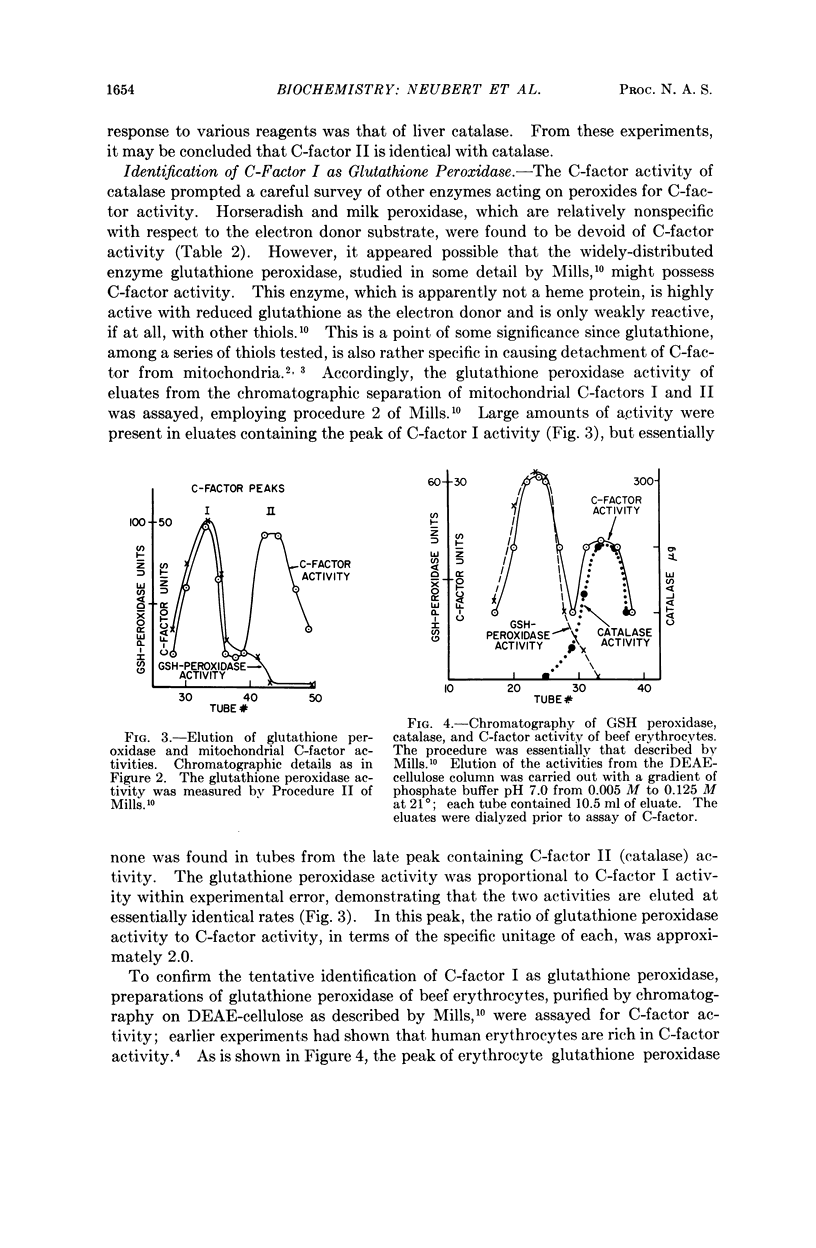




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B. The reactions of catalase in the presence of the notatin system. Biochem J. 1950 Apr;46(4):387–402. doi: 10.1042/bj0460387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORWIN L. M. Studies on peroxidation in vitamin E-deficient rat liver homogenates. Arch Biochem Biophys. 1962 Apr;97:51–58. doi: 10.1016/0003-9861(62)90043-7. [DOI] [PubMed] [Google Scholar]
- GREENFIELD R. E., PRICE V. E. Liver catalase. III. Isolation of catalase from mitochondrial fractions of polyvinylpyrrolidonesucrose homogenates. J Biol Chem. 1956 Jun;220(2):607–618. [PubMed] [Google Scholar]
- HOFFSTEN P. E., HUNTER F. E., Jr, GEBICKI J. M., WEINSTEIN J. Formation of "lipid peroxide" under conditions which lead to swelling and lysis of rat liver mitochondria. Biochem Biophys Res Commun. 1962 May 4;7:276–280. doi: 10.1016/0006-291x(62)90190-0. [DOI] [PubMed] [Google Scholar]
- Herbert D., Pinsent J. Crystalline bacterial catalase. Biochem J. 1948;43(2):193–202. doi: 10.1042/bj0430193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INADA Y., KUROZUMI T., SHIBATA K. Peroxidase activity of hemoproteins. I. Generation of activity by acid or alkali denaturation of methemoglobin and catalase. Arch Biochem Biophys. 1961 Apr;93:30–36. doi: 10.1016/0003-9861(61)90311-3. [DOI] [PubMed] [Google Scholar]
- LEHNINGER A. L. A heat-labile factor required in extrusion of water from mitochondria. J Biol Chem. 1962 Mar;237:946–951. [PubMed] [Google Scholar]
- LEHNINGER A. L., NEUBERT D. Effect of oxytocin, vasopressin, and other disulfide hormones on uptake and extrusion of water by mitochondria. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1929–1936. doi: 10.1073/pnas.47.12.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHNINGER A. L. Oxidative phosphorylation in submitochondrial systems. Fed Proc. 1960 Dec;19:952–962. [PubMed] [Google Scholar]
- LEHNINGER A. L., REMMERT L. F. An endogenous uncoupling and swelling agent in liver mitochondria and its enzymic formation. J Biol Chem. 1959 Sep;234:2459–2464. [PubMed] [Google Scholar]
- MARCUS A., FEELEY J. Inhibition of catalase by reducing agents in the presence of phenazine methosulfate. J Biol Chem. 1962 Jan;237:221–223. [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A. A study of the inhibition of catalase by 3-amino-1:2:4:-triazole. Biochem J. 1958 Mar;68(3):468–475. doi: 10.1042/bj0680468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLS G. C. Hemoglobin catabolism. I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J Biol Chem. 1957 Nov;229(1):189–197. [PubMed] [Google Scholar]
- MILLS G. C. The purification and properties of glutathione peroxidase of erythrocytes. J Biol Chem. 1959 Mar;234(3):502–506. [PubMed] [Google Scholar]
- NEUBERT D., LEHNINGER A. L. The effect of thiols and disulfides on water uptake and extrusion by rat liver mitochondria. J Biol Chem. 1962 Mar;237:952–958. [PubMed] [Google Scholar]
- NEUBERT D., ROSE T. H., LEHNINGER A. L. Assay and cellular distribution of mitochondrial "contraction factor". J Biol Chem. 1962 Jun;237:2025–2031. [PubMed] [Google Scholar]
- OTTOLENGHI A., BERNHEIM F., WILBUR K. M. The inhibition of certain mitochondrial enzymes by fatty acids oxidized by ultraviolet light or ascorbic acid. Arch Biochem Biophys. 1955 May;56(1):157–164. doi: 10.1016/0003-9861(55)90345-3. [DOI] [PubMed] [Google Scholar]
- PETERSON L. H. Introduction to the principles of digital and analogue computers. Fed Proc. 1962 Jan-Feb;21:69–74. [PubMed] [Google Scholar]
- SAMEJIMA T., SHIBATA K. Denaturation of catalase by formamide and urea related to the subunit make-up of the molecule. Arch Biochem Biophys. 1961 May;93:407–412. doi: 10.1016/0003-9861(61)90286-7. [DOI] [PubMed] [Google Scholar]
- THIELE E. H., HUFF J. W. Quantitative measurements of lipide peroxide formation by normal liver mitochondria under various conditions. Arch Biochem Biophys. 1960 Jun;88:203–207. doi: 10.1016/0003-9861(60)90222-8. [DOI] [PubMed] [Google Scholar]
- WILBUR K. M., BERNHEIM F., SHAPIRO O. W. The thiobarbituric acid reagent as a test for the oxidation of unsaturated fatty acids by various agents. Arch Biochem. 1949 Dec;24(2):305–313. [PubMed] [Google Scholar]
- WOJTCZAK L., LEHNINGER A. L. Formation and disappearance of an endogenous uncoupling factor during swelling and contraction of mitochondria. Biochim Biophys Acta. 1961 Aug 19;51:442–456. doi: 10.1016/0006-3002(61)90600-x. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., OTA A., OKUNUKI K. Activation of Pseudomonas cytochrome oxidase by catalase. J Biochem. 1961 May;49:414–420. doi: 10.1093/oxfordjournals.jbchem.a127319. [DOI] [PubMed] [Google Scholar]
- ZALKIN H., TAPPEL A. L. Studies of the mechanism of vitamin E action. IV. Lipide peroxidation in the vitamin E-deficient rabbit. Arch Biochem Biophys. 1960 May;88:113–117. doi: 10.1016/0003-9861(60)90205-8. [DOI] [PubMed] [Google Scholar]



