Abstract
We analyzed the cytolytic activity of intraepithelial T cells (IEL) isolated from the small intestines of 2- to 3-month-old mutant mice rendered deficient in different gene(s) in which the number of IEL expressing either T cell receptor (TCR)-αβ (αβ-IEL) or TCR-γδ (γδ-IEL) were absent or markedly diminished. When compared with wild-type littermates, cytolytic activity of γδ-IEL was sharply attenuated in TCR-β mutant mice but remained unaltered in TCR-α mutant mice in which a minor population of dull TCR-β+ (βdim)-IEL was also present. Cytolytic activity of γδ-IEL was maintained in mice doubly homozygous for β2-microglobulin and transporter associated with antigen processing 1 gene mutations in which a conspicuous decrease was noted in absolute numbers of αβ-IEL. In contrast, both TCR-δ and IL-7 receptor-α gene mutations that lead to lack of γδ-IEL generation did not affect the development or cytolytic activity of the remaining αβ-IEL. The anti-CD3 and anti-TCR-γδ mAb-induced IFN-γ production of γδ-IEL showed the same TCR-α and TCR-β mutation-dependent variability. These results indicate that cytolytic and IFN-γ-producing activities of γδ T cells in mouse intestinal epithelium are TCR-β-chain-dependent.
In the mouse small intestine, numerous T cells (≈5 × 107) expressing either T cell receptor (TCR)-αβ (40–70%) or TCR-γδ (30–60%) reside above the basement membrane together with the columnar epithelial cells (intestinal intraepithelial T lymphocytes; IEL). IEL are unusual among mouse peripheral T cells in that freshly isolated IEL are capable of killing Fc-receptor-bearing target cells after bridging them with anti-CD3 or anti-TCR mAbs (1–3) and in that most γδ-IEL and many αβ-IEL, unlike thymus-derived T cells, express a unique CD8αα homodimer (4–7) instead of a CD8αβ heterodimer and develop extrathymically in the intestinal mucosa (4, 5, 7–13). Nevertheless, the functional role of IEL and the precise extrathymic developmental events involving the segregation of αβ- and γδ-IEL lineages are not well understood.
Owing to the successful generation of TCR-α (14, 15), -β (15), and -δ (16) gene mutant mice (α−/−, β−/−, and δ−/− mice, respectively), we have learned much about intrathymic differentiation of αβ and γδ T cells and about their biological functions in the peripheral lymphoid tissues. For instance, TCR-β, but not TCR-α, gene rearrangement or expression is mandatory not only for the generation but also for the expansion of the pool of CD4+CD8+ thymocytes (15), αβ and γδ T cell development occurs in a mutually independent fashion (16), and both α−/− and β−/− mice spontaneously develop inflammatory bowel disease (IBD) (17), although the IBD is more severe and present more consistently in α−/− mice than in β−/− mice (17). In addition, taking advantage of the fact that murine IEL compartment is enriched with γδ T cells, the biological significance of these poorly defined T cells has been investigated in δ−/− mice and several distinctive functions of γδ-IEL in the intestinal mucosa were revealed (18–20).
We have previously shown that the cytolytic activity of γδ-IEL is strain-dependent in conventional mice (3) and that this strain-dependent variability is unaltered in the germ-free condition (21). In contrast, the cytolytic activity of αβ-IEL is the hallmark of in situ activation by intestinal microorganisms, which is absent in germ-free mice (1, 2, 21). In the present study, we found that γδ-IEL from 2- to 3-month-old β−/− mice fail to display cytolytic activity, whereas γδ-IEL from α−/− mice and αβ-IEL from δ−/− mice display a vigorous cytolytic activity comparable to that displayed by γδ- and αβ-IEL isolated from the small intestine of wild-type (wt) littermate mice. Similar TCR-α and TCR-β gene-dependent variability was also seen in IFN-γ production on polyclonal stimulation of γδ-IEL. These findings indicate that TCR-β gene expression, most likely the presence of αβ- or βdim-IEL, is critical for the differentiation of γδ-IEL into constitutively activated T cells in the intestinal mucosal microenvironment.
MATERIALS AND METHODS
Mice.
The development of TCR-β mutant (β−/−) mice (15), TCR-α mutant (α−/−) mice (15), and TCR-δ mutant (δ−/−) mice (16) has been described. These mutant strains were backcrossed 12–14 times to the C57BL/6J Jcl parent (CLEA Japan, Tokyo) in our animal facility. We obtained wt and β−/− mice by crossing β+/− and β−/− mice, and wt and α−/− mice by crossing α+/− and α−/− mice. wt, β−/−, and α−/− littermate mice were also obtained from the F2 generation of an intercross between β−/− and α−/− mice. Mice were typed by using PCR analysis of tail DNA with a set of primers for the neomycin resistance gene (5′-CTTGGGTGGAGAGGCTATTC-3′ and 5′-AGGTGAGATGACAGGAGATC-3′, 280-bp PCR fragment), for the wt TCR-β gene (5′-AAGGTCTCCTTGTTTGAGCC-3′ and 5′-GCTATAATTGCTCTCCTTGT-3′, 180-bp PCR fragment), and for the wt TCR-α gene (5′-TCCAGAACCCAGAACCCTGCTGTG-3′ and 5′-CCTGAACTGGGGTAGGTGGCG-3′, 259-bp PCR fragment). These wt and TCR-β and TCR-α mutant mice of both sexes, 2 to 3 or 4 to 8 months of age, were used in the experiments, and the experimental observations made in the present study were consistent irrespective of the origins of the wt, β−/−, and α−/− mice. wt and δ−/− mice used were obtained by crossing δ+/− and δ−/− mice. Mice were typed by using PCR analysis of tail DNA with a set of primers for the neomycin resistance gene (see above) and for the wt TCR-δ gene (5′-AAAAGCCAGCCTCCGGCCAAA-3′ and 5′-AACTGAACATGTCACTGAATT-3′, 222-bp PCR fragment). Mice with a disrupted gene encoding IL-7 receptor α-chain (IL-7R−/− mice) have been described (22). By intercrossing mice with a mutated β2-microglobulin (β2M) gene (23) and those with a mutated transporter associated with antigen processing 1 (TAP1) gene (24), we (25) generated mice doubly homozygous for β2M and TAP1 mutations (named β2TA mice) in which the development of αβ-IEL was severely impaired because of the drastic decrease of MHC class I molecule expression (25). These wt and mutant mice were used at 2 to 3 months of age.
Antibodies.
The following mAbs were used: Anti-CD3 mAb 145-2C11 (PharMingen), anti-pan TCR-αβ mAb H57–597 (PharMingen), anti-pan TCR-γδ mAb GL-3 (PharMingen), anti-pan TCR-γδ mAb 3A10 (3), anti-Vγ1 mAb (26), anti-Vγ4 mAb UC3–10A6 (PharMingen), anti-Vγ7 mAb (provided by L. Lefrancois, University of Connecticut Health Center), anti-Vδ4 mAb GL-2 (PharMingen), anti-Thy-1.2 mAb 30H-12 (Becton Dickinson), anti-CD4 mAb GK 1.5 (Becton Dickinson), anti-CD8α mAb 53.6.7 (Becton Dickinson), and anti-CD8β mAb 53.5.8 (PharMingen). Essentially the same results were obtained in flow cytometric and redirected cytotoxicity analyses by using anti-pan TCRγδ mAbs GL-3 and 3A10, and, unless otherwise stated, the data presented were obtained with the mAb GL-3.
Isolation of Mouse IEL.
We isolated IEL according to the method described previously (21, 25). In brief, small intestine free of the lumen content was turned inside out with the aid of polyethylene tubing. The inverted intestine was cut into four or five segments, and the segments were transferred to a 50-ml conical tube (Falcon 2070) containing 45 ml of RPMI 1640 medium including 5% FCS, 25 mM Hepes, penicillin at 100 units/ml, and streptomycin at 100 μg/ml. The tube was shaken at 37°C for 45 min (horizontal position; orbital shaker at 150 rpm). Cell suspensions were collected in a new 50-ml conical tube and passed through a glass-wool column to deplete cell debris and sticky cells (crude cell preparation). Subsequently, the cells were suspended in 30% Percoll solution and centrifuged for 20 min at 560 × g. After centrifugation, cells at the bottom of the solution were subjected to Percoll discontinuous-gradient centrifugation, and IEL were recovered at the interphase of 44% and 70% Percoll solutions.
Immunofluorescence Analysis.
IEL were stained with various combinations of appropriate mAbs described above. To eliminate the dead cells from the data, we used propidium iodide. The data were analyzed by using an EPICS Elite flow cytometer.
Redirected Cytotoxicity Assay.
Redirected cytolytic activity of freshly isolated IEL was measured by using a standard 51Cr-release assay. The fresh effector IEL were incubated with 3 × 103 51Cr-labeled Fc receptor-positive P815 mastocytoma target cells for 6 hr at 37°C without addition of any mAbs or in the presence of anti-CD3 mAb (0.2 μg/ml), anti-TCR-αβ mAb (0.2 μg/ml), or anti-TCR-γδ mAb (1 μg/ml) in 75 μl of complete medium (RPMI 1640 medium containing 10% FCS, 10 mM Hepes, 5 × 10−5 M 2-mercaptoethanol, 4 mM glutamine, penicillin at 100 units/ml, and streptomycin at 100 μg/ml) in each well of flat-bottom 96-well microtiter plates. Then, 200 μl of complete medium was added, and 100 μl of supernatant was collected after centrifugation for assay of released 51Cr. All determinations were carried out at least two E/T ratios. The percent specific 51Cr release was calculated from the following formula: (experimental release − spontaneous release)/(detergent-induced release − spontaneous release) × 100.
IFN-γ Production by IEL.
Freshly isolated IEL (2 × 105 cells per well) were stimulated with plate-coated anti-CD3 mAb or anti-TCR-γδ mAb in each well of flat-bottom 96-well microtiter plates. After 72 hr, supernatants were collected, and IFN-γ in the supernatants was measured by using ELISA (27). To quantitate IFN-γ, plates were coated with anti-IFN-γ mAb R4–6A2 (American Type Culture Collection) and detected by using biotinylated anti-IFN-γ mAb XMG 1.2 (a gift from J. S. Abrams, DNAX, Palo Alto, CA) after incubation with streptavidin conjugated with alkaline phosphatase for development with disodium p-nitrophenylphosphate.
RESULTS
Cytolytic Activity of γδ-IEL in wt, β−/−, and α−/− Mice.
Starting at 4–5 months of age in our animal facility, progressive wasting syndrome, hunched posture, diarrhea, and/or anorectal prolapse have been observed in ≈70–80% of α−/− mice of both sexes. In contrast to the previous report (17), however, most of the β−/− mice have remained healthy without symptoms in the 4- to 8-month period of observation. In an attempt to investigate whether any immunological disorder extends from the inflamed colonic mucosa to the seemingly normal small intestine of α−/− mice, we analyzed the cytolytic activity of IEL isolated from the small intestines of 4- to 8-month-old wt, β−/−, and α−/− mice. Consistent with the previous findings (28), γδ-IEL from β−/− mice exhibited minimal cytolytic activity when lysis was induced with anti-CD3 mAb or anti-TCR-γδ mAb, and in fact the activity was almost absent in γδ-IEL in one-fifth of the β−/− mice. In marked contrast, γδ-IEL from α−/− mice with or without IBD exhibited normal, even augmented, levels of cytolytic activity.
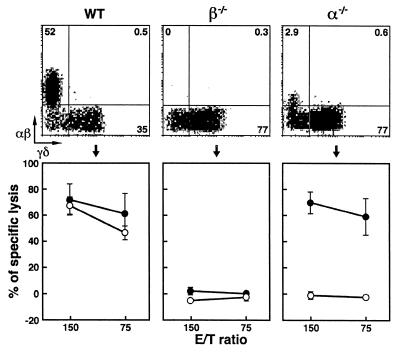
We next evaluated the cytolytic activity of IEL isolated from young adult animals at 2–3 months of age, during which time most α−/− mice do not exhibit the onset of IBD. As shown in Fig. 1, γδ-IEL from wt and α−/− mice displayed significant and comparable levels of cytolytic activity, whereas γδ-IEL from β−/− mice displayed negligible cytotoxicity. The only difference between β−/− and α−/− mice in terms of the composition of αβ- and γδ-IEL was the presence of a small population of βdim-IEL in the latter α−/− mice (Fig. 1).
Figure 1.
Two-color flow cytometric analysis and cytolytic activity of IEL isolated from wt, β−/− and α−/− mice at 2–3 months of age. (Upper) Representative staining of αβ- and γδ-IEL. IEL were incubated first with anti-TCR-αβ mAb (biotinylated) and then with streptavidin–phycoerythrin (Caltag, South San Francisco, CA) and anti-TCR-γδ mAb (FITC-conjugated). Percentage of positive cells in the corresponding quadrants is shown. (Lower) The redirected lysis assay was done in the presence of anti-TCR-αβ mAb (○) or anti-TCR-γδ mAb (●). The results are means ± SD of data obtained from three independent analyses of five mice per group.
Phenotypic and Vγ/Vδ Gene Usage Analyses of γδ-IEL in wt, β−/−, and α−/− Mice.
Two major CD8αα+ and CD4−CD8− subsets are present in the mouse γδ-IEL when classified on the basis of expression of CD4, CD8α, and/or CD8β molecules (25, 29). No significant change in the relative proportion of these two major subsets was seen between γδ-IEL from wt, β−/−, and α−/− mice (data not shown). In contrast, 2-fold expansion of the Thy-1+ γδ-IEL subset was noted in α−/− mice when compared with wt and β−/− mice (Table 1), suggesting that α−/− mice harbor an increased number of constitutively activated γδ-IEL (1, 11) in the intestine.
Table 1.
γδ-IEL isolated from various mouse strains
| Mice n | γδ-IEL per mouse, × 106 | Subset of γδ-IEL, %
|
||||
|---|---|---|---|---|---|---|
| Thy-1+ | Vγ1+ | Vγ4+ | Vγ7+ | Vδ4+ | ||
| wt 8 | 3.05 ± 0.68 | 27.5 ± 10.9 | 34.7 ± 5.7 | 10.3 ± 4.1 | 52.8 ± 11.8 | 14.0 ± 3.6 |
| β−/− 9 | 4.24 ± 1.06 | 23.1 ± 1.6 | 55.3 ± 4.5 | 8.2 ± 1.4 | 38.9 ± 7.0 | 11.3 ± 1.4 |
| α−/− 8 | 4.90 ± 1.91 | 43.4 ± 12.0 | 45.5 ± 13.6 | 9.1 ± 7.6 | 43.5 ± 11.3 | 21.9 ± 5.8 |
IEL isolated from wt, β−/− and α−/− mice were incubated first with anti-Thy-1.2 mAb (biotinylated) and then with streptavidin–phycoerythrin and anti-TCR-γδ mAb (FITC-conjugated). These IEL were also incubated first with anti-Vγ1, anti-Vγ4, anti-Vγ7, or anti-Vδ4 mAb. After washing, the IEL were incubated with biotinylated goat anti-hamster IgG and subsequently counterstained with streptavidin-phycoerythrin and anti-TCR-γδ mAb (FITC-conjugated). The proportion of Thy-1+ cells in α−/− γδ-IEL was significantly higher than those in wt (P < 0.05) and β−/− (P < 0.01) γδ-IEL. The proportion of Vδ4+ cells in α−/− γδ-IEL was significantly higher than those in wt (P < 0.01) and β−/− (P < 0.01) γδ-IEL.
The data presented so far indicate that mutations of TCR-β and -α genes have little effect on the colonization of γδ-IEL but exert markedly different effects on the cytolytic function and Thy-1 expression of γδ-IEL. In this regard, it is reasonable to consider the possibility that the TCR repertoire of γδ-IEL in α−/− mice differs from that in β−/− mice. In an attempt to address this possibility, we conducted two-color immunofluorescence analysis on γδ-IEL isolated from wt, β−/− and α−/− mice, by using FITC-conjugated anti-pan-γδ mAb GL-3 and one of the four different V segment-specific mAbs listed in Materials and Methods. No significant differences , if any, were observed between the wt and mutant conditions in Vγ1, Vγ4, and Vγ7 gene segment use, whereas the proportion of Vδ4+ subset in total γδ-IEL was expanded 2-fold in α−/− mice (Table 1).
Cytolytic Activity of γδ-IEL in β2TA mice.
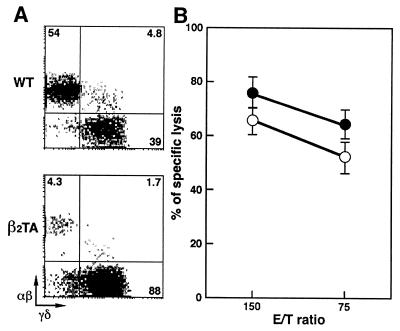
We have previously shown (25) that mice doubly homozygous for β2M and TAP1 gene mutations (named β2TA mice) basically fail to express major histocompatibility complex class I molecules, lack thymus-derived CD8+ T cells, and have conspicuously decreased numbers of αβ-IEL associated with a concomitant increase in colonization of γδ-IEL. Therefore, it is important to evaluate the cytolytic activity of γδ-IEL in β2TA mice, and the results obtained are shown in Fig. 2. Although the population of αβ-IEL was reduced drastically in size (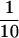 ; Fig. 2A) in β2TA mice, cytolytic activity of γδ-IEL was maintained in this mutant condition (Fig. 2B).
; Fig. 2A) in β2TA mice, cytolytic activity of γδ-IEL was maintained in this mutant condition (Fig. 2B).
Figure 2.
Two-color flow cytometric analysis and cytolytic activity of IEL isolated from wt and β2TA mice at 2–3 months of age. (A) Representative staining of αβ- and γδ-IEL. IEL were incubated first with anti-TCR-αβ mAb (biotinylated) and then with streptavidin–phycoerythrin and anti-TCR-γδ mAb (FITC-conjugated). Percentage of positive cells in the corresponding quadrants is shown. (B) Redirected cytolytic activity of γδ-IEL from wt (○) and β2TA (●) mice in the presence of anti-TCR-γδ mAb. The results are means ± SD of data obtained from two independent analyses of four mice per group.
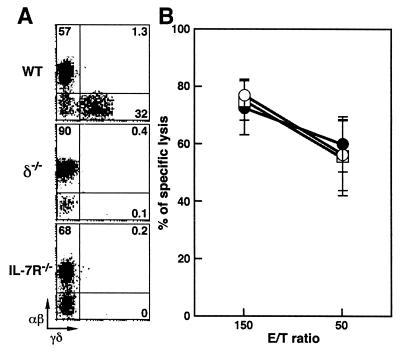
Cytolytic Activity of αβ-IEL from δ−/− and IL-7R−/− mice.
γδ-IEL from 2- to 3-month-old β−/− mice that lacked αβ-IEL failed to display cytolytic activity (Fig. 1). In contrast, it was demonstrated that αβ-IEL from δ−/− mice that lack γδ-IEL exhibit normal cytolytic activity (28). To reconfirm whether cytolytic activity of αβ-IEL is totally independent of γδ-IEL, we examined αβ-IEL isolated from δ−/− and IL-7R−/− mice. Although the mechanisms are quite different between the two mutations, development of γδ-IEL was also completely hampered in the IL-7R−/− mice (Fig. 3A; refs. 22 and 30). As shown in Fig. 3B, the constitutive cytolytic activity of αβ-IEL was maintained normally in both δ−/− and IL-7R−/− mice. Taken together, our results indicated that αβ T cells, most likely αβ-IEL, were necessary for the induction of cytolytic activity of γδ-IEL, whereas γδ-IEL were irrelevant to the cytolytic activity of αβ-IEL in mice at 2–3 months of age.
Figure 3.
Two-color flow cytometric analysis and cytolytic activity of IEL isolated from wt, δ−/−, and IL-7R−/− mice at 2–3 months of age. (A) Representative staining of αβ- and γδ-IEL. IEL were incubated first with anti-TCR-αβ mAb (biotinylated) and then with streptavidin–phycoerythrin and anti-TCR-γδ mAb (FITC-conjugated). Percentage of positive cells in the corresponding quadrants is shown. (B) Redirected cytolytic activity of αβ-IEL from wt (○), δ−/− (●), and IL-7R−/− (□) mice in the presence of anti-TCR-αβ mAb. The results are means ± SD of data obtained from three independent analyses of four to five mice per group.
IFN-γ Production by in Vitro-Activated γδ-IEL from wt, β−/−, and α−/− mice.
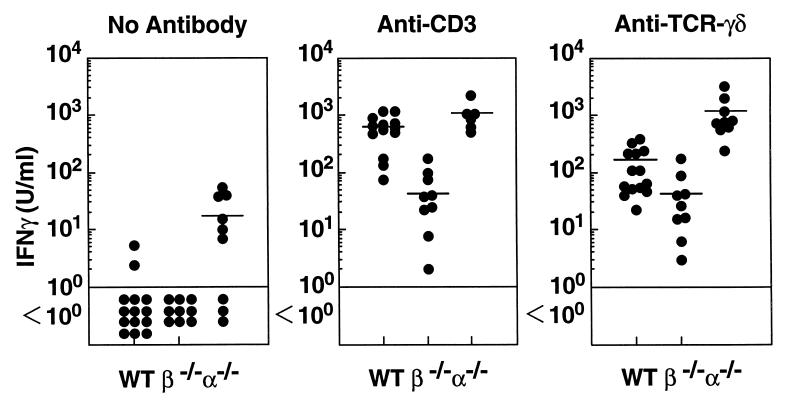
We attempted to determine whether IFN-γ production by γδ-IEL exhibits a mutation-dependent variability similar to that of the cytolytic activity. IEL were stimulated in vitro with immobilized anti-CD3 mAb or anti-TCR-γδ mAb for 3 days, and the IFN-γ present in the culture supernatants was measured by using ELISA. Consistent with our previous observations (31), absolute amounts of IFN-γ produced by IEL from β−/− mice (γδ-IEL) were about 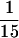 of those produced by IEL from wt mice (αβ- and γδ-IEL) after stimulation with ant-CD3 mAb (Fig. 4). Fig. 4 shows that, surprisingly, in the presence of anti-γδ TCR mAb, the amount of IFN-γ produced by γδ-IEL from α−/− mice was more than 20-fold greater than that produced by γδ-IEL from β−/− mice. These results indicated that γδ-IEL from α−/− mice retained a far greater IFN-γ producing activity than that retained by γδ-IEL from β−/− mice.
of those produced by IEL from wt mice (αβ- and γδ-IEL) after stimulation with ant-CD3 mAb (Fig. 4). Fig. 4 shows that, surprisingly, in the presence of anti-γδ TCR mAb, the amount of IFN-γ produced by γδ-IEL from α−/− mice was more than 20-fold greater than that produced by γδ-IEL from β−/− mice. These results indicated that γδ-IEL from α−/− mice retained a far greater IFN-γ producing activity than that retained by γδ-IEL from β−/− mice.
Figure 4.
IFN-γ production by IEL isolated from wt (n = 14), β−/− (n = 9) and α−/− (n = 9) mice at 2–3 months of age. Two × 105 IEL were cultured in either uncoated, ant-CD3 mAb-coated, or anti-TCR-γδ mAb-coated culture plates. Supernatants were collected on day 3, and the concentration of IFN-γ in the corresponding supernatants was measured by ELISA.
DISCUSSION
Analysis of TCR gene knock-out mice which lack either αβ T cells (β−/−) or γδ T cells (δ−/−) revealed the mutually independent development and tissue localization of these two distinct T cells (14, 15). Regarding the functional level of crosstalk, however, Huleatt and Lefrancois (28) demonstrated that the generally low cytolytic activity of γδ-IEL from β−/− mice contrasts with the cytolytic activity of γδ-IEL from normal mice. Our present results have confirmed and extended their observations by showing that although cytolytic and IFN-γ-producing activities of γδ-IEL are sharply attenuated in β−/− mice, the γδ-IEL activities remain the same or even increase in α−/− mice.
The compensatory increase in the number of γδ-IEL was not associated with any gross alteration of major CD8αα+ and CD4−8− γδ-IEL subsets in β−/− and α−/− mice. However, the ratio of Thy-1+ to Thy-1− γδ-IEL and composition of the Vδ4+ subset were 2-fold higher in α−/− mice than in β−/− and wt mice (Table 1). Mechanisms underlying the increase in Vδ4+ and Thy-1+ γδ-IEL populations in α−/− mice are not known. One possibility for causes of the increase might be a still unappreciated function of βdim-IEL present in α−/− mice. Marginal differences in Vγ1, Vγ4, and Vγ7 gene segment use were also noted between γδ-IEL from β−/−, α−/−, and wt mice (Table 1). The expansion of the Thy-1+ γδ-IEL population and vigorous cytolytic activity of γδ-IEL in α−/− mice are consistent with previous studies (1, 11) in which it was shown that Thy-1+ IEL subset contains constitutively activated cytolytic T cells. It is also noteworthy that the cytolytic activity of γδ-IEL (Fig. 2) and their subset composition, as defined by the expression of CD4 and CD8α chains (25) in β2TA mice, were comparable with those in wt mice although a conspicuous decrease in absolute numbers of αβ-IEL was noted in β2TA mice (Fig. 2). Collectively, these results indicate that although intraepithelial compartmentalization of γδ-IEL takes place in the absence of αβ-IEL and TCR-β gene expression, the presence of at least a small number of αβ- or βdim-IEL is critical for the development of cytolytic and IFN-γ-producing γδ-IEL during relatively late differentiation steps that convert precursor γδ-IEL into the constitutively activated state.
In contrast to β−/− mice, α−/− mice are predisposed to a marked increase in antibody producing B cells secreting autoantibodies (32–36) and to germinal center formation (34). In fact, the traits are comparable to or even exceed those of age-matched wt mice (32, 33, 35), and the cellular mass of lymphoid tissues in α−/− mice becomes greater than that in control wt mice, either after exposure to environmental antigens (37) or with age (35, 38). This study revealed that, even during 2–3 months of young adult age, cytolytic and IFN-γ-producing activities of γδ-IEL were uniformly high in α−/− mice but very low in β−/− mice. Analysis of large intestinal γδ-IEL from 2- to 3-month-old α−/− and β−/− mice indicated that they were also cytolytic in the former but not in the latter mutant mice (unpublished observations). In this context, the persistent colonization of constitutively activated Thy-1+ γδ-IEL subset in the intestinal epithelia of young α−/− mice probably has important implications for the subsequent development of IBD in older α−/− mice. In conclusion, not only the βdim T cells (35, 36, 39, 40), but also possibly the activated γδ T cells in the intestinal mucosa, may play an important role in the early stages of development of chronic IBD in α−/− mice.
Acknowledgments
We thank Drs. A. K. Bhan, E. Mizoguchi, and A. Mizoguchi for their critical reading of the manuscript and K. Ishimaru for her technical assistance. This work was supported by the Agency of Science and Technology, Japan and by the Japan Society for the Promotion of Science (JSPS-RFTF 97L00701).
ABBREVIATIONS
- α−/−
TCR-α gene mutant
- β−/−
TCR-β gene mutant
- βdim
TCR-β dull positive
- β2TA
homozygous for β2-microglobulin and transporter associated with antigen-processing 1 gene mutations
- δ−/−
TCR-δ gene mutant
- IBD
inflammatory bowel disease
- IEL
intestinal intraepithelial T lymphocytes
- wt
wild type
References
- 1.Lefrancois L, Goodman T. Science. 1989;243:1716–1718. doi: 10.1126/science.2564701. [DOI] [PubMed] [Google Scholar]
- 2.Guy-Grand D, Malassis-Seris M, Briottet C, Vassalli P. J Exp Med. 1991;173:1549–1552. doi: 10.1084/jem.173.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishikawa H, Li Y, Abeliovich A, Yamamoto S, Kaufmann S H E, Tonegawa S. Proc Natl Acad Sci USA. 1993;90:8204–8208. doi: 10.1073/pnas.90.17.8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefrancois L. Immunol Today. 1991;12:436–438. doi: 10.1016/0167-5699(91)90015-L. [DOI] [PubMed] [Google Scholar]
- 5.Rocha B, Vassali P, Guy-Grand D. Immunol Today. 1992;13:449–454. doi: 10.1016/0167-5699(92)90074-H. [DOI] [PubMed] [Google Scholar]
- 6.Barrett T A, Gajewski T F, Danielpour D, Chang E B, Beagley K W, Bluestone J A. J Immunol. 1992;149:1124–1130. [PubMed] [Google Scholar]
- 7.Poussier P, Edouard P, Lee C, Binnie M, Julius M. J Exp Med. 1992;176:187–199. doi: 10.1084/jem.176.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosley R L, Styre D, Klein J R. J Immunol. 1990;145:1369–1375. [PubMed] [Google Scholar]
- 9.Lefrancois L, LeCorre R, Mayo J, Bluestone J A, Goodman T. Cell. 1990;63:333–340. doi: 10.1016/0092-8674(90)90166-c. [DOI] [PubMed] [Google Scholar]
- 10.Bandeira A, Itohara S, Bonneville M, Burlen-Defranoux O, Mota-Santos T, Coutinho A, Tonegawa S. Proc Natl Acad Sci USA. 1991;88:43–47. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guy-Grand D, Cerf-Bensussan N, Malissen B, Malassis-Seris M, Briottet C, Vassali P. J Exp Med. 1991;173:471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. J Exp Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito H, Kanamori Y, Takemori T, Nariuchi H, Kubota E, Takahashi-Iwanaga H, Iwanaga T, Ishikawa H. Science. 1998;280:275–278. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- 14.Philpott K L, Viney J L, Kay G, Rastan S, Gardner E M, Chae S, Hayday A C, Owen M J. Science. 1992;256:1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 15.Mombaerts P, Clarke A R, Rudnicki M A, Iacomini J, Itohara S, Lafaille J J, Wang Y, Ichikawa Y, Jaenisch R, Hooper M L, et al. Nature (London) 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 16.Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke A R, Hooper M L, Farr A, Tonegawa S. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 17.Mombaerts P, Mizoguchi E, Grusby M J, Glimcher L H, Bhan A K, Tonegawa S. Cell. 1993;75:275–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 18.Komano H, Fujiura Y, Kawaguchi M, Matsumoto S, Hashimoto Y, Obana S, Mombaerts P, Tonegawa S, Yamamoto H, Itohara S, et al. Proc Natl Acad Sci USA. 1995;92:6147–6151. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujihashi K, McGhee J R, Kweon M-N, Cooper M D, Tonegawa S, Takahashi I, Hiroi T, J, Mestecky J, Kiyono H. J Exp Med. 1996;183:1929–1935. doi: 10.1084/jem.183.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts S J, Smith A L, West A B, Wen L, Findly R C, Owen M J, Hayday A C. Proc Natl Acad Sci USA. 1996;93:11774–11779. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawaguchi M, Nanno M, Umesaki Y, Matsumoto S, Okada Y, Cai Z, Shimamura T, Matsuoka Y, Ohwaki M, Ishikawa H. Proc Natl Acad Sci USA. 1993;90:8591–8594. doi: 10.1073/pnas.90.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maki K, Sunaga S, Komagata Y, Kodaira Y, Mabuchi A, Karasuyama H, Yokomuro K, Miyazaki J, Ikuta K. Proc Natl Acad Sci USA. 1996;93:7172–7177. doi: 10.1073/pnas.93.14.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zijlstra M, Bix M, Simister N E, Loring J M, Raulet D H, Jaenisch R. Nature (London) 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- 24.Van Kaer L, Ashton-Rickard P G, Ploegh H L, Tonegawa S. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 25.Fujiura Y, Kawaguchi M, Kondo Y, Obana S, Yamamoto H, Nanno M, Ishikawa H. J Immunol. 1996;156:2710–2715. [PubMed] [Google Scholar]
- 26.Pereira P, Gerber D, Huang S Y, Tonegawa S. J Exp Med. 1995;182:1921–1930. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishijima K, Hisatsune T, Minai Y, Kohyama M, Kaminogawa K. Cell Immunol. 1994;154:193–201. doi: 10.1006/cimm.1994.1068. [DOI] [PubMed] [Google Scholar]
- 28.Huleatt J W, Lefrancois L. Immunity. 1996;5:263–273. doi: 10.1016/s1074-7613(00)80321-0. [DOI] [PubMed] [Google Scholar]
- 29.Nanno M, Matsumoto S, Koike R, Miyasaka M, Kawaguchi M, Masuda T, Miyawaki S, Cai Z, Shimamura T, Fujiura Y, et al. J Immunol. 1994;153:2014–2020. [PubMed] [Google Scholar]
- 30.Maki K, Sunaga S, Ikuta K. J Exp Med. 1996;184:2423–2427. doi: 10.1084/jem.184.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohyama M, Hachimura S, Nanno M, Ishikawa H, Kaminogawa S. Microbiol Immunol. 1997;41:353–359. doi: 10.1111/j.1348-0421.1997.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 32.Wen L, Roberts S J, Viney J L, Wong F S, Mallick C, Findly R C, Peng Q, Craft J E, Owen M J, Hayday A C. Nature (London) 1994;369:654–658. doi: 10.1038/369654a0. [DOI] [PubMed] [Google Scholar]
- 33.Mizoguchi A, Mizoguchi E, Tonegawa S, Bhan A K. Int Immunol. 1996;8:1387–1394. doi: 10.1093/intimm/8.9.1387. [DOI] [PubMed] [Google Scholar]
- 34.Dianda L, Gulbranson-Judge A, Pao W, Hayday A C, MacLennan I C M, Owen M J. Eur J Immunol. 1996;26:1603–1607. doi: 10.1002/eji.1830260729. [DOI] [PubMed] [Google Scholar]
- 35.Mizoguchi A, Mizoguchi E, Chiba C, Spiekermann G M, Tonegawa S, Nagler-Anderson C, Bhan A K. J Exp Med. 1996;183:847–856. doi: 10.1084/jem.183.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi I, Kiyono H, Hamada S. Gastroenterology. 1997;112:1876–1886. doi: 10.1053/gast.1997.v112.pm9178680. [DOI] [PubMed] [Google Scholar]
- 37.Viney J L, Dianda L, Roberts S L, Wen L, Mallick C A, Hayday A C, Owen M J. Proc Natl Acad Sci USA. 1994;91:11948–11952. doi: 10.1073/pnas.91.25.11948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizoguchi A, Mizoguchi E, Chiba C, Bhan A K. J Exp Med. 1996;184:707–715. doi: 10.1084/jem.184.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mombaerts P, Mizoguchi E, Ljunggren H-G, Iacomini J, Ishikawa H, Wang L, Grusby M J, Glimcher L H, Winn H J, Bhan A K, et al. Int Immunol. 1994;6:1061–1070. doi: 10.1093/intimm/6.7.1061. [DOI] [PubMed] [Google Scholar]
- 40.Mizoguchi E, Mizoguchi A, Bhan A K. Lab Invest. 1997;76:385–397. [PubMed] [Google Scholar]