Abstract
We have sequenced 870 kilobases of the FHIT/FRA3B locus, from FHIT intron 3 to intron 7. The locus is AT rich (61.5%) and Alu poor (6.2%), and it apparently does not harbor other genes. In a detailed analysis of the 308-kilobase region between FHIT exon 5 and the telomeric end of intron 3, a region known to encompass a human papillomavirus-16 integration site and two clusters of aphidicolin-induced chromosome 3p14.2 breakpoints, we have precisely mapped 10 deletion and translocation endpoints in cancer-derived cell lines relative to positions of specific repetitive elements, regions of high genome flexibility and aphidicolin-induced breakpoints. Conclusions are (i) that aphidicolin-induced breakpoint clusters fall close to high-flexibility sequences, suggesting that these sequences contribute directly to aphidicolin-induced fragility; (ii) that 9 of the 10 FHIT allelic deletions in cancer cell lines resulted in loss of exons, with 7 deletion endpoints near long interspersed nuclear elements or long terminal repeat elements; and (iii) that cancer-specific deletions encompass multiple high-flexibility genomic regions, suggesting that fragile breaks may occur at these regions, whereas repair of the breaks involves homologous pairing of flanking sequences with concomitant deletion of the damaged fragile sequence.
Fragile sites are specific chromosomal regions that appear as gaps or breaks on metaphase chromosomes after the exposure of cells to specific reagents. Fragile sites may be inherited or constitutive, and representative fragile sites of both types have been studied at the molecular level (1, 2). FRA3B, FRA7G, and FRA7H, 3 of the 84 common fragile sites listed in the Genome Database have been studied in detail (2). The molecular basis for occurrence of common fragile sites is unknown, although it has been suggested that late replication of these chromosome regions contributes to their fragility (3–5). Because the candidate tumor suppressor gene, FHIT, encompasses the FRA3B and is inactivated by deletion in a large fraction of human cancers, we have been interested in understanding the relationship between fragility and cancer susceptibility. Thus, we have continued our detailed analysis of cancer cell-specific molecular alterations within and near FHIT intron 4, arguably the most fragile 250 kilobases (kb) of the human genome (6).
As a prerequisite for these molecular studies, we have sequenced more than 870 kb of the FHIT/FRA3B locus and, in a previous detailed analysis of FHIT alteration in intron 5, showed that carcinogen-induced damage at fragile sites flanking exon 5 frequently resulted in long interspersed nuclear elements (LINE)-mediated homologous recombination and damage repair with attendant FHIT region loss (7). The previous study did not provide clues to mechanisms underlying fragility or to positions of many of the FRA3B landmarks (7, 8). We have now mapped positions of FRA3B landmarks as well as deletion/translocation breakpoints in cervical, lung, and esophageal cancer cells. Furthermore, to analyze structural characteristics of the DNA sequences that might be associated with fragility, we performed structural analysis by using the computer program flexstab, developed by Mishmar et al. (9); this program measures the flexibility parameter, which is expressed as fluctuation in the twist angle, and was developed for analysis of constitutive fragile site FRA7H. Flexibility plays an important role in protein–DNA interactions and affects chromatin condensation (9, 10); high-flexibility regions were proposed to be susceptible to fragility through lack of completion of DNA replication. Such regions may also be susceptible to chromosomal rearrangements, integration of viral sequences, or hybrid breaks (9).
The goal of our study was to provide further clues to mechanisms underlying fragility at FRA3B and the contribution of FRA3B fragility to cancer susceptibility. Our approach was to determine precise positions of FRA3B landmarks and cancer-specific FHIT deletions/translocations relative to the structural features of the intron 4/intron 3 sequence. Therefore, we determined the positions of LINE1 and other repetitive elements relative to cancer deletion breaks. Previously, we proposed that such repetitive elements participate in homologous recombination-mediated repair of damaged fragile regions (7). Additionally, we determined the frequency of flexible peak clusters and their relation to positions of fragile landmarks and cancer cell deletion endpoints in intron 4.
MATERIALS AND METHODS
DNA Sequencing Templates.
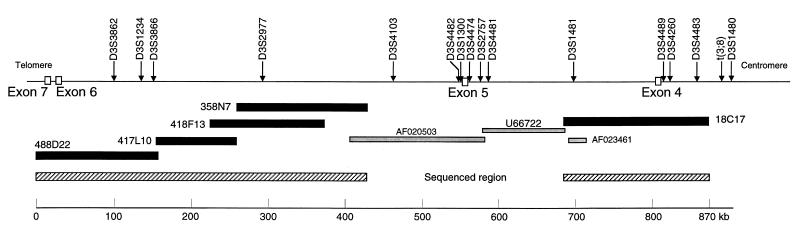
Bacterial artificial chromosome (BAC) clones 18C17 (for intron 4) as well as 358N7, 418F13, 417L10, and 488D22 (for intron 5), overlapping the previously sequenced regions (AF020503 and U66722), were obtained from Research Genetics (Huntsville, AL; CITB B & C Library). Positions of templates within the FHIT gene are shown in Fig. 1.
Figure 1.
The FHIT locus: intron 3 through intron 7. The hatched bar on the right represents the 180-kb sequenced region that covers 120 kb of intron 4 and 60 kb of intron 3 but not the t(3;8) chromosome translocation break. The hatched bar on the left shows the 400-kb sequence that covers 360.9 kb of intron 5, 2.6 kb of intron 6, and 36.5 kb of intron 7. The closed bars represent the five BACs that were sequenced to assemble the complete sequence. AF020503, U66722, and AF023461 are GenBank accession numbers for the previously sequenced portions of FRA3B. The loci shown across the top were placed on the map by sequence homology.
Cell Lines.
The human cancer cell lines Siha and HeLa (cervical carcinoma-derived) were obtained from the American Type Culture Collection, and H460/H211 and H1573 (lung carcinoma-derived) were obtained from John Minna (University of Texas Southwestern Medical Center, Dallas). Previously, we have shown that H460 and H211 are identical genetically (11). The following 27 esophageal carcinoma cell lines were obtained from the Department of Surgery, Medical Institute of Bioregulation, Kyushu University, Beppu, Japan: TE1, TE2, TE3, TE4, TE5, TE6, TE7, TE8, TE9, TE11, TE12, TE13, TE14, TE15, KYSE110, KYSE140, KYSE150, KYSE170, KYSE180, KYSE190, KYSE270, KYSE300, KYSE410, KYSE450, KYSE500, KYSE510, and KYSE700. Alterations of FRA3B/FHIT in esophageal cancer have been reported (12–14). We selected TE8, which has a homozygous deletion in intron 4, to use for determination of deletion endpoints of both alleles. TE8, KYSE170, and KYSE270 showed absence of FHIT transcripts by reverse transcription–PCR with primers UR5 and 06 (15).
Sequencing.
BAC DNA was prepared by Qiagen (Chatsworth, CA) Mini or Maxi prep kit according to the manufacturer’s protocol. BAC DNA libraries for shotgun sequencing were constructed and sequenced as described (7).
The final sequence of introns 4 and 5 was analyzed by using blast, repeatmasker, grailii, genie, genefinder (fgeneh), and genescan programs (16–21).
Deletion Analysis.
For determination of positions of cancer cell-specific homozygous deletions in intron 4, oligonucleotide primer pairs at 20-kb intervals (in repeat-free regions) were prepared for PCR amplification on cellular DNA templates. Positions of deletion endpoints were then narrowed to within several kilobases by using primer pairs at 3-kb intervals. PCRs were carried out as described (7).
Inverse PCR.
We performed inverse PCR according to our previously described method (7), which was a modified version of the original method (22). In brief, 3 μg of source DNA was digested with 50 units of restriction enzymes with 4- to 6-bp recognition sites. After filling in incompatible cohesive ends with T4 DNA polymerase (New England Biolabs), cleaved DNAs were isolated after phenol-chloroform extraction and ethanol precipitation. For higher efficiency self-ligation (circularization), we introduced the following modification: the digested DNA was ligated in a 500-μl reaction volume by using 10 units of T4 DNA ligase (Boehringer Mannheim), 10× ligation buffer [20 mM Tris⋅HCl, pH 7.5/1 mM EDTA/5 mM DTT/60 mM KCl/50% (vol/vol) glycerol], and incubation at 14°C overnight. After ethanol precipitation, the DNA was dissolved in 30 μl of distilled water. The circular products of the ligation reactions were used as templates for PCR amplification as described (7).
Analysis of DNA Helix Flexibility.
Potential local variations in the DNA structure, referred to as DNA flexibility, were measured by using the flexstab program; flexibility, expressed as fluctuations in the twist angle, was originally determined for the FRA7H fragile region by Mishmar et al. (9). We used flexstab, obtained from the web site of Hebrew University of Jerusalem (10), for our analysis of the intron 4 sequence. The overlapping windows were 100 bp, and dinucleotide value was added along the window and averaged by the window length.
Immunohistochemistry.
Expression of Fhit protein in fixed tumor sections was detected by immunohistochemical staining with a polyclonal serum specific for human Fhit as described (23). The lung-cancer sections were kindly provided by Gabriella Sozzi (Istituto Nazionale Tumori, Milan, Italy) and Louise Fong (Thomas Jefferson University, Philadelphia).
RESULTS AND DISCUSSION
Sequence of the FHIT Gene from Intron 3 Through Intron 7.
About 300 kb of the FHIT gene, covering FHIT exon 5 and portions of flanking introns 4 and 5 were sequenced previously (7, 8). We have now sequenced the two contiguous flanking regions to supply a complete, assembled FHIT sequence from mid intron 3 to mid intron 7, a region of 870,169 nucleotides, including 63.7 kb of intron 3, the 243.9-kb intron 4, the 522.8-kb intron 5, the 2.6-kb intron 6, and a 36.5-kb portion of intron 7. The BACs 18C17, 358N7, 418F13, 417L10, and 488D22 were sequenced completely and assembled to obtain the final sequence. Positions of the BACs, previously sequenced regions, and the newly sequenced regions are illustrated in Fig. 1. As also illustrated in Fig. 1, a number of markers that were mapped previously (24–30) in the vicinity or within the FHIT gene have now been placed precisely within the sequenced region. Several markers within intron 3 had been mapped into intron 4 (30). Likewise, marker D3S1234 had been thought to be in intron 8 (15) but is now mapped into intron 5. Two previously identified expressed sequence tags, D3S3866 and D3S3862 (29), flank D3S1234 in intron 5. These expressed sequence tags were noted by Lux et al. (29) to be within the FHIT locus and were suggested as targets of loss in the region. These assembled expressed sequence tags are colinear with the genomic DNA sequence and do not show hallmarks of genes. Wang et al. (30) used primer pairs for many of these sequenced tagged markers to seek homozygous deletions in kidney and pancreatic cancer cell lines. We also tested some of the same kidney (A704, A498, Caki1, Caki2, and ACHN) and pancreatic (CFPACI, SU86, BXPC3, ASPCI, and MIAPACA2) cancer cell lines for markers D3S2977, D3S1300, exon 5, D3S2757, U39804, exon 4, D3S4260, and other markers. We did not observe the homozygous deletions in kidney cancer cell lines reported by Wang et al. (30). We did observe a large homozygous deletion in pancreatic cancer cell line BXPC3, although the deletion included exon 5 as reported by others (31, 32), whereas Wang et al. (30) reported retention of exon 5. It seems unlikely that variation in cell lines is an explanation for these differences.
The new 180-kb sequence surrounding exon 4 was extensively analyzed for repetitive elements and regions with coding potential. No apparent CpG island was observed in this region, nor were other hallmarks of coding regions. As shown in Table 1, the sequence of the first 120 kb of intron 4 was reported previously (accession nos. AF020503 and U66722). The 120- to 308-kb region represents the newly sequenced region. Our numbering of the intron 4 sequences begins with the last nucleotide of exon 5 serving as nucleotide 1 for our illustrations and discussion of the intron 4 and intron 3 sequence. LINE1 sequences are notably more frequent in the 120- to 180-kb region (36.3%) than in other regions. The total LINE1 content is higher in intron 4 (16.5%) than in the previously sequenced region (15.1%) flanking exon 5. LTR/retroviral elements are most frequent (5.7%) in the 180- to 240-kb region, especially in clusters around exon 4. No CGG/CCG or other expanded repeats were identified in intron 4. Thus, as suggested by previous data, the molecular basis for fragility of FRA3B is different from that of the rare fragile sites (7, 8). There must be other components or structures that contribute to fragility.
Table 1.
Comparison of frequency of repetitive elements
| Results |
AF020503 and U66722
|
BAC 18C17
|
Intron 4 (and 3) (total 308 kb) | Intron 5 (total 150 kb) | Intron 5–7 (total 411 kb) | |||
|---|---|---|---|---|---|---|---|---|
| 0–60 | 60–120 | 120–180 | 180–240 | 240–308 | ||||
| SINEs | ||||||||
| Alus | 2,640 (4.4)* | 3,893 (6.5) | 3,748 (6.2) | 2,540 (4.2) | 5,309 (7.8) | 18,130 (5.9) | 8,370 (5.6) | 27,855 (6.8) |
| MIRs | 1,440 (2.4) | 1,216 (2.0) | 776 (1.3) | 1,734 (2.9) | 1,585 (2.3) | 6,751 (2.2) | 4,630 (3.1) | 11,800 (2.9) |
| LINEs: | ||||||||
| L1 | 9,056 (15.1) | 6,937 (11.6) | 21,806 (36.3) | 3,313 (5.5) | 9,956 (14.6) | 51,068 (16.6) | 22,679 (15.1) | 27,458 (6.7) |
| L2 | 1,227 (2.0) | 1,002 (1.7) | 70 (0.1) | 3,362 (5.6) | 2,423 (3.5) | 8,084 (2.6) | 2,078 (1.4) | 15,913 (3.9) |
| LTR elements | ||||||||
| MaLRs | 0 (0) | 1,260 (2.1) | 1,632 (2.7) | 369 (0.6) | 2,738 (4.0) | 5,999 (2.0) | 3,173 (2.1) | 17,174 (4.2) |
| Retroviral | 207 (0.3) | 457 (0.8) | 1,181 (2.0) | 3,434 (5.7) | 638 (0.9) | 5,917 (1.9) | 1,083 (0.7) | 3,248 (0.8) |
| DNA elements | ||||||||
| MER1 | 1,442 (2.4) | 621 (1.0) | 1,625 (2.7) | 890 (1.4) | 875 (1.3) | 5,453 (1.8) | 2,451 (1.6) | 8,048 (2.0) |
| MER2 | 2,845 (4.7) | 0 (0) | 3,181 (5.3) | 960 (1.6) | 641 (0.9) | 7,627 (2.5) | 2,779 (1.9) | 3,060 (0.7) |
| Mariners | 0 (0) | 66 (0.1) | 79 (0.1) | 0 (0) | 0 (0) | 145 (0) | 1,368 (0.9) | 91 (0) |
| Unclassified | 779 (1.3) | 0 (0) | 526 (0.9) | 763 (1.3) | 525 (0.8) | 2,593 (0.8) | 1,028 (0.7) | 0 (0) |
| Total repeats | 20,290 (33.8) | 15,757 (26.3) | 35,013 (58.4) | 20,022 (33.4) | 29,032 (42.4) | 120,114 (38.9) | 49,986 (33.3) | 128,170 (31.2) |
| GC content, % | 38.9 | 38.9 | 36.8 | 40.3 | 38.2 | 38.6 | 38.3 | 38.6 |
Data are shown as total and percentage of (in parentheses) nucleotides. The sequence of the 0- to 120-kb region of intron 4 was reported previously (accession nos. AF020503 and U66722). The 120- to 308-kb region represents the newly sequenced region. Alu sequences are most frequent in the 240- to 308-kb region (7.8%). LINE1 sequences are notably more frequent in the 120- to 180-kb region (36.3%) than in other regions. The total LINE1 content is higher in intron 4 (16.6%) than in the previously sequenced region of intron 5 (15.1%). Long terminal repeat (LTR/retroviral) elements are most frequent (5.7%) in the 180- to 240-kb region, especially in clusters around exon 4. MIR, mammalian-wide interspersed repeat; SINE, short interspersed nuclear elements; MaLR, mammalian LTR retrosequences; MER, medium reiteration frequency element.
Landmarks and Sequence Flexibility.
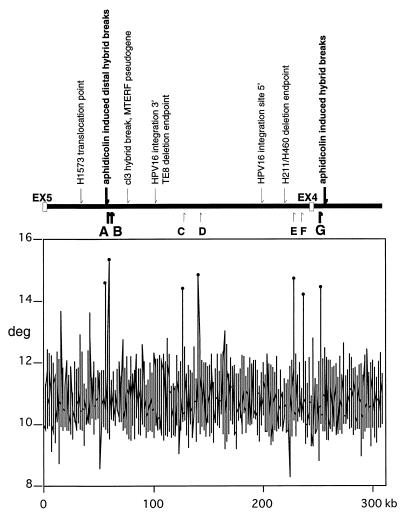
Several groups have published sequence for regions of FRA3B/FHIT, because it was a site for human papillomavirus-16 (HPV16) integration (24, 25), aphidicolin-induced hybrid breaks (26, 27), or cancer cell line deletion endpoints (7, 8). We are now able to pinpoint the location of these breaks and insertions relative to nearby repeats and regions of high flexibility. As illustrated in Fig. 2, seven spikes, A through G, of high flexibility were observed in FHIT intron 4; those peaks are at 55.4, 58.7, 126.3, 140.8, 228.0, 235.6, and 251.7 kb from exon 5 and vary from 14.2 to 15.4 degrees of flexibility. Peaks A and B are 0.35 kb and 3.7 kb centromeric of the distal aphidicolin-induced hybrid breakpoints; peak G is about 7 kb telomeric of the proximal aphidicolin-induced hybrid break cluster in intron 3 (see Fig. 2 and Table 2). The spontaneous cl3 hybrid break and the MTERF pseudogene are 21 kb from peak B. The flanking sequences of the HPV16 DNA cervical cancer integration site sequences (M33614 and M33613) are more than 20 kb from peaks C and E, respectively. Two lung cancer cell line deletion endpoints in intron 4, H1573 at 26.7 kb and H460/H211 at 222 kb, are 29 kb and 6 kb, respectively, from the nearest peaks.
Figure 2.
Relationship between fragile-region landmarks and flexible regions. (Upper) Previously identified landmarks are indicated above the line representing the FHIT intron 4 locus. Small arrows indicate nucleotide positions of landmarks in intron 4 and intron 3. They designate sequences of a lung cancer cell line translocation point (H1573) at 26.7 kb; a spontaneous hybrid break and the MTERF pseudogene at 79 kb; HPV16 integration site endpoints at 101 and 198 kb; and a deletion endpoint of lung carcinoma cell line (H460/H211) at 222 kb. Bold arrows indicate the distal and proximal aphidicolin-induced hybrid breaks at positions 55 and 257 kb. Helix flexibility was evaluated by using a previously developed computer program (flexstab). This program measures the flexibility parameter, which is expressed as fluctuation in the twist angle. Regions of helix flexibility may be fragile points within the FRA3B, which includes introns 4 and 5. (Lower) The vertical axis shows degrees of inclination in the twist angle, and the horizontal axis indicates nucleotide position of each 100-bp window. The peak values relative to the average were considered as potential flexible regions and drawn as spikes. Positions of the seven spikes, A to G, are shown by bold and small arrows below the locus. The thick arrows representing spikes A, B, and G emphasize the observation that the aphidicolin-induced distal and proximal hybrid breaks are located close to flexibility spikes (0.35 kb from spike A and 7.1–7.6 kb from spike G, respectively).
Table 2.
Characterization of intron 4 regions of high flexibility
| Peak | Distance from exon 5, kb | Hybrid breaks | Distance from peak, kb | L1 and LTR (size in kb) | Distance from peak, kb |
|---|---|---|---|---|---|
| A | 55.4 | Aphidicolin-induced | 0.35 | L1 (0.3)/t | 2.5 |
| distal hybrid breaks/t | |||||
| B | 58.7 | cl3 hybrid break/c | 21 | ||
| C | 126.3 | Within L1 (0.7) | |||
| L1 (1.7)/t | 0.4 | ||||
| L1 (1.4)/c | |||||
| D | 140.8 | Within L1 (0.5) | |||
| L1 (0.4)/t | 0.1 | ||||
| L1 (1.5)/t | 2.4 | ||||
| E | 228 | LTR (0.5)/t | 6.1 | ||
| LTR (0.4)/t | 1.5 | ||||
| LTR (0.5)/c | 4.3 | ||||
| F | 235.6 | LTR (0.6)/t | 2.6 | ||
| LTR (0.5)/c | 0.6 | ||||
| G | 251.7 | Aphidicolin-induced | 7.1–7.6 | LTR (0.2)/t | 4 |
| proximal hybridbreaks/c | LTR (0.1)/t | 3.5 |
Positions of landmark hybrid breaks, cancer breaks, and repetitive sequences (LINE1 and LTR) near the seven flexibility peaks are listed. Sequences of peaks C and G showed 90% homology with each other over 40 bp. Aphidicolin-induced distal and proximal hybrid breaks (underlined) are close to peak A (0.35 kb) and not far from peak G (7.1–7.6 kb), respectively. /t, telomeric; /c, centromeric of indicated flexible peak.
Mishmar et al. (9) previously analyzed the flexibility of a portion of intron 5. We have also used their program to analyze flexibility of all of intron 5 and have observed that the pSV2neo integration flanking site (accession no. HSU6118; ref. 28) is within 1.8 kb of a peak with 13.1 degrees of flexibility (data not shown).
The aphidicolin-induced hybrid breaks and pSV2neo integration sites have been considered definitive markers of the FRA3B fragile region, and thus, their positions near the region of high genome flexibility suggest that such flexibility does indeed contribute to fragility and to the recombinogenic activity of this highly active common fragile region. The spontaneous hybrid break in hybrid cl3 is not at a flexible peak, and neither are cancer breaks and HPV integration sites. Thus, it may be that different carcinogenic agents break the region in different sequences or that after repair of fragile gaps, we cannot determine, in most cases, the sequence where the original break occurred.
Cancer Cell Deletion Endpoints.
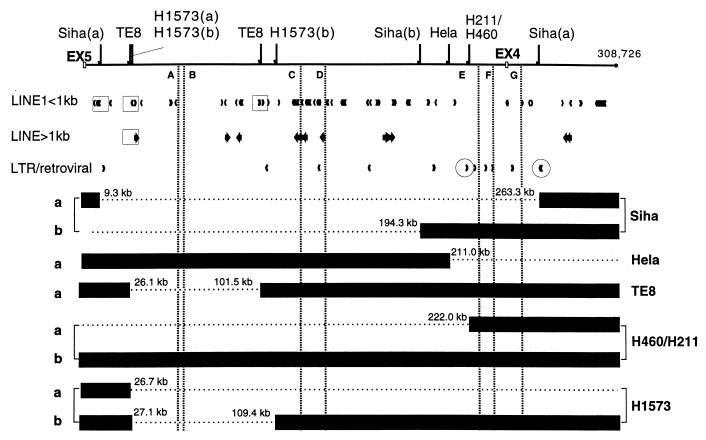
Esophageal cancer cell lines were examined for deletions, and six (TE8, KYSE170, KYSE190, KYSE270, KYSE410, and KYSE450) showed homozygous deletions in introns 4 and 5. We selected TE8 with a homozygous deletion in intron 4 for detailed analysis of deletion endpoints. Two cervical cancer cell lines, Siha and HeLa, and two lung cancer cell lines, H460/H211 and H1573, were also analyzed for homozygous deletions in intron 4. We sequenced and mapped positions of 10 breakpoints of these five cancer cell lines, and results are illustrated in Fig. 3.
Figure 3.
Fragile region topography near cancer cell breakpoints. Depiction of 10 deletion endpoints relative to L1 elements (L1 sequences of >1 kb, bold arrows; L1 sequences of <1 kb, small arrowheads) and LTR/retroviral elements. The four squares enclosing L1 elements emphasize that the TE8 breakpoints, the telomeric Siha breakpoint, the translocation point, and the telomeric breakpoint of H1573 are located within or near L1 elements. The two circles enclosing LTR arrowheads stress that the H460/H211 breakpoints and the centromeric breakpoint of Siha allele a are near LTR/retroviral elements. The dashed vertical lines represent the flexibility spikes A–G (shown in Fig. 2) and show the position of flexibility/fragility spikes relative to cancer cell deletions. For the HeLa and TE8 cell lines, the exact structure of the b alleles in this region is not yet known.
In Siha cells, independent deletions of 254 kb and 213.1 kb occurred on the two alleles at positions 9.3–263.3 kb for allele a and 18.8 kb telomeric to the splice donor site of intron 5 to 194.3 kb for allele b, with a 185-kb overlapping region of homozygous deletion at 9.3–194.3 kb. In HeLa cells, one breakpoint for allele a was observed at position 211 kb. In TE8 cells, two breakpoints were found at positions 26.1 kb and 101.5 kb for allele a, which was observed as a homozygous deletion. In H460/H211 cells, deletion of 238 kb was observed from 16.4 kb telomeric of the splice donor site of intron 5 to 222 kb for allele a. In H1573 cells, independent deletions were observed at positions 26.7 kb for allele a and 27.1–109.4 kb for allele b, with a 82.3-kb overlapping region of deletion at 27.1–109.4 kb.
Cancer Breakpoints and Repetitive Sequences.
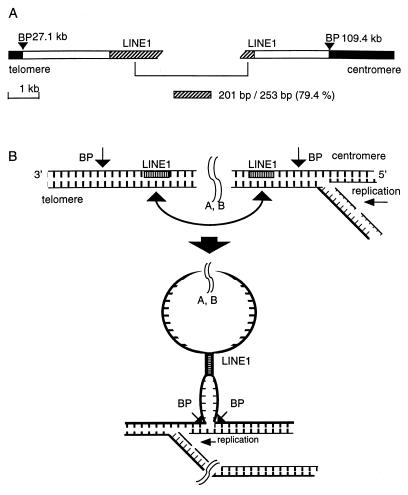
As detailed in Table 2, the deletion/translocation breakpoints in the five cancer cell lines are not positioned near flexibility peaks as were the aphidicolin-induced hybrid breaks. Thus, for the cancer cell line deletion endpoints, we looked for nearby repetitive sequences that might have played a role in repair of carcinogen-induced damage to FHIT/FRA3B. A recent study reported an important role for LINE1 in repair of deleted regions by nonhomologous recombination or unequal homologous recombination (33), and previously, we have shown that 16 of 21 cancer cell deletion endpoints were in or near LINE1 elements (7). In TE8 cells, both deletion endpoints on allele a were close to L1 elements (Fig. 3). In Siha allele a, the deletion endpoint positioned at 9.3 kb was located near an L1 element. The two breakpoints of H1573 allele b were located near or within L1 elements. The H460/H211 allele a endpoint and the centromeric breakpoint of Siha allele a were near LTR/retroviral elements, as summarized in Fig. 3. H1573 allele b showed high sequence homology (79.4%) of LINE1 elements near both breakpoints (Fig. 4A). Similarly, in the TE8 allele a, LINE1 sequences near the two breakpoints were 79.5% homologous with each other. In Siha allele a, the telomeric breakpoint was located near LINE1 sequences, and two homologous unique sequences were observed near the two deletion endpoints. Thus, 6 of 10 deletion endpoints were near L1 sequences. Siha allele b showed 79.4% homologous unique sequences within Alu repeats near both breakpoints. We have shown a simple model (Fig. 4B) suggesting how homologous regions might contribute to deletion/repair. Both H1573 alleles and a TE8 allele show one very similar deletion endpoint within 1.0 kb of each other near a LINE1 (>1 kb) element at 26–27 kb. Interestingly, deletion endpoints near this same L1 element have been reported for three deleted cancer alleles, two in colon cancer and one in a nasopharyngeal cancer cell line (7), reinforcing the role of L1 homology in recombination at these loci. Thus, there are very frequently homologous sequences near deletion endpoints in rearranged FHIT alleles of cancer cells; LINE1 elements are frequently but not invariably involved.
Figure 4.
Model for the role of homologous sequence elements in repair of double-strand breaks within the fragile region. Carcinogen-induced damage in the fragile region may cause double- or single-strand breaks at flexibility peaks, necessitating repair during replication. (A) The H1573 allele b is shown with black bars representing sequences retained in the cell, BP representing positions of deletion endpoints, and hatched regions representing the 79.4% homologous LINE1 elements near the breakpoints. (B) This simple model suggests how the homologous LINE1 elements might participate in a repair mechanism that would allow replication of the deleted H1573b allele.
As summarized in Table 2, the nucleotide positions of deletion endpoints were not located near peaks of high flexibility. Only the aphidicolin-induced fragile region landmarks, such as the hybrid breaks and pSVneo integration sites (not shown), mapped at flexibility peaks. If flexibility peaks truly represent fragile sites, each of the other breaks mapped may represent the loci after the damage to the fragile sites has been repaired. Thus, it may be that how the locus is repaired and what the homologous region uses in repair depends on where the original damage to the fragile locus occurs. For example, if the sequence at a flexible peak is damaged by carcinogen metabolites, the type of repair may depend on the types of repeats flanking the damaged peak.
In addition, it is possible that the specific fragile sites/peaks most susceptible to damage may depend on tissue type and the inducing carcinogen. Primer sequences flanking the flexible peaks and other fragile region landmarks may be used to look for polymorphisms across the fragile region in the human population and to seek correlations of specific polymorphism classes with cancer susceptibility.
Effect of Deletions on Expression of Fhit.
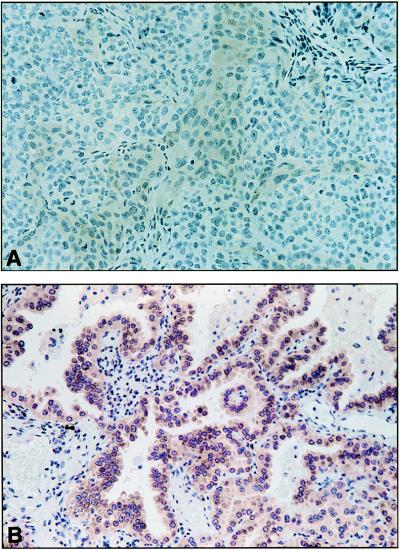
The H460/H211 cells show a homozygous deletion within FHIT intron 5 (not shown), but that homozygous deletion represents the overlap region of independent deletions of the two alleles. Allele b retains all of the region from intron 3 through exon 5 shown in Fig. 3 but is missing exons 6–8 as described (11). Allele a, as shown in Fig. 3, is missing exon 5 and most of intron 4; thus, these cells cannot produce Fhit protein. However, allele a of Siha and allele b of H1573 retain exons 5–9 of FHIT (not shown) but express very little Fhit protein (see Fig. 5, for example), suggesting that alterations within FHIT introns can affect the level of protein expression, possibly through an effect on mRNA stability or efficiency of processing.
Figure 5.
Absence of Fhit protein in H1573 lung cancer cells. The H1573 lung cancer cell line allele a has a translocation between chromosome 3 and 4 (11) at 26.7 kb centromeric to exon 5 so that exons 5–9 are present (with a homozygous deletion within intron 5, ref. 11). Allele b, as shown in Fig. 3, has sustained a deletion within intron 4 (and another in intron 5, ref. 11). Thus, none of the protein-coding exons have been lost through deletion; however, the H1573 cells make very little Fhit protein. (A) Immunostaining with Fhit-specific antiserum of a section of a nude mouse H1573 tumor. (B) Immunostaining (the brown chromagen) of a rare human lung cancer that is positive for Fhit expression.
Acknowledgments
This work was supported by Outstanding Investigator Grant CA39860 and U.S. Public Health Service Grant CA21124 from the National Cancer Institute.
ABBREVIATIONS
- kb
kilobase
- BAC
bacterial artificial chromosome
- LTR
long terminal repeat
- HPV
human papillomavirus
- LINE
long interspersed nuclear elements
Footnotes
References
- 1.Sinden R R. Am J Hum Genet. 1999;64:346–353. doi: 10.1086/302271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutherland G R, Richards R I. Am J Hum Genet. 1999;64:354–359. doi: 10.1086/302267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selig S, Okumura K, Ward D C, Cedar H. EMBO J. 1992;11:1217–1225. doi: 10.1002/j.1460-2075.1992.tb05162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Beau M M, Rassool F V, Neilly M E, Espinosa R, III, Glover T W, Smith D I, McKeithan T W. Hum Mol Genet. 1998;7:755–761. doi: 10.1093/hmg/7.4.755. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Darling J, Zhang J-S, Huang H, Liu W, Smith D I. Hum Mol Genet. 1999;8:431–437. doi: 10.1093/hmg/8.3.431. [DOI] [PubMed] [Google Scholar]
- 6.Glover T, Stein C. Am J Hum Genet. 1988;43:265–273. [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue H, Ishii H, Alder H, Snyder E, Druck T, Huebner K, Croce C M. Proc Natl Acad Sci USA. 1997;94:14584–14589. doi: 10.1073/pnas.94.26.14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boldog F, Gemmill R M, West J, Robinson M, Robinson L, Li E, Roche J, Todd S, Waggoner B, Lundstrom R, et al. Hum Mol Genet. 1997;6:193–203. doi: 10.1093/hmg/6.2.193. [DOI] [PubMed] [Google Scholar]
- 9.Mishmar D, Rahat A, Scherer S W, Nyakatura G, Hinzman B, Kohwi Y, Mandel-Gutfroind Y, Lee J R, Drescher B, Sas D E, et al. Proc Natl Acad Sci USA. 1998;95:8141–8146. doi: 10.1073/pnas.95.14.8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishmar D, Mandel-Gutfreund Y, Margalit H, Rahat A, Kerem B. Am J Hum Genet. 1999;64:908–910. doi: 10.1086/302299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Druck T, Berk L, Huebner K. Oncology Res. 1998;10:341–345. [PubMed] [Google Scholar]
- 12.Ohta M, Inoue H, Cotticelli M G, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, et al. Cell. 1996;84:587–597. doi: 10.1016/s0092-8674(00)81034-x. [DOI] [PubMed] [Google Scholar]
- 13.Michael D, Beer D G, Wilke C W, Miller D E, Glover T W. Oncogene. 1997;15:1653–1659. doi: 10.1038/sj.onc.1201330. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka H, Shimada Y, Harada H, Shinoda M, Hatooka S, Imamura M, Ishizaki K. Cancer Res. 1998;58:3429–3434. [PubMed] [Google Scholar]
- 15.Druck T, Hadaczek P, Fu T B, Ohta M, Siprashvili Z, Baffa R, Negrini M, Kastury M, Kastury K, Veronese M L, et al. Cancer Res. 1997;57:504–512. [PubMed] [Google Scholar]
- 16.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 17.Smit A F. Curr Opin Genet Dev. 1996;6:743–748. doi: 10.1016/s0959-437x(96)80030-x. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Einstein J R, Mural R J, Shah M, Uberbacher E C. Ismb. 1994;2:376–384. [PubMed] [Google Scholar]
- 19.Kulp D, Haussler D, Reese M G, Eeckman F H. Ismb. 1996;4:134–142. [PubMed] [Google Scholar]
- 20.Solovyev V V, Salamov A A, Lawrence C B. Nucleic Acids Res. 1994;22:5156–5163. doi: 10.1093/nar/22.24.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burge C, Karlin S. J Mol Biol. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- 22.Ochman H, Gerber A S, Hartl D L. Genetics. 1988;120:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadaczek P, Siprashvili Z, Markiewski M, Domagala W, Druck T, McCue P A, Pekarsky Y, Ohta M, Huebner K, Lubinski J. Cancer Res. 1998;58:2946–2951. [PubMed] [Google Scholar]
- 24.Wilke C M, Hall B K, Hoge A, Paradee W, Smith D I, Glover T W. Hum Mol Genet. 1996;5:187–195. doi: 10.1093/hmg/5.2.187. [DOI] [PubMed] [Google Scholar]
- 25.Wagatsuma M, Hashimoto K, Matsukura T. J Virol. 1995;64:813–821. doi: 10.1128/jvi.64.2.813-821.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paradee W, Wilke C M, Wang L, Shridhar R, Mullins C M, Hoge A, Glover T W, Smith D I. Genomics. 1996;35:87–93. doi: 10.1006/geno.1996.0326. [DOI] [PubMed] [Google Scholar]
- 27.Wang K, Paradee W, Mullins C, Shridhar R, Rosati R, Wilke C M, Glover T W, Smith D I. Genomics. 1997;41:485–488. doi: 10.1006/geno.1997.4690. [DOI] [PubMed] [Google Scholar]
- 28.Rassool F W, LeBeau M M, Shen M-L, Neilly M E, Espinosa R, III, Ong S T, Boldog F, Drabkin H, McCarroll R, McKeithan T W. Genomics. 1996;35:109–117. doi: 10.1006/geno.1996.0329. [DOI] [PubMed] [Google Scholar]
- 29.Lux A, Bardenheuer W, Michael D, Brocker F, Julicher K, Vieten L, Michaelis S, Seeber S, Opalka B, Schutte J. Hum Genet. 1997;100:90–95. doi: 10.1007/s004390050471. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Darling J, Zhang J-S, Qian C-P, Hartmann L, Conover C, Jenkins R, Smith D I. Oncogene. 1998;16:635–642. doi: 10.1038/sj.onc.1201576. [DOI] [PubMed] [Google Scholar]
- 31.Simon B, Bartsch D, Prasnika N, Munch K, Blum A, Arnold R, Goke B. Cancer Res. 1998;58:1538–1587. [PubMed] [Google Scholar]
- 32.Sorio C, Baron A, Orlandini S, Zamboni G, Pederzoli P, Huebner K, Scarpa A. Cancer Res. 1999;59:1308–1314. [PubMed] [Google Scholar]
- 33.Segal Y, Peissel B, Renieri A, Marchi M D, Ballabio A, Pei Y, Zhou J. Am J Hum Genet. 1999;64:62–69. doi: 10.1086/302213. [DOI] [PMC free article] [PubMed] [Google Scholar]