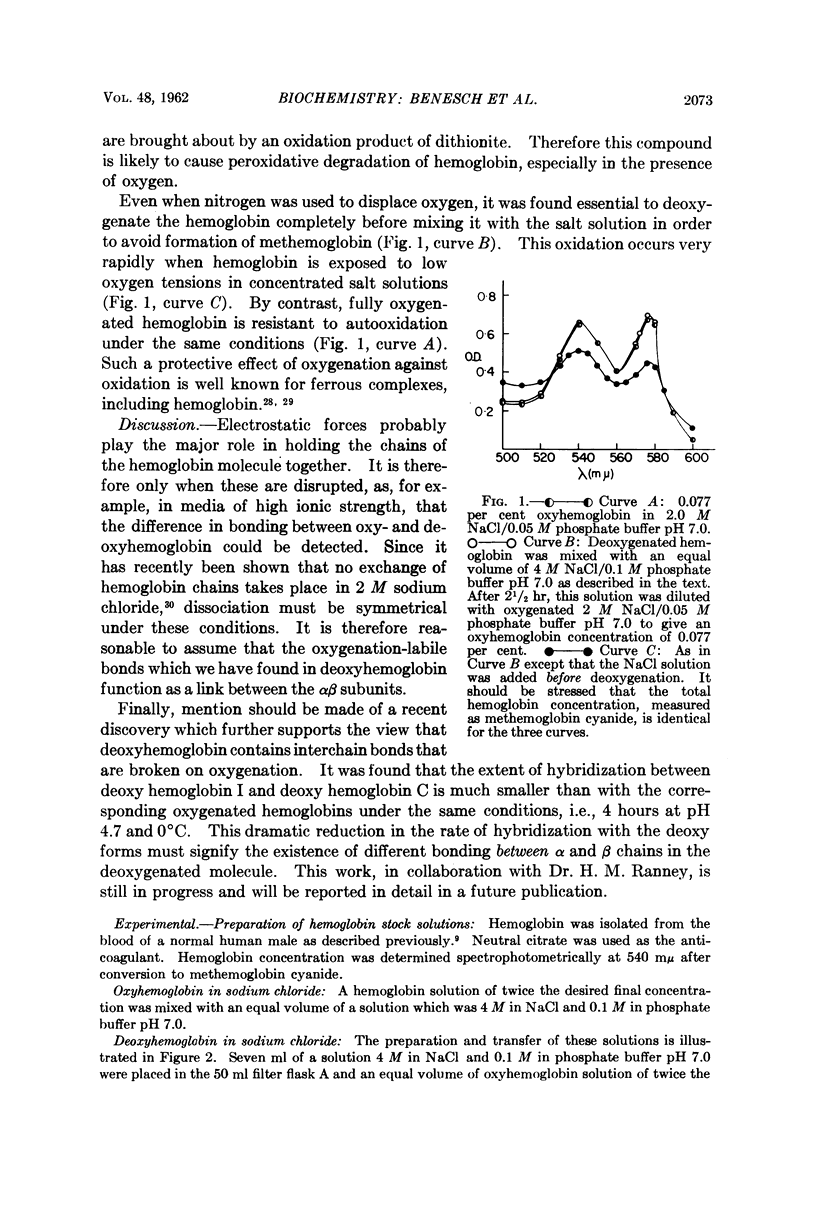
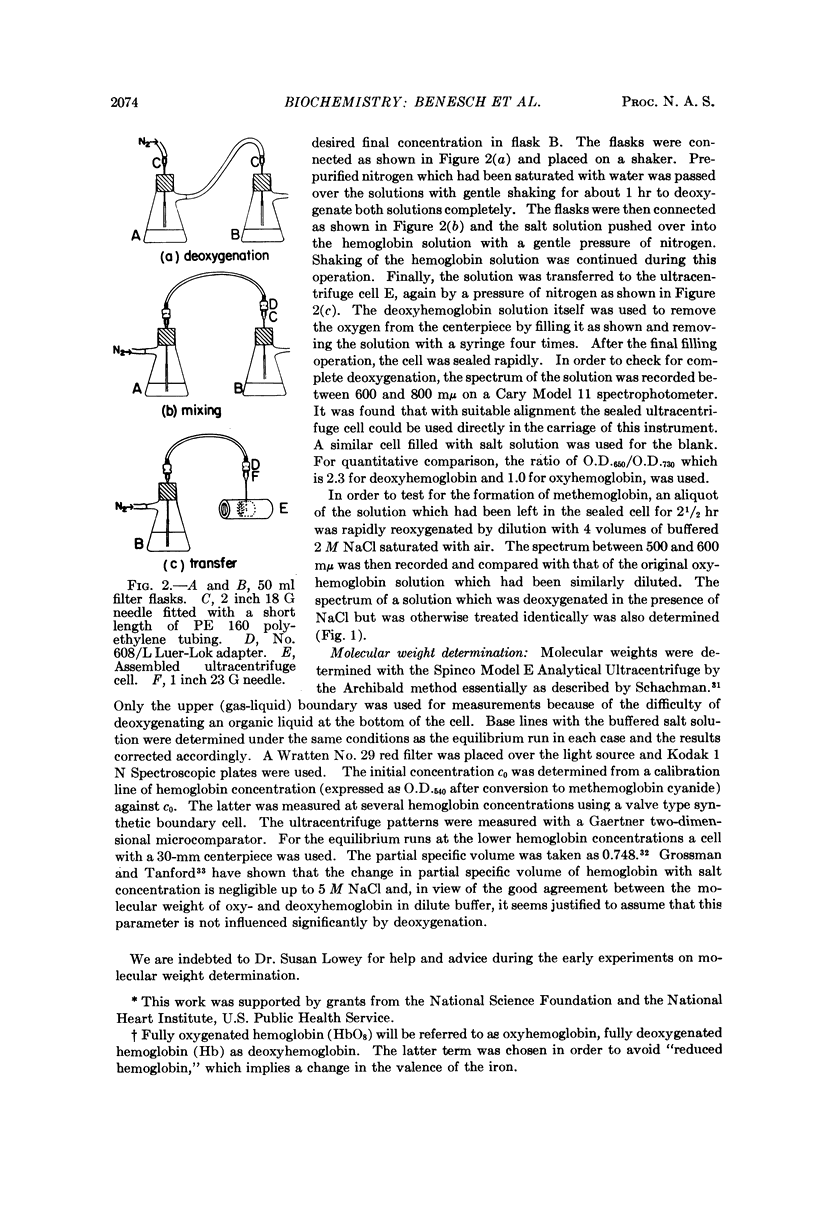
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONINI E., WYMAN J., BUCCI E., FRONTICELLI C., ROSSI-FANELLI A. The dissociation and recombination of subunits of human, horse, and canine hemoglobin. J Mol Biol. 1962 May;4:368–375. doi: 10.1016/s0022-2836(62)80017-5. [DOI] [PubMed] [Google Scholar]
- BENESCH R. E., BENESCH R. The influence of oxygenation on the reactivity of the--SH groups of hemoglobin. Biochemistry. 1962 Sep;1:735–738. doi: 10.1021/bi00911a002. [DOI] [PubMed] [Google Scholar]
- DALZIEL K., O'BRIEN J. R. Side reactions in the deoxygenation of dilute oxyhaemoglobin solutions by sodium dithionite. Biochem J. 1957 Sep;67(1):119–124. [PMC free article] [PubMed] [Google Scholar]
- FIELD E. O., O'BRIEN J. R. Dissociation of human haemoglobin at low pH. Biochem J. 1955 Aug;60(4):656–661. doi: 10.1042/bj0600656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIELD E. O., OGSTON A. G. Boundary spreading in the migration of a solute in rapid dissociation equilibrium; theory and its application to the case of human haemoglobin. Biochem J. 1955 Aug;60(4):661–665. doi: 10.1042/bj0600661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUISMAN T. H., DOZY A. M. Studies on the heterogeneity of hemoglobin. V. Binding of hemoglobin with oxidized glutathione. J Lab Clin Med. 1962 Aug;60:302–319. [PubMed] [Google Scholar]
- KENDREW J. C., BODO G., DINTZIS H. M., PARRISH R. G., WYCKOFF H., PHILLIPS D. C. A three-dimensional model of the myoglobin molecule obtained by x-ray analysis. Nature. 1958 Mar 8;181(4610):662–666. doi: 10.1038/181662a0. [DOI] [PubMed] [Google Scholar]
- PERUTZ M. F., MITCHISON J. M. State of haemoglobin in sickle-cell anaemia. Nature. 1950 Oct 21;166(4225):677–679. doi: 10.1038/166677a0. [DOI] [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E., CAPUTO A. Studies on the relations between molecular and functional properties of hemoglobin. I. The effect of salts on the molecular weight of human hemoglobin. J Biol Chem. 1961 Feb;236:391–396. [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E., CAPUTO A. Studies on the relations between molecular and functional properties of hemoglobin. II. The effect of salts on the oxygen equilibrium of human hemoglobin. J Biol Chem. 1961 Feb;236:397–400. [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E., CAPUTO A. The oxygen equilibrium of reconstituted hemoglobins. III. Human mesohemoglobin. Arch Biochem Biophys. 1959 Nov;85:37–42. doi: 10.1016/0003-9861(59)90444-8. [DOI] [PubMed] [Google Scholar]
- SCOULOUDI H. The myoglobin molecule. Nature. 1959 Feb 7;183(4658):374–376. doi: 10.1038/183374a0. [DOI] [PubMed] [Google Scholar]
- YAGI K., OZAWA T. Complex formation of apo-enzyme, coenzyme and substrate of D-amino acid oxidase. IV. Changes in physico-chemical properties of the protein. Biochim Biophys Acta. 1962 Aug 13;62:397–401. doi: 10.1016/0006-3002(62)90269-x. [DOI] [PubMed] [Google Scholar]



