During the next 3 years, a demographic milestone will be reached across North America as baby boomers, who are currently in their late 50s, begin to reach 65 years of age. The proportion of the Canadian population aged 65 or older will rise from 11.6% in 1991 to 16% in 2016 and will reach 23% in 2041. As this demographic shift occurs, the health problems affecting elderly people will take centre stage as never before. The prevalence of dementia (both general types of dementia and Alzheimer disease) dramatically increases with age. The prevalence of Alzheimer disease is 1% among people aged 65–74 years, 6.9% among those 75–84 years and 26% among those aged 85 years and older.1 Although the evidence is dated, it is expected that in the next decade there will be a large increase in the number of patients who present with memory loss and dementia in Canada (Figure 1) and the United States. In Canada, there are 60 150 new cases of dementia each year,2 and there are currently about 450 000 people with all forms of dementia. This figure is expected to double over the next 30 years.1,3 The increase in vascular dementia will be less dramatic compared with all types of dementia (because of improved hypertension control). The prevalence of Alzheimer disease is expected to increase sharply unless dramatic new preventative therapies emerge.
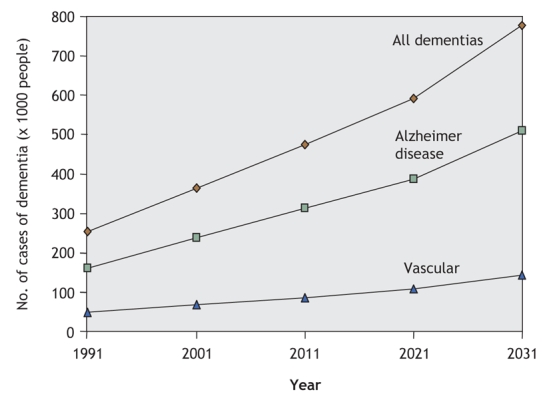
Figure 1: Projected prevalence of all dementias, Alzheimer disease and vascular dementia in Canada.2,3
The dementias are a family of diseases defined in clinical terms (Box 1.) Our understanding of the dementia subcategories continues to evolve as the fields of imaging and neuropathology supply new levels of detail. Forty years ago, dementias were classified as cortical or subcortical.5 Such localization approaches are highly inaccurate but serve to stress the difference between dementias without motor features (e.g., Alzheimer disease, frontotemporal dementias) and most other dementias (characterized by the presence of accompanying motor and gait abnormalities). Figure 2 shows the types of dementia commonly seen in Canadian dementia clinics.6 In this type of classification, mixed dementia (usually Alzheimer disease and vascular dementia) is common. Individuals with mixed dementias have 2 separate brain conditions (degenerative disease and vascular insults) that are synergistic in producing clinical symptoms of dementia at an earlier age than either alone.7
Box 1.
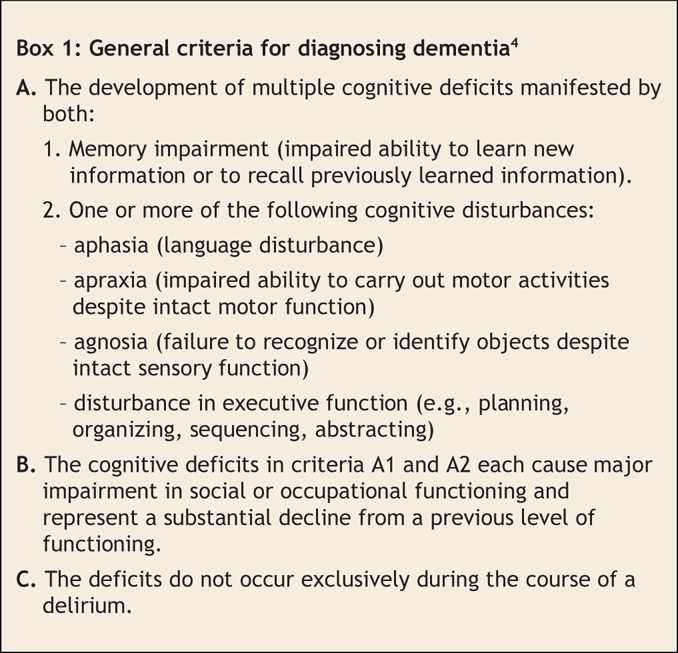
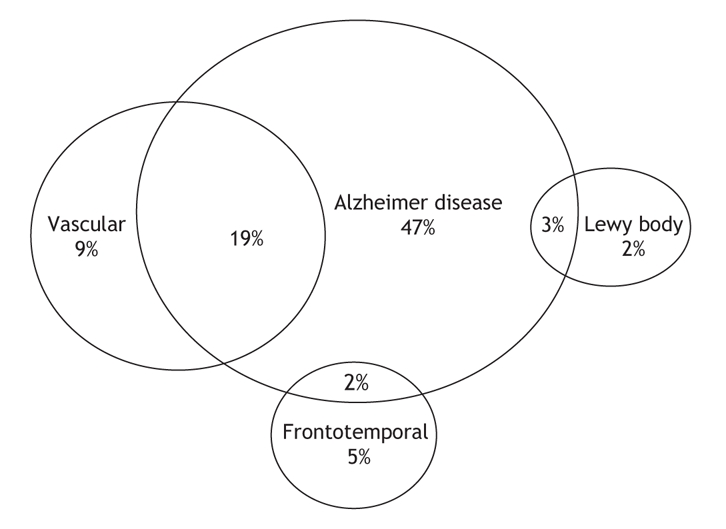
Figure 2: Types of dementia commonly seen in Canadian memory clinics.6 Note: Other mixed types of dementia make up 10% of the total number of cases.
Alzheimer disease is not only devastating to both patients and affected families but also is a considerable financial burden on society, particularly as the disease progresses. It is estimated that the annual economic burden is Can$10 000 for each person with mild Alzheimer disease and $37 000 for severe Alzheimer disease.8 For every person with dementia who does not live in a long-term care facility, there is also the burden of caregiving, which usually falls on the patient's spouse or children. Supporting caregivers and managing and treating their stress will also be increasing problems for Canadian physicians.
New developments in dementia
Following publication of the recommendations from the Second Canadian Consensus Conference on Dementia in 1999,9,10 there have been developments in many fields, including biology and genetics, risk assessment, and management. There have been substantial improvements in neuroimaging, such as magnetic resonance imaging (MRI) hippocampal volume measures,11 and positron emission tomography (PET) scanning with flourodeoxyglucose and amyloid ligands.12 Other developments include the recognition of the potential role of homocysteine in the pathogenesis of Alzheimer disease13 and the discovery of new genes associated with sporadic, late-onset Alzheimer disease14 and a form of frontotemporal dementia.15,16 The recognition that vascular risk factors (e.g., hypertension, hyperlipidemia) increase the likelihood of Alzheimer disease and vascular dementia offers enhanced opportunities for prevention of dementia. These recent advances raise questions about the optimal approach for diagnosing dementia.
In addition to improvements in diagnosing dementia, we have also made progress in its management. New types of nonpharmacologic treatments have been developed and studied, despite the inherent difficulties of research in this area. Guidelines on dementia and driving have been introduced. Cholinesterase inhibitors have been widely adopted into practice, although their usefulness continues to be debated. Memantine (Ebixa) has been introduced in Canada. This antagonist to glutamate N-methyl-D-aspartate (NMDA) receptors has a small beneficial effect in cases of moderate to severe Alzheimer disease, but it has not been shown to be effective in the mild stages.17 Many physicians remain uncertain about its place in Alzheimer disease therapy. The use of vitamin E as a therapeutic agent received dissenting support at the second consensus conference; however, its routine use was not recommended in the published guidelines. Since then, vitamin E has come under attack as a therapeutic intervention.
The Third Canadian Consensus Conference on the Diagnosis and Treatment of Dementia
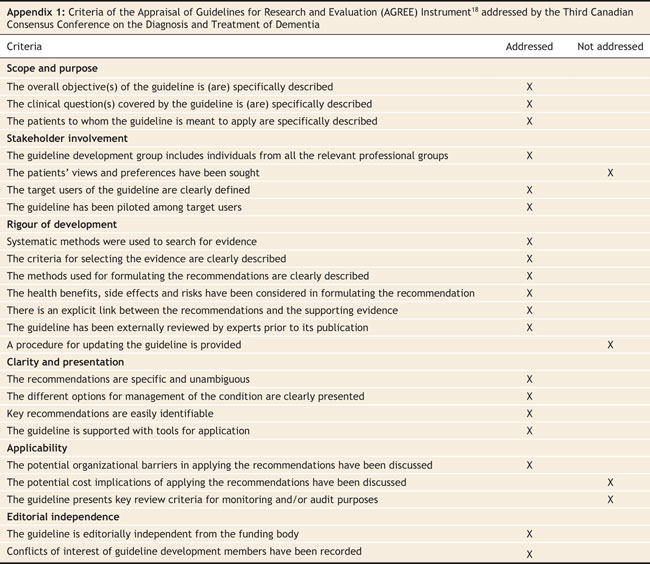
Faced with significant advances and new evidence on existing diagnostics and therapeutics, the Third Canadian Consensus Conference on the Diagnosis and Treatment of Dementia (CCCDTD3) was held in March 2006. The organization, planning and structure of the conference adhered as much as possible to the guidelines established by the Appraisal of Guidelines Research and Evaluation (AGREE) collaboration,18 and we met 19 of the 23 criteria (Appendix 1). A steering committee that included nationally eminent experts in different aspects of dementia organized this meeting.
The conference was funded by government, industry and not-for-profit charities (Appendix 2). The total contribution of pharmaceutical companies was less than 50% of the total cost of the conference. All grants were unrestricted, and no organization or company had any input into the form or content of the recommendations.
Guideline-creation process
We established communication with the College of Family Physicians of Canada early in the process, and a representative of the College was included in the organizing committee. Participants in the working groups were chosen because of their expertise in dementia or a related area and their reputation as a national leader. Participants were also chosen based on their reputation for delivering high-quality work and for being open-minded and collaborative. We included a family physician in each working group to review the recommendations from a family-practice perspective. Each participant provided a written conflict-of-interest statement.
Each working group performed literature searches, prepared background papers and agreed to a series of draft recommendations. The papers and recommendations were posted to a website, and feedback was solicited from all participants. This process lasted several months. We performed a standardized analysis of the quality of the evidence for therapy, which included a rating of the quality of the studies by use of the Jadad scale19 and such quantitative aspects as statistical significance and magnitude of effects.20
The quality of the available evidence was graded using a system adapted from the Canadian Task Force on Preventive Health Care (Box 2, Box 3).21 We assessed the strength of the recommendations using the system that had been used in the Second Canadian Consensus Conference on Dementia.9,22 Ideally, grade A and E recommendations were supported by level 1 evidence; however, level 2 evidence was sometimes deemed sufficient. A grade C recommendation did not imply that the manoeuvre was useless or harmful. Instead, it meant that there was insufficient evidence to make a stronger recommendation or that studies to date have reported conflicting results. In the series of articles to be published in CMAJ in the coming months, the grading and strength of supporting evidence is given.
Box 2.

Box 3.

Conference participants read the background papers prepared by each working group and voted on the recommendations (80% of votes were required for consensus). Recommendations that did not achieve consensus were either modified (and the amended recommendation brought forward for further discussion) or discarded. In 5 cases, the final vote on the amended recommendation received between 60%–80% of votes. These recommendations were stated to have achieved only weak consensus.
At a 2-day meeting attended by all members of the working groups (including 2 family practitioners designated by the College of Family Physicians of Canada, delegates from the Canadian Institutes of Health Research and the Alzheimer Society of Canada, and observers from other partners including pharmaceutical companies), each working group provided a brief overview, followed by a presentation, discussion and voting on each modified recommendation. Voting was open and transparent, and the process for formulating recommendations was outlined in detail before the conference. Several recommendations had to be reworked to achieve group approval.
A total of 215 recommendations were initially posted on the website. Of these, 146 were ultimately approved with strong consensus. Five achieved weak consensus, and the remaining recommendations were rejected (or modified and incorporated into other recommendations). This process (and the recommendation list) was unique in its scope and breadth, its success at achieving agreement among physicians and specialists from different domains, and its determination to develop practical guidelines and recommendations that are relevant to general physicians. The full set of recommendations is available on the websites of the Third Canadian Consensus Conference on the Diagnosis and Treatment of Dementia (www.cccdtd.ca) and the Alzheimer Society of Canada (www.alzheimer.ca).
Dissemination of the recommendations
In the coming months, CMAJ will present a 5-part case-based series highlighting new advances and their role in practice. This series will provide clinicians with a practical, evidence-based approach to the management of dementia. The series focuses on the following topics:
1. Risk factors and prevention of Alzheimer disease and dementia. Some of the genes involved in early-and late-onset Alzheimer disease are known: should genetic testing be performed? We also have considerable knowledge of the environmental risk factors for dementia. What advice should physicians give to their patients who want to preserve their memory into old age?
2. Investigating and diagnosing dementia. Alzheimer disease is the most serious illness for which we lack a simple blood test, definitive imaging or biopsy guidance for diagnosis. What current tests should family physicians and specialists use in diagnosis? What lies in the future?
3. Mild cognitive impairment and cognitive impairment no dementia. Attempts have now been made to define, diagnose and manage these early stages of mild memory loss. How do these developments affect clinical practice?
4. Managing mild and moderate dementia. Given the recent progress in symptomatic therapy, improved knowledge of the vascular aspects of dementia and new approaches to changing progression, what treatments are recommended?
5. Managing severe dementia. What pharmacologic and non-pharmacologic approaches should be used to manage cognitive and behavioural problems at this stage of illness?
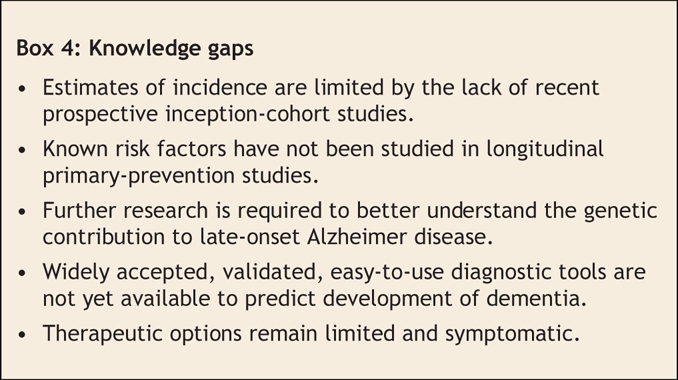
Knowledge gaps
Although this series of articles defines the current status of management, there is no doubt that there are knowledge gaps (Box 4) and that the future will bring more developments in the clinical care of dementia. Simple diagnostic and monitoring tests are sorely needed. A decade from now, we hope to have a “cognitive blood-pressure cuff” or a biomarker that allows much earlier diagnosis of Alzheimer disease. We hope to have clear nonpharmacologic therapeutic approaches and more effective therapies to modulate and prevent this disease. We also hope to have improved medical manpower and reformed health-care funding to allow physicians more time to devote to diagnosing, managing and treating elderly patients with dementia as well providing support to their caregivers.
Box 4.
As with all guidelines for major diseases, it is difficult to identify and synthesize large bodies of literature. It is even more difficult to draw clear conclusions and make recommendations when the evidence is either limited or altogether lacking. Although we made every attempt to ensure that our methodology was rigorous, that our evidence base complete and that our recommendations were made using an unbiased and transparent decision-making process, expert opinion and judgement were frequently required. Some of our recommendations may be dated as more recent information becomes available, and quite frequently, clear unambiguous recommendations could not be made. We also recognize that implementation of all 146 recommendations may be difficult because of unrecognized barriers.
We chose not to develop separate sets of recommendations for different levels of specialization. In addition, this series of articles has a clear focus on Alzheimer and vascular dementia, rather than on more rare forms of dementia (e.g., Lewy body disease, normal pressure hydrocephalus). The background articles for the recommendations provide considerably more detail about less frequent forms of dementia and their management.
It is our hope that these guidelines and their dissemination through CMAJ will improve the assessment of risk, diagnosis and care of patients with dementia. We encourage CMAJ readers to provide feedback to our working group, so that further recommendations can be based on a wide appreciation of needs among health care professionals.
Appendix 1.
Appendix 2.
Footnotes
Steering Committee members (alphabetical order): Dr. Howard Bergman (Joseph Kaufmann Professor of Geriatric Medicine, McGill University; president of the Canadian Geriatric Society; director of the Quebec Research Network on Aging); Dr. Howard Chertkow (professor of Neurology and Geriatric Medicine, McGill University; past president of the Consortium of Canadian Centres for Clinical Cognitive Research; chair of the steering committee); Dr. John W. Feightner (family physician; professor of Family Practice, University of Western Ontario); Dr. Howard Feldman (professor and head of the Division of Neurology, University of British Columbia; liaison to the Canadian Neurological Society); Dr. Serge G. Gauthier (professor of Neurology, Medicine, and Psychiatry, McGill University; chief of the Alzheimer's disease research unit at the McGill Centre for Studies in Aging); Dr. Nathan Herrmann (geriatric psychiatrist; professor and director of Geriatric Psychiatry, Sunnybrook Hospital, University of Toronto; liaison to the Canadian Academy of Geriatric Psychiatry); Dr. David Hogan (Brenda Stafford Foundation Chair; professor in Geriatric Medicine, University of Calgary); Dr. Yves Joanette (neuropsychology and speech-language pathologist; directeur, Centre de recherche de l'Institut universitaire de gériatrie de Montréal, Université de Montréal); Dr. Christopher J. Patterson (professor of Geriatric Medicine; McMaster University).
Working Group members: Risk factors and prevention: Christopher Patterson MD (coordinator), John Feightner MD MSc, Angeles Garcia MD PhD and Christopher MacKnight MD MSc. Mild memory loss states: Howard Chertkow MD (coordinator), Ziad Nasreddine MD, Yves Joanette PhD, Valérie Drolet PhD, John Kirk MD, Fadi Massoud MD, Sylvie Belleville PhD, Morris Freedman MD and Howard Bergman MD. Diagnosis: Howard Feldman MD (coordinator), Claudia Jacova PhD, Andrew Kertesz MD, Mervin Blair BSc, Michael Borrie MD, Hyman Schipper MD, PhD, John Fisk PhD, Alain Robillard MD, Tiffany Chow MD and Angeles Garcia MD PhD. Genetics: Ging-Yuek Robin Hsiung MD (coordinator) and A. Dessa Sadovnick PhD (coordinators). Mild and moderate dementia: David Hogan MD (coordinator), Peter Bailey MD, Anne Carswell MSc PhD, Barry Clarke MD, Carole Cohen BA MD, Dorothy Forbes RN PhD, Malcolm Man-Son-Hing MSc MD, Krista Lanctôt PhD, Debra Morgan PhD and Lilian Thorpe MD.M Severe dementia: Nathan Herrmann MD (coordinator), Serge Gauthier MD (coordinator) and Paul Lysy MD. Dementia with a vascular component: Christian Bocti MD (coordinator), Sandra Black MD (coordinator) and Chris Frank MD. Ethics: John Fisk PhD (coordinator), Lynn Beattie MD (coordinator), Martha Donnelly MD, Anna Byszewski MEd MD and Frank J. Molnar MD. Future revision of criteria: Ken Rockwood MD (coordinator), Rémi Bouchard MD (coordinator), Richard Camicioli MD and Gabriel Léger MD.
Editor's Note: The 18 background papers with supporting evidence for the recommendations were published in the October 2007 issue of Alzheimer's and Dementia and are available at www.alzheimersanddementia.org. These articles are also freely available at www.cccdtd.ca (through agreement with Elsevier).
This article has been peer reviewed.
Competing interests: Howard Chertkow has received support from Pfizer Canada (advisory board, speaker, grant recipient), Neurochem Inc. (advisory board) and Lundbeck Canada (advisory board, speaker). Dr. Chertkow is supported by operating grants from the Canadian Institutes for Health Research, the Alzheimer Society of Canada, and the Fonds de la recherche en santé du Québec. Dr. Chertkow is a chercheur national of the Fonds de la recherche en santé du Québec.
Correspondence to: Dr. Howard Chertkow, Bloomfield Centre for Research in Aging, Rm 408, Sir Mortimer B. Davis–Jewish General Hospital, 3755 Chemin Côte Ste–Catherine, Montréal QC H3T 1E2; fax 514 340-8295; howard.chertkow@mcgill.ca
REFERENCES
- 1.Canadian Study of Health and Aging: Risk factors for Alzheimer's disease in Canada. Neurology 1994;44:2073-80. [DOI] [PubMed]
- 2.The incidence of dementia in Canada. The Canadian Study of Health and Aging Working Group. Neurology 2000;55:66-73. [PubMed]
- 3.Canadian study of health and aging: study methods and prevalence of dementia. CMAJ 1994;150:899-913. [PMC free article] [PubMed]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington: The Association; 1994.
- 5.Wells CE. Differential diagnosis of Alzheimer's dementia: affective disorder. In: Reisberg B, ed. Alzheimer's Disease: The Standard Reference. New York: Free Press; 1983. p.193-7.
- 6.Feldman H, Levy AR, Hsiung GY, et al. A Canadian cohort study of cognitive impairment and related dementias (ACCORD): study methods and baseline results. Neuroepidemiology 2003;22:265-74. [DOI] [PubMed]
- 7.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 1997;277:813-7. [PubMed]
- 8.Hux MJ, O'Brien BJ, Iskedjian M, et al. Relation between severity of Alzheimer's disease and costs of caring. CMAJ 1998;159:457-65. [PMC free article] [PubMed]
- 9.Patterson CJ, Gauthier S, Bergman H, et al. The recognition, assessment and management of dementing disorders: conclusions from the Canadian Consensus Conference on Dementia. CMAJ 1999;160(Suppl):S1-15. [PMC free article] [PubMed]
- 10.Patterson CJ, Gauthier S, Bergman H, et al. Canadian Consensus Conference on Dementia: a physician's guide to using the recommendations. CMAJ 1999;160:1738-42. [PMC free article] [PubMed]
- 11.Jack CR Jr, Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology 2000;55:484-9. [DOI] [PMC free article] [PubMed]
- 12.Klunk WE, Price JC, DeKosky ST, et al. The applications of amyloid-imaging to the diagnosis and treatment of Alzheimer's disease. Alzheimers Demen 2005;1(Suppl):5.
- 13.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med 2002;346:476-83. [DOI] [PubMed]
- 14.Rogaeva E, Meng Y, Lee JH, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet 2007;39:168-77. [DOI] [PMC free article] [PubMed]
- 15.Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006;442:916-9. [DOI] [PubMed]
- 16.Mackenzie IR, Baker M, West G, et al. A family with tau-negative frontotemporal dementia and neuronal intranuclear inclusions linked to chromosome 17. Brain 2006;129:853-67. [DOI] [PubMed]
- 17.Areosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Systematic Reviews 2005 Jul 20; (3): CD003154. [DOI] [PubMed]
- 18.The Appraisal of Guidelines Research and Evaluation (AGREE) Collaboration. The AGREE Instrument. London (UK): The Collaboration; 2001. Available: www.agreecollaboration.org/pdf/agreeinstrumentfinal.pdf (accessed 2007 Dec 20).
- 19.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [DOI] [PubMed]
- 20.Natural Standard. Explanation of Columns in Natural Standard Evidence Table. Cambridge (MA): The Standard. www.naturalstandard.com/explanation_columns.html (accessed 2007 Dec 20).
- 21.Woolf SH, Battista RN, Anderson GM, et al. Assessing the clinical effectiveness of preventive maneuvers: analytic principles and systematic methods in reviewing evidence and developing clinical practice recommendations. A report by the Canadian Task Force on the Periodic Health Examination. J Clin Epidemiol 1990;43:891-905. [DOI] [PubMed]
- 22.Patterson C, Gauthier S, Bergman H, et al. The recognition, assessment and management of dementing disorders: conclusions from the Canadian Consensus Conference on Dementia. Can J Neurol Sci 2001;28(Suppl 1):S3-16. [DOI] [PubMed]