Abstract
An appropriate animal model that can eliminate confounding factors of diet would be very helpful for evaluation of the health effects of nutrients such as n-3 fatty acids. We recently generated a fat-1 transgenic mouse expressing the C. elegans fat-1 gene encoding an n-3 fatty acid desaturase that converts n-6 to n-3 fatty acids (which is absent in mammals). The fat-1 transgenic mice are capable of producing n-3 fatty acids from the n-6 type, leading to abundant n-3 fatty acids with reduced levels of n-6 fatty acids in their organs and tissues, without the need of a dietary n-3 supply. Feeding an identical diet (high in n-6) to the transgenic and wild type littermates can produce different fatty acid profiles in these animals. Thus, this model allows well-controlled studies to be performed, without the interference of the potential confounding factors of diet. The transgenic mice are now being used widely and are emerging as a new tool for studying the benefits of n-3 fatty acids and the molecular mechanisms of their action.
1. Challenges and limitations of dietary supplementation in nutritional studies
Dietary supplementation is a traditional approach to modify tissue nutrient composition (e.g. fatty acid profile) in animal studies of nutrition. Feeding animals with different diets that consist of many components derived from different materials can bring in many variations between the experimental groups. Although the total energy can be balanced, it is difficult to make all the dietary components identical (in both quality and quantity) between two diets. The inevitable differences between diets act as confounding factors in nutritional studies and may contribute to inconsistent or conflicting results. This is the case particularly for studies in the field of lipid (especially n-3 fat) nutrition (1).
For example, to examine the effects of two different n-6/n-3 fatty acid ratios, two different diets must be utilized to feed the animals in order to establish the different fatty acid profiles. At present, no pure n-3 and n-6 fatty acids are available for animal diet. Generally, fish oils and plant seed/vegetable oils are used to provide n-3 and n-6 fatty acids respectively. These fatty acids are derived from different sources and may contain other bioactive compounds that affect the study outcomes of interest In addition to the levels of n-6 and n-3 fatty acids, the amounts of other components, including saturated fatty acids, monounsaturated fatty acids, cholesterol, antioxidants, contaminants and other unidentified substances, are also different between the two kinds of oils. Even for the same kind of oil, the purity and freshness can sometimes vary greatly from one product to another. It is well recognized that the polyunsaturated fatty acids are highly unstable and susceptible to oxidation. Oxidation can occur even when the diet is left in the cage for a couple of days at room temperature. Therefore, many variables arising from the diet and feeding procedure can potentially impose confounding effects on the fatty acid ratio.
It is apparent that diets or nutritional supplements contain many nutrients and other components that may interact and therefore add further complexity to their evaluation. However, factors such as background components of oils, preparation and storage of diet and feeding procedures, were rarely taken into consideration in most n-3-related studies. In this context, additional studies done without significant methodological improvements might not be likely to meaningfully improve the state of science with respect to the health effects of nutrients (such as essential fatty acids) (1). Thus, developing plausible models of defined contents of essential fatty acids in animals are warranted.
2. The fat-1 transgenic mouse
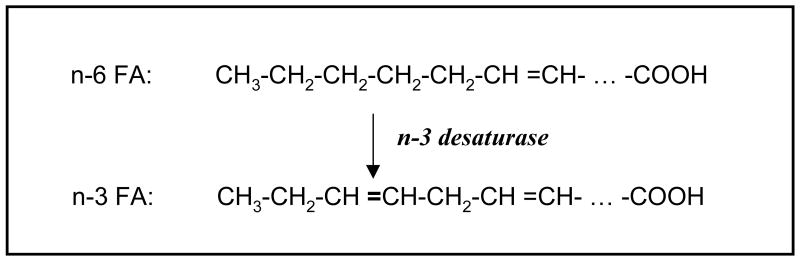
An approach to eliminate the need of n-3 fatty acid supplementation is to produce n-3 fatty acids endogenously, ideally from n-6 fatty acids. Mammals normally cannot convert n-6 to n-3 fatty acids because they lack the gene for the feat. However, some lower life such as the roundworm C. elegans harbors a gene called fat-1 (2), which encodes an n-3 fatty acid desaturase that can introduce a double bond into n-6 fatty acids at the n-3 position of their hydrocarbon chains to form n-3 fatty acids (3) (See Fig. 1 for illustration).
Fig. 1.
Conversion of n-6 fatty acids (FA) to n-3 fatty acids by an n-3 desaturase that does not exist in mammalian cells. The n-3 desaturase can catalyze introduction of a double bond into n-6 fatty acids at the n-3 position of their hydrocarbon chains to form n-3 fatty acids.
Hence, we tried to generate a transgenic mouse capable of converting n-6 to n-3 fatty acids with the fat-1 gene (4). To heterologously express the C. elegans n-3 fatty acid desaturase in mice, we modified the fat-1 gene encoding this protein by optimization of codon usage for mammalian cells and coupled it to a chicken beta-actin promoter (which allows high-level and broad expression of the transgene in mice). We then microinjected the expression vector into fertilized eggs to produce transgenic mouse lines (4).
Both transgenic and wild type mice are maintained on a diet rich in n-6 fatty acids (mainly linoleic acid) with very little n-3 fatty acids (~0.1% of total fat supplied). Under this dietary regime, wild-type mice have little or no n-3 fatty acid in their tissues because they cannot naturally produce n-3 from n-6 fatty acids, whereas the fat–1 transgenic mice have significant amounts of n-3 fatty acids (derived from n-6 fatty acids) in their tissues (4). Figure 2 shows the differential fatty acid profiles of total lipids extracted from skeletal muscles of age and sex-matched wild type and transgenic mice. In the wild type animals, the polyunsaturated fatty acids found in the tissues are mainly (98%) the n-6 linoleic acid (LA, 18:n-6) and arachidonic acid (AA, 20:4n-6) with trace (or undetectable) amount of n-3 fatty acids. In contrast, there are large amounts of n-3 polyunsaturated fatty acids, including linolenic acid (ALA, 18:3n-3), eicosapentaenoic acid (EPA, 20:5n-3), docosapentaenoic acid (DPA, 22:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), in the tissues of transgenic mice. Accordingly, the levels of n-6 fatty acids LA and AA in transgenic tissues are significantly reduced, indicating a conversion of n-6 to n-3 fatty acids. The resulting ratio of n-6 to n-3 fatty acids in the tissues of transgenic animals is close to 1. This n-3 rich profile of lipid with a balanced ratio of n-6 to n-3 and an even more balanced AA/(EPA+DPA+DHA) can be observed in all of the organs/tissues, including muscle and milk (4). These data unequivocally demonstrate that the fat-1 transgenic mice are capable of producing n-3 fatty acids from n-6 fatty acids, resulting in enrichment of n-3 fatty acids in their organs /tissues without the need of dietary n-3 supply, which is impossible in wild type mammals. The transgenic mice are normal and healthy. To date, many generations of transgenic mouse lines have been examined and their tissue fatty acid profiles showed consistently high levels of n-3 fatty acids, indicating the transgene is transmittable.
Fig. 2.
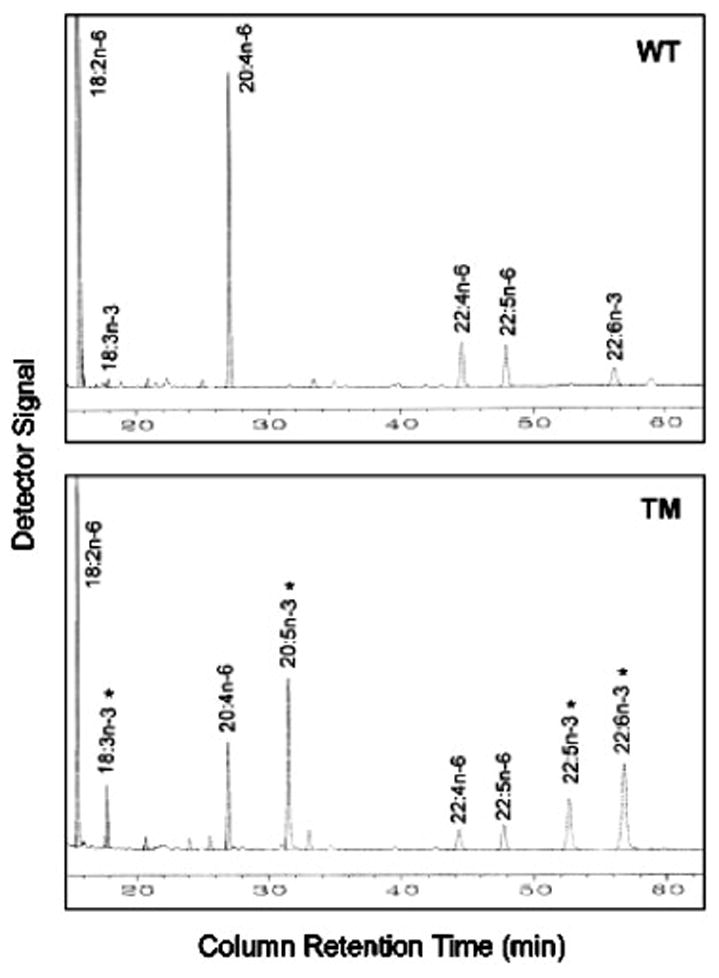
Partial gas chromatograph traces showing the polyunsaturated fatty acid profiles of total lipids extracted from skeletal muscles of a wild-type mouse (WT, upper panel) and a fat-1 transgenic mouse (TM, lower panel). Both the wild-type and transgenic mice were 8-week-old females and fed on the same diet, which is high in n-6 but low in n-3 fatty acids. Note, the levels of n-6 fatty acids (18:2n-6, 20:4n-6, 22:4n-6 and 22:5n-6) are remarkably lower whereas n-3 fatty acids (marked with *) are abundant in the transgenic muscle (lower panel) compared with the wild type muscle in which there is very little n-3 fatty acids (upper panel).
3. Advantages of the fat-1 mouse model over conventional dietary supplementation
This genetic approach is highly effective in altering the tissue ratio of n-6 to n-3 fatty acids because it can not only increase the absolute amount of n-3 fatty acids but also significantly decrease the tissue level of n-6 fatty acids, leading to a balanced n-6/n-3 fatty acid ratio in body tissues without changing the mass of tissue fatty acids (i.e. the total amount of n-6 plus n-3 is the same except the n-6/n-3 ratio in comparison with non-transgenic mice, which conventional dietary supplementation hardly achieves). Thus, this model is ideal for addressing the effects of tissue n-6/n-3 ratio in the body.
The use of this fat-1 mouse model allows us to produce two different fatty acid profiles (i.e. high vs. low 6/n-3 ratio) in a litter of mice born to the same mother by using just a single diet (e.g. a high n-6 diet). Specifically, mating a fat-1 male hetrozygote with a wild type female mouse can produce offspring with two different genotypes: a half of them being fat-1 transgenic and other half being wild type. Then these littermates can be fed just an identical diet but they exhibit distinct fatty acid profiles (high vs. low 6/n-3 ratio in wild type and fat-1 mice, respectively). Thus, this will eliminate the need of two different diets for a comparative study so that the potential variations of the impurities, flavor, caloric and other components in the supplemented oils as well as other factors derived from dietary supplementation (such as inconsistent preparation, storage conditions, timing and duration of feeding) can be avoided. Thus, use of this model may provide more reliable and definitive results compared to dietary manipulation.
In addition, use of the fat-1 mouse model can save a lot of troubles, time and money that are required for a lengthy feeding (usually for 2–3 months or longer) of different diets in the dietary supplementation studies. In the transgenic mice, change in fatty acid composition (conversion of n-6 to n-3) occurs as early as in the embryo stage and lasts for the whole life. Thus, with this mouse model it’s convenient for one to examine the health effects of n-3 fatty acids at different ages or time points.
More attractively, fat-1 transgenic mouse lines can be used to genetically cross with established disease models (transgenic or knockout animals, such as ob/ob obesity model and ApoE-/- atherosclerosis model) to generate combined (fat-1 plus a diseased gene) models, which allow proper evaluation of the effects of n-3 fatty acids and or n-6/n-3 ratio on disease development and progression. Therefore, fat-1 transgenic mice will serve as the new tool for omega-3 fatty acid research.
4. Recent studies utilizing the fat-1 mice
At present, more than 30 laboratories worldwide are using the fat-1 transgenic mice to study the effects of n-3 fatty acids on various health problems. Some of them have been published recently (5–13) and I would like to briefly summarize them to illustrate the utility of this model in omega-3 research.
In order to delineate the role played by tissue status of n-3 fatty acids and the resultant anti-inflammatory mediators (particularly the newly discovered resolvins and protectins) in retarding inflammatory disease development and progression, we utilized the fat-1 mice and their counterpart littermates to interpret inflammatory responses in the colons following DSS (Dextrane sodium sulphate) treatment (5). The ratio of the long-chain n-6 fatty acid (20:4n-6, 22:4n-6 & 22:5n-6) to the long-chain n-3 fatty acids (20:5n-3, 22:5n-3 & 22:6n-3) was 1.7 in fat-1 transgenics and 30.1 in wild type mice. Our results demonstrated that DSS-induced colonic inflammation evaluated in terms of both clinical manifestations and pathology, was significantly less severe in fat-1 transgenic mice than that in wild type littermates. The protection afforded against colitis in fat-1 mice is correlated with enhanced formation of anti-inflammatory derivatives of n-3 fatty acids (RvE1 and RvD3, and PD1), down-regulation of proinflammatory factors/cytokines (NFκB, TNFα, iNOS and IL-1β) and upregulation of mucoprotective factors (TFF3, TOLLIP and ZO-1) in the colons of these animals (5). Similarly, we utilized another acute inflammation model (D-GalN/LPS-induced hepatitis) to demonstrate less severe inflammatory injury as measured by serum aminotransferase levels and histological liver damage in fat-1 mice. This was accompanied by reduced hepatic gene expression of the pro-inflammatory cytokines TNF-alpha, IL-1beta, IFN-gamma and IL-6 and reduced apoptosis in liver cells as indicated by DAPI-staining (6). In another study (7), Fernandes et al showed that the lower n-6/n-3 ratios in splenocytes from calorie-restricted fat-1 mice were associated with significant reductions in the activities of NF-κB and AP-1 and the subsequent secretion of IL-6 and TNF-α following LPS treatment (7). These findings demonstrate a role played by an increased tissue status of n-3 fatty acids as well as decreased n-6/n-3 ratio in protection against inflammation through alterations of gene expression mediated, probably, by anti-inflammatory lipid mediators of the n-3 fatty acids.
The relationship between tissue n-6/n-3 fatty acid ratio and tumorigenesis remains to be clarified in well-controlled experimental models. Therefore, we recently implanted mouse melanoma B16 cells into fat-1 and wild type littermates to examine the incidence of tumor formation and tumor growth rate (8). The results showed a striking reduction in melanoma development and progression in fat-1 transgenic mice. The levels of n-3 fatty acids and their metabolite PGE3 were much higher (but the n-6/n-3 ratio is much lower) in the tumor and surrounding tissues of fat-1 mice compared to wild type animals. Also, the PTEN gene (a tumor suppressor) was significantly up-regulated in the fat-1 mice. In addition, in vitro data also suggest the anti-melanoma effect of n-3 fatty acids through, at least in part, PGE-3 mediated PTEN pathway (8). In another study, we examined the role of endogenous n-3 fatty acids in colon tissue in preventing colon tumorigenesis. The results showed that fat-1 transgenic mice had lower incidence and growth rate of colon tumors induced by inflammation (dextrane sodium sulfate, DSS) plus treatment with carcinogen (azoxymethane, AOM) (9). There was also evidence of lower activity of NF-κB, higher expression of transforming growth factor beta (TGF-β) in the colons, and lower expression of inducible nitric oxide synthase (iNOS) in the tumors of fat-1 animals (9). Furthermore, to determine the influence of n-6 and n-3 fatty acids on prostate cancer risk in animals with a defined genetic lesion (Pten- deletion), Chen and his colleagues crossed the fat-1 mice with prostate-specific Pten-knockout mice (a prostate cancer model) to generate compound mice (Pten-knockout plus fat-1) (10). They found that the hybrid mice (Pten-knockout plus fat-1, which have higher levels of n-3 fatty acids) had a significantly lower rate of tumor growth and survived longer compared to Pten-knockout mice (without fat-1) (10). Tumors from mice with high n-3 had lower proportions of phosphorylated Bad, resulting in increased cell apoptosis (10). They concluded that n-3 and n-6 fatty acids have opposite effects on tumor growth. Similar protective effect of n-3 fatty acids in reducing the risk of breast cancer by promoting mammary gland differentiation have also been observed in the fat-1 transgenic mice (11, 12).
To elucidate the protective effect of n-3 fatty acids against hypoxia-induced pathological neovascularization, Smith and colleagues used the fat-1 transgenic mouse model to examine oxygen-induced retinopathy (13). They found that increased n-3-FA tissue levels in fat-1 transgenic mice decreased the retinal avascular area by increasing vessel regrowth after injury and thereby reducing the hypoxic stimulus for neovascularization (13). The protective effect of n-3-fatty acids and their bioactive metabolites (resolvins and protectins) was mediated, in part, through suppression of tumor necrosis factor-α (13). These findings suggest that increasing the sources of n-3-fatty acids or their bioactive products reduces pathological angiogenesis.
Many studies using fat-1 transgenic mice are still under way and more data on the effects of n-3 fatty acids derived from this model will be available in the near future.
5. Conclusions
The use of fat-1 transgenic mice can eliminate the need of dietary manipulation and thereby avoid the potential confounding effects stemming from dietary supplementation in the n-3 fatty acid studies. This mouse model can provide a well-controlled experimental condition (an unadulterated system) for studying the beneficial effects of n-3 fatty acids and is thus a valuable addition to the conventional methods used in this field. Application of the fat-1 model, in combination with the modern technologies of lipidomics and proteomics, to elucidate the interaction between the fatty acids or their derivates and genes/proteins may have a great impact on the development of omega-3 research in the future.
Acknowledgments
This work was supported by grants from the American Cancer Society (RSG-03-140-01-CNE) and the National Institute of Health (R01CA-113605).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balk EM, Horsley TA, Newberry SJ, Lichtenstein AH, Yetley EA, Schachter HM, Moher D, MacLean CH, Lau J. A collaborative effort to apply the evidence-based review process to the field of nutrition: challenges, benefits, and lessons learned. Am J Clin Nutr. 2007;85(6):1448–1456. doi: 10.1093/ajcn/85.6.1448. [DOI] [PubMed] [Google Scholar]
- 2.Spychalla JP, Kinney AJ, Browse J. Identification of an animal omega-3 fatty acid desaturase by heterologous expression in Arabidopsis. Proc Natl Acad Sci USA. 1997;94(4):1142–1147. doi: 10.1073/pnas.94.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang ZB, Ge Y, Chen ZH, Brown J, Laposata M, Leaf A, Kang JX. Adenoviral gene transfer of C. elegans n-3 fatty acid desaturase optimizes fatty acid composition in mammalian cells. Proc Natl Acad Sci USA. 2001;98:4050–4054. doi: 10.1073/pnas.061040198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang JX, Wang J, Wu L, Kang ZB. Fat-1 transgenic mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 5.Hudert C, Weylandt KH, Wang J, Lu Y, Song H, Dignass A, Serhan CN, Kang JX. Fat-1 transgenic mice are protected from experimental colitis. Proc Natl Acad Sci USA. 2006;103(30):11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmöcker C, Weylandt KH, Kahlke L, Wang J, Lobeck H, Tiegs G, Berg T, Kang JX. Omega-3 fatty acids alleviate D-GaIN/LPS induced acute hepatitis by suppression of cytokines. Hepatology. 2007;45:864–869. doi: 10.1002/hep.21626. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya A, Chandrasekar B, Rahman MM, Banu J, Kang JX, Fernandes G. Inhibition of inflammatory response in transgenic fat-1 mice on a calorie-restricted diet. Biochem Biophys Res Commun. 2006;349(3):925–930. doi: 10.1016/j.bbrc.2006.08.093. [DOI] [PubMed] [Google Scholar]
- 8.Xia SH, Wang J, Lu Y, Song H, Serhan CN, Kang JX. The growth of melanoma is reduced in Fat-1 transgenic mice: Impact of n-6/n-3 essential fatty acids. Proc Natl Acad Sci USA. 2006;103(33):12499–12504. doi: 10.1073/pnas.0605394103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowark J, Weylandt KH, Habbel P, Wang J, Dignass A, Glickman JN, Kang JX. Colon tumorigenesis is suppressed in transgenic mice rich in endogenous n-3 fatty acids. Carcinogenesis. 2007 doi: 10.1093/carcin/bgm166. (In press) [Epub ahead of print on Jul 18 2007] PMID: 17634405. [DOI] [PubMed] [Google Scholar]
- 10.Berquin IM, Min Y, Wu R, Wu J, Perry D, Cline JM, Thomas MJ, Thornberg T, Smith A, Edwards IJ, D’Agostino R, Zhang H, Wu H, Kang JX, Chen YQ. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Investig. 2007;117:1866–1875. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma DWL, Ngo V, Huot P, Kang JX. Omega-3 polyunsaturated fatty acids endogenously synthesized in fat-1 mice are enriched in the mammary gland. Lipids. 2006;41(1):35–39. doi: 10.1007/s11745-006-5067-9. [DOI] [PubMed] [Google Scholar]
- 12.Liu YE, Pu W, Wang J, Kang JX, Shi YE. Activation of Stat5 and induction of a pregnancy-like mammary gland differentiation by eicosapentaenoic and docosapentaenoic omega-3 fatty acids. FEBS Journal. 2007;274(13):3351–3362. doi: 10.1111/j.1742-4658.2007.05869.x. [DOI] [PubMed] [Google Scholar]
- 13.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda E, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Serhan CN, Smith LEH. Increasing dietary intake of ω-3 PUFA reduces pathological retinal angiogenesis. Nature Medicine. 2007;13(7):868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]