Abstract
Mechanical loading is a powerful regulator of tissue properties in engineered cardiovascular tissues. To ultimately regulate the biochemical processes, it is essential to quantify the effect of mechanical loading on the properties of engineered cardiovascular constructs. In this study the Flexercell FX-4000T (Flexcell Int. Corp., USA) straining system was modified to simultaneously apply various strain magnitudes to individual samples during one experiment. In addition, porous polyglycolic acid (PGA) scaffolds, coated with poly-4-hydroxybutyrate (P4HB), were partially embedded in a silicone layer to allow long-term uniaxial cyclic mechanical straining of cardiovascular engineered constructs. The constructs were subjected to two different strain magnitudes and showed differences in biochemical properties, mechanical properties and organization of the microstructure compared to the unstrained constructs. The results suggest that when the tissues are exposed to prolonged mechanical stimulation, the production of collagen with a higher fraction of crosslinks is induced. However, straining with a large strain magnitude resulted in a negative effect on the mechanical properties of the tissue. In addition, dynamic straining induced a different alignment of cells and collagen in the superficial layers compared to the deeper layers of the construct. The presented model system can be used to systematically optimize culture protocols for engineered cardiovascular tissues.
keyword: Tissue engineering, Collagen organization, Cell orientation, Biochemical properties, Mechanical properties
Introduction
Load bearing (soft) tissues are composed of a highly organized extracellular matrix (ECM), which primarily consists of collagen, elastin and proteoglycans. Predominantly, the collagen architecture (i.e., content, crosslinks, and orientation) determines the mechanical behavior of these tissues.3,8 In addition, model studies show that the collagen organization is strongly coupled to the mechanical loading condition of these tissues.9,16 Several load bearing tissues become dysfunctional and need replacement. Frequently replaced load bearing cardiovascular soft tissues include coronary arteries and heart valves.49 The currently used replacement strategies all have several shortcomings35,45 and the most important drawback of the replacement tissue is the inability to remodel in response to the dynamic biological environment. Tissue engineering (TE) is a promising technique that has the potential to overcome these shortcomings. However, engineered tissues often lack sufficient amounts of properly organized ECM and consequently do not meet mechanical demands.
An often applied technique in TE comprises the use of autologous cell sources and biodegradable carrier materials (synthetic or biological). These tissue engineered constructs are most often mechanically conditioned and are ultimately placed in the human body. Successful attempts have been performed to create strong vessels by engineering vessels using the self assembled vascular graft approach,34 growing vessels in the recipients peritoneal cavity12 and using a porous biodegradable scaffold seeded with cells.26,41 Tissue engineered heart valves have been cultured using either a biological scaffold that will attract endogenous cells1,43 or using a biodegradable synthetic scaffold on which cells are seeded.19,25 Recently, Mol et al.38 have cultured tissue engineered heart valves that demonstrated sufficient mechanical strength for placement at the aortic position. The valves were cultured using mechanical stimulation of cell seeded scaffolds in a bioreactor by mimicking the diastolic phase of the heart cycle. However, the mechanical stimulation protocol for cardiovascular engineered constructs can be further improved to ultimately approach the mechanical properties of the native tissue (e.g., anisotropy) and reduce culture times.
The effect of mechanical conditioning on the biosynthetic activity of cells has been studied both in two- and three-dimensional culture conditions and this effect showed to be dependent on the nature of the ECM.48 Cells have been stimulated by using a variety of culture systems such as longitudinal stretching devices and vacuum driven devices.11 Several studies have been performed looking at the effect of mechanical stimulation on tissue remodeling in complex geometries.25,38 Similar experiments in well defined simple geometries were performed.2,19,36,46 Seliktar et al.46,47 cultured fibroblast populated collagen gels (tubular constructs) in the presence of accurately controlled deformation. Only a limited amount of studies have focused on the effect of well defined mechanical loading on the properties of engineered cardiovascular tissues,36,41 in which de novo formation of ECM is studied (e.g., polyglycolic acid (PGA) scaffolds seeded with cells). Mechanical control of tissue properties in engineered constructs is desired. This requires a detailed study on the effects of well defined loading conditions on ECM synthesis and ECM organization in order to optimize the loading regimen.
The first aim of this paper was to develop a model system, which allowed long term dynamic loading of cardiovascular engineered constructs. Previously, PGA in combination with cells deformed plastically and straining of the constructs required continuous adjustment of the applied strain.29,36 By supporting the scaffolds with a silicone layer, a technique was developed that enabled continuous straining of engineered cardiovascular constructs. Local tissue strains were measured during culture using digital image correlation (DIC). These constructs, consisting of PGA coated with poly-4-hydroxybutyrate (P4HB) and seeded with human saphenous vein cells (HSVC), were attached to flexible membranes, which were strained using a Flexercell FX-4000T (Flexcell Int. Corp., USA) straining system.4,21 The system was modified to apply various strain magnitudes to individual samples. The second aim of this paper was to investigate the influence of different strain magnitudes on ECM organization. Different strain magnitudes were applied to cardiovascular engineered constructs in order to demonstrate the effect of mechanical loading on the ECM organization. The cultured constructs were analyzed biochemically and the microstructure was visualized using multiphoton microscopy.
Materials And Methods
Straining System
The straining system consisted of a modified version of a Flexercell FX-4000T (Flexcell International, USA). This setup allowed easy access to straining of cells and tissue constructs using a vacuum controlled deformation of Bioflex (Flexcell International, USA) sixwell plates.21 The original Flexercell system controlled the strain magnitude by the amount of vacuum that was applied. This system was modified in order to apply various strain magnitudes to individual samples by controlling the amount of membrane displacement when a vacuum was applied.
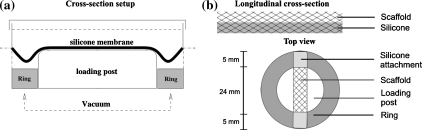
In Fig. 1a a schematic overview of the modified straining setup is shown. When a vacuum is applied to the flexible membrane of the Bioflex plate, the membrane will deform at the locations where it is not supported by the loading post. Polycarbonate rings of varying height were placed around the original loading posts. When a maximum vacuum is applied in the presence of the polycarbonate rings, the rings limit the deformation of the flexible silicone membrane. By varying the height of these rings the deformation of the membrane can be varied.
Figure 1.
(a) Schematic cross-section of modified Flexcell system. This schematic shows the polycarbonate rings (gray) placed around the loading post, which limit the deformation of the membrane when a vacuum is applied. (b) Reinforcement of the polyglycolic acid scaffold with an elastic silicone layer. In the upper part a longitudinal cross-section of the PGA/P4HB scaffold embedded in an elastic silicone layer. A layer of 0.5 mm thick, consisting of MDX4-4210 (Dow Corning, USA). In the lower part rectangular scaffolds (34 × 5 mm2 × ±1–1.2 mm) were attached to Bioflex culture wells (Flexcell Int. Corp., USA) by applying MDX4-4210 to the outer 5 mm of these constructs
The applied strain fields were validated by using DIC. A random dot pattern was sprayed on the flexible silicone membrane of the Bioflex wells. These membranes were deformed by applying a square waveform with a magnitude of maximum vacuum pressure at a frequency of 1 Hz. During loading, images of the deformed state were recorded at 60 frames per second using a Phantom v5.1 high speed camera (Vision Research Inc., USA). The recorded images were analysed using Aramis DIC software (Gom mbh., Germany). Strain fields were calibrated for three specific ring heights (8.16, 7.47, and 7.05 mm), corresponding to approximately 4, 8, and 12% strain (n = 6).
Straining PGA Based Constructs
The scaffold consisted of non-woven PGA scaffold (density 72.76 mg/cm3; Cellon, Luxemburg), which was coated with 1% (w/v) poly-4-hydroxybutyrate (P4HB; Symetis Inc., Switzerland) dissolved in tetrahydrofuran (THF; Merck, Germany).25 The scaffold was supported by a 0.5 mm thick layer of elastic silicone (Fig. 1b), which was created by partly pressing the PGA scaffold into uncured silicone (Silastic MDX4-4210, Dow Corning, USA). After curing, rectangular scaffolds were cut (34 × 5 × 1 mm3) and the constructs were attached at the outer 5 mm to the flexible membrane by using Silastic MDX4-4210 (Fig. 1b). The strain applied to the constructs was quantified (n = 6) after 2 weeks of dynamic culture using a similar protocol as was used for the membrane strains in the section "Straining System".
Cell and Tissue Culture
Human saphenous vein cells (HSVC, myofibroblasts) were acquired from a 44-year-old woman and were grown using regular cell culture methods.44 The cells were expanded in medium consisting of advanced Dulbecco’s Modified Eagle Medium (DMEM; Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Greiner Bio one, The Netherlands), 1% l-glutamax (Gibco, USA) and 0.1% gentamycin (Biochrom, Germany).
The scaffolds were vacuum dried for 48 h, followed by exposure to ultra violet light for 1 h and were subsequently placed in 70% ethanol for 4–5 h to obtain sterility. The scaffolds were allowed to dry overnight, followed by washing three times with phosphate buffered saline (PBS; Sigma, USA). Prior to cell seeding, tissue culture medium was added to the scaffolds for 16 h to facilitate cell attachment. HSVC cells at passage 7 were seeded on these scaffolds using fibrin gel (Sigma, Germany) as a cell carrier.37 The cells were seeded at a concentration of approximately 20 million cells per cm3 and the tissue constructs were subsequently cultured in tissue culture medium (at 37 °C and 5% CO2). The tissue culture medium was changed every 3–4 days and consisted of advanced DMEM (Gibco, USA) supplemented with 10% FBS, 1% l-glutamax, 0.3% gentamycin and l-ascorbic acid 2-phosphate (0.25 mg/L; Sigma, USA).
Straining Protocol
The engineered cardiovascular constructs were cultured for 3 weeks, comprising 6 days of no applied loading to allow the cells to adapt after seeding, followed by 2 weeks of dynamic straining at a frequency of 1 Hz. Three different strain conditions were applied: 0% strain, 4% dynamic strain and 8% dynamic strain (n = 8). The measurements of the applied strain were performed on separate samples strained at 4 and 8% strain for 2 and 3 weeks (n = 6).
Mechanical Testing
The constructs were sacrificed after 3 weeks of culture and were tested for their mechanical properties within 1 h (n = 6). The silicone layer was removed from the samples and the remaining tissues were placed in tissue culture medium to moisturize the samples. Thickness and width of the samples were determined using a Plμ 2300 non contact optical image profiler (Sensofar Tech S.L., Spain). A representative area measuring 8.35 × 7.85 mm2 was scanned using a 5× objective (Nikon, Japan) at a scanspeed of 1×. Thickness and width were obtained by averaging over the representative area.
Uniaxial tensile tests in the longitudinal direction were performed on the engineered tissues using a tensile stage (Kammrath & Weiss Gmbh, Germany) equipped with a 20 N loadcell. The tissues were tested at a constant strainrate of 1/60 s−1 and were tested until break. Simultaneously, the force and elongation were measured, which were converted to Cauchy stress and strain, respectively. The linear slope of the resulting curve was defined as the tangent stiffness of the sample.
Biochemical Assays on Tissue Formation
The tissues that were used for mechanical testing (n = 6) were subsequently analyzed with biochemical assays for the amount of DNA, glycosaminoglycan (GAG), collagen and hydroxylysyl pyridinoline (HP) cross-links. The amount GAG and hydroxyproline (Hyp) were expressed per μg DNA and the amount of DNA was expressed per mg dryweight. Furthermore, the amount of collagen cross-links was normalized per collagen triple helix.
The lyophelized samples were digested in papain solution (100 mM phosphate buffer, 5 mM l-cystein, 5 mM ethylenediaminetetraacetic acid (EDTA) and 125–140 μg papain per mL) at 60 °C for 16 h. The samples were then centrifuged and subsequently analysed. A portion of the supernatant was used for the DNA assay, a portion was used for the GAG assay and another portion was used for Hyp and cross-link analysis. The amount of DNA was determined using the Hoechst dye method.13 In short, TE buffer (10 mM Tris, 1 mM EDTA, pH 7.4) was used to dilute the samples and 100 μL was pipetted into a black 96 wells plate (Corning, USA). An equal amount of working solution containing the Hoechst dye (10 mM Tris, 1 mM EDTA, 2 M NaCl and 2.5 μg Hoechst dye per mL) was added to each well. To allow binding of the Hoechst dye to the DNA, the plate was incubated at room temperature for 10 min and was protected from light. Subsequently, the fluorescence was measured (excitation wavelength 355 nm, emission wavelength 460 nm). The amount of DNA in the samples was determined from a standard curve prepared from calf thymus DNA (Sigma, USA).
The GAG content was determined using a modification of the assay described by Farndale et al.20 Briefly, 40 μL of each sample was pipetted in duplo into a flat bottom 96 wells plate. 150 μL of DMMB color reagens (46 μM dimethylmethylene blue, 40.5 mM glycin, 40.5 mM NaCl, pH 3.0) was added to each well and the plate was gently shaken. The absorbencies at 540 and 595 nm were read within 5–10 min and extracted from one another. The amount of GAG in the samples was determined from a standard curve prepared from chrondroitin sulfate of shark cartilage (Sigma, USA).
Digested tissue samples were hydrolyzed by adding 25% HCl (Merck, Germany) to 200 μL of sample volume to obtain a final concentration of 6 M HCl. These samples were hydrolysed at 110 °C for 22 h and were subsequently used for amino acid and cross-link analyses. Hyp residues were measured on the acid hydrolysates by reverse-phase high-performance liquid chromatography (HPLC) after derivatization with 9-fluorenylmethyl chloroformate (FMOC, Fluka, Switzerland) as previously described by Bank et al.6 Residues of the same samples were used to measure the number of HP cross-links using HPLC as described by Bank et al.5 The amount of Hyp was converted to the amount of collagen using a conversion factor of 7.46.40
Histology and Multiphoton Microscopy
One sample per group was used for histology. Tissues were fixed in phosphate-buffered formalin (Fluka, USA) and embedded in paraffin. 10 μm transverse sections were cut and these sections were stained with Haematoxylin and Eosin (H&E) to study general tissue formation.
In addition, one living sample per group was stained for multiphoton microscopy. Cell Tracker Blue CMAC (CTB; Invitrogen, the Netherlands) and CNA35-OG48810,32 were used as specific fluorescent markers for cell cytoplasm and collagen, respectively. CTB and CNA35-OG488 were excitable with two-photon microscopy and exhibited broad emission spectra at 466 and 520 nm. Labeling of the tissue was performed with tissue culture medium, which was supplemented with CTB (15.0 μM) and CNA35-OG488 (2.0 μM). The CTB solution was applied for 5 h, followed by CNA35-OG488 (3.0 μM) for 16 h. The samples were then placed in tissue culture medium. An inverted Zeiss Axiovert 200 microscope (Carl Zeiss, Germany) coupled to an LSM 510 Meta (Carl Zeiss, Germany) laser scanning microscope was used to image the tissue engineered construct. A chameleon ultra 140 fs pulsed Ti:Sapphire laser (Coherent, USA), was tuned to 760 nm to image the applied fluorescent probes. A 63× water-immersion objective (1.2 N.A.; Carl Zeiss, Germany) was used and the channels for the two photo multiplier tubes (PMT) were defined as follows: 435–485 nm, CTB (PMT1) and 500–530 nm, CNA35-OG488 (PMT2). Separate images were obtained from each PMT (coded blue and green, respectively) and combined into a single image.
Statistics
Quantitative data are represented as mean ± standard deviation (Table 1), except for graphical representations, where data are depicted as mean ± standard error of the mean (SEM, n = 6). The data were analysed with an analysis of variance (ANOVA), followed by a Bonferroni post-hoc test to indicate significant differences between experimental groups (SPSS 12.01, SPSS Inc., USA).
Table 1.
Results of biochemical assays and mechanical testing for each loading condition
| Tissue properties | Straining conditions | ||
|---|---|---|---|
| 0% | 4% | 8% | |
| DNA/dry weight (μg/mg) | 1.51 ± 0.38 | 1.62 ± 0.13 | 1.59 ± 0.12 |
| GAG/DNA (−) | 7.78 ± 0.58 | 6.92 ± 0.33* | 6.80 ± 0.48* |
| Collagen/DNA (−) | 58.78 ± 13.35 | 40.81 ± 1.57** | 39.76 ± 2.31** |
| Crosslinks (−) | 0.16 ± 0.021 | 0.25 ± 0.024** | 0.25 ± 0.031** |
| Stiffness (MPa) | 2.64 ± 0.47 | 2.80 ± 0.50 | 1.92 ± 0.16*,+ |
Abbreviations are defined as follows: Hyp = Hydroxyproline and GAG = Glycosaminoglycan. *Indicates significant difference with reference condition 0% (*p < 0.05, **p < 0.01), +indicates significant difference with respect to condition 4% (+p < 0.05)
Results
Strain Field Characterization
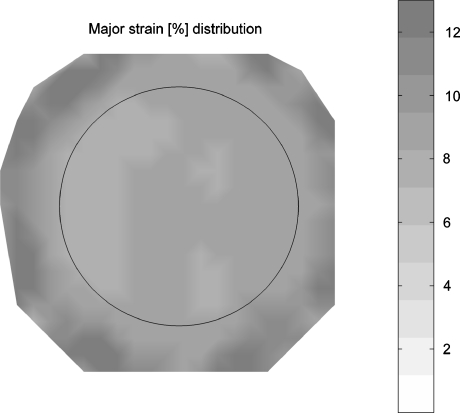
By placing polycarbonate rings around the Flexcell loading posts, the straining system was modified to simultaneously apply various strain magnitudes to individual samples during one experiment. The strain profile, applied to the membranes, was calibrated for three different ring heights (8.16, 7.47, and 7.05 mm) using round loading post geometries and showed reproducible homogeneous strain fields (Fig. 2). The average strains (%) and standard deviation were 3.84 ± 0.61, 8.08 ± 0.72 and 12.48 ± 0.45, respectively.
Figure 2.
Representative two dimensional strain (%) distribution of the Bioflex flexible membrane in case of the use of a 7.47 mm high ring. Circle indicates edges of the loading post
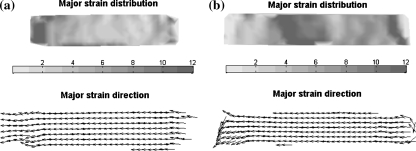
The strain fields at the surface of the strained engineered cardiovascular constructs (n = 6) were validated for two different strain rings (8.16 and 7.47 mm) after 2 weeks of dynamic culture. After 2 weeks of culture the average strain (%) measured 4.57 ± 1.34 and 8.04 ± 2.81, respectively. Typical strain fields after 2 weeks of culture for both ring heights (Figs. 3a and 3b, respectively) showed a more inhomogeneous distribution than the strain applied to the membrane without the tissue construct. The measured average applied strain in the tissue constructs is nearly equal to the membrane only situation (two dimensional), but the standard deviations are larger, reflecting the more inhomogeneous nature of the strain field. Furthermore, the major strain direction for both straining conditions (Fig. 3) is uniaxial.
Figure 3.
Representative two dimensional tissue strain distributions on the surface of an engineered tissue. (a) Representative two dimensional strain (%) distribution in case of a 8.16 mm ring. (b) Representative two dimensional strain (%) distribution in case of a 7.47 mm ring
Effect of Cyclic Strain on Tissue Properties
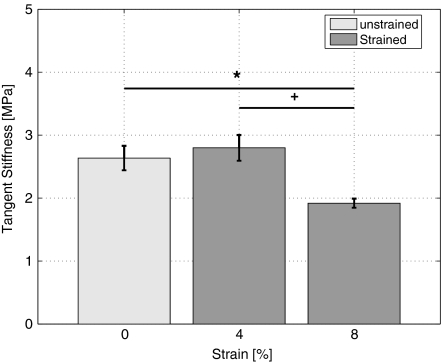
To characterize the effect of different strain magnitudes on engineered cardiovascular tissue properties, the tangent stiffness of the constructs and the amount of DNA, GAG, collagen, and HP crosslinks (Table 1) were quantified. Cyclic strain did not increase the amount of DNA per dryweight within the engineered tissue constructs relative to unstrained engineered tissue constructs. However, collagen per DNA and GAG per DNA in mechanically strained constructs, were significantly reduced compared to unstrained tissue constructs. The number of HP crosslinks per triple helix on the other hand were significantly increased for both strain conditions. The tangent stiffness of the 4% strained constructs was equal to the stiffness of the unstrained tissue constructs, whereas the 8% strained constructs showed a significant reduction in stiffness relative to both the unstrained and the 4% strained constructs (Fig. 4).
Figure 4.
Tangent stiffness (MPa) of 3-weeks-old engineered cardiovascular constructs as a function of different strain magnitudes. *Indicates significant difference with reference condition 0% (*p < 0.05) and +indicates significant difference with 4% loading condition (+p < 0.05)
Histology and Multiphoton Microscopy
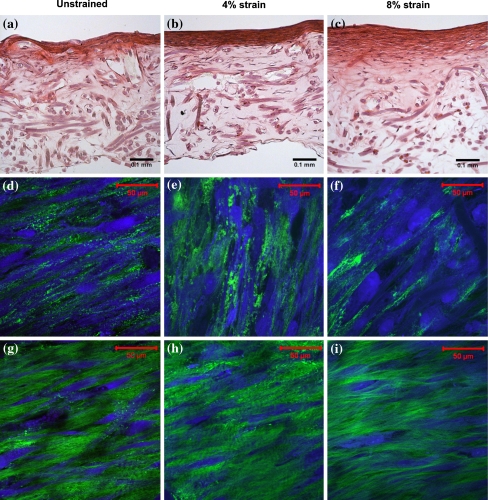
The H&E stain was performed to obtain a general overview of matrix formation within the engineered cardiovascular constructs after 3 weeks of culture (Figs. 5a–c). In general the constructs showed a dense layer of cells and ECM at the surface of the construct. Deeper into the tissues less cells and a faint staining of the ECM was seen, which illustrates a reduced production of ECM per cell. The bottom of the engineered tissue, which was attached to the silicone support layer, did not show much production of matrix due to an insufficient supply of nutrients. Comparing the different straining conditions showed that the superficial layer appeared thinner in unstrained tissues relative to the strained tissues. In addition, the superficial layer of the 4% strained construct appeared more dense compared to the superficial layer of the 8% strained construct.
Figure 5.
Histology and microstructure of 3-weeks-old engineered cardiovascular constructs. The straining direction is from left to right. (a–c) H&E staining of engineered cardiovascular constructs cultured at 0, 4, and 8% strain, respectively. (d–f) Multiphoton images of cells (blue) and collagen (green) visualized at the surface of the engineered constructs, approximately 3 μm into the tissue. (g–i) Multiphoton images of cells and collagen visualized approximately 25 μm into the engineered tissue constructs
Multiphoton microscopy was performed to visualize the organization of cells and collagen within the engineered cardiovascular tissues. The surface of the construct was covered with a layer of cells with a small amount of collagen in between the cells (Figs. 5d–f). The cells and collagen at the surface of the strained tissues were aligned oblique or perpendicular with respect to the strain direction. However, the cells and the collagen at the surface of the unstrained construct were oriented more parallel to the longitudinal axis of the construct compared to the cells in the strained construct. Slightly deeper into the tissue more collagen and less cells (Figs. 5g–i) were present, which were both aligned almost parallel to the straining direction.
Discussion
Mechanical loading is an important regulator of biochemical processes within living tissues.42 In order to regulate these biochemical processes, it is essential to quantify the effect of mechanical loading on the properties of engineered cardiovascular constructs. In this study an experimental framework was presented in which an adapted version of the Flexercell FX-4000T (Flexcell Int. Corp., USA) straining system was used to simultaneously apply various strain magnitudes to individual samples during one experiment. The flexercell system allows a high throughput of samples and is easily kept sterile for prolonged periods of time. Porous PGA/P4HB scaffolds were partially embedded in a silicone layer, which allowed repeated and uniaxial long term mechanical straining of these constructs. The constructs were attached to Bioflex wells (Flexcell Int. Corp., USA), subjected to two different strain magnitudes for several weeks and showed differences in biochemical properties, mechanical properties and organization of the microstructure compared to the unstrained constructs. The results suggest that prolonged mechanical stimulation induces the production of collagen with a higher fraction of crosslinks, which improves the intrinsic mechanical properties of the collagen. However, too large straining resulted in detrimental effects of straining on the mechanical properties of the tissue. In addition, straining induced a different alignment of cells and collagen in the superficial layers compared to the deeper layers of the construct.
By using polycarbonate rings of different height placed around the Flexercell loading posts, various strain magnitudes could be applied to individual samples while only one vacuum pressure was applied. This eliminates the risk of inter-experimental differences, as the number of experiments to culture cells at different strain magnitudes can be reduced. However, using these rings imposes limitations to the applied waveform, as with vacuum controlled devices the shape of the vacuum waveform can be controlled. When rings are used to limit deformation this type of waveform control is pointless. Therefore, an input signal of a square waveform with an amplitude of maximum vacuum pressure was chosen.
In order to allow stretching of three dimensional engineered cardiovascular constructs the model system was further developed. One of the components of the tissue construct was PGA coated with P4HB, which previously did not show elastic material behavior.29,51 Therefore, part of the porous PGA scaffold was embedded in a thin layer of liquid silicone. This allowed dynamic stretching of these engineered tissues. The strain fields were validated after 2 weeks of culture (1 week strain) and the presence of the supporting silicone layer resulted in an elastic response of the engineered constructs, which is illustrated by the fact that after 2 weeks of culture the initially applied strain was preserved. However, the strain distribution after 3 weeks could not be ascertained as constructs partially detached from the supporting silicone layer, which approximately corresponds to the loss of mechanical integrity of PGA.29 Although the strain was not entirely defined, a dynamic component was still present. This means that controlled strain can ultimately be applied over a period of 2.5–3 weeks. Analyzing the different strain fields showed that the average strain constitutes the applied strain, which was validated for the membrane without tissue construct. However, the strain distribution was much more inhomogeneous for the tissue constructs. Possibly, the inhomogeneous deformation can be attributed to inhomogeneous tissue properties resulting from non-uniform tissue formation. A limitation to the setup is that the tissue formation (Figs. 5a–c) is constricted to the surface of the construct due to the reduced supply of nutrients in the presence of the silicone layer.
In the present study dynamic straining did not have an effect on proliferation. The amount of DNA per dryweight did not differ between the dynamically strained constructs and the unstrained constructs. Previously, cell proliferation was not influenced by mechanical loading in relatively long term studies.2,28,38 However, Mol et al.38 did show an elevated level of proliferation in free floating engineered cardiovascular constructs, which were not constrained mechanically. It is essential to realize that in this study the unstrained constructs were not entirely stress free. The HSVC belong to the myofibroblast phenotype 25,38 and these myofibroblasts produce a continuous isometric tension.24 An internal tension is generated in the construct due to the fact that the engineered construct is restricted to contract by the supporting silicone layer. The internal stress generated by (myo)fibroblast contraction is sufficient to limit cell proliferation.
In general, studies on biosynthesis of collagen and GAG most often show upregulation in response to mechanical straining.31,33 However, these results are often observed in short term studies, whereas for prolonged straining of engineered tissue constructs an increase in collagen production is not always observed.28,38 Our results show that the production of both GAGs and collagen per cell was even reduced in response to strain. This reduced collagen production per DNA was similar to Mol et al.,36 who also observed a decrease in collagen production per DNA. In contrast, the fraction of HP crosslinks per collagen triple helix were increased compared to the unstrained culture condition. Crosslinks are often related to the mechanical properties of tissues.3,14 The mechanical properties of the 4% strained constructs were equal to the unstrained constructs, whereas the tangent stiffness of the 8% strained constructs was significantly reduced. Equal mechanical properties combined with an increased fraction of crosslinks and a decreased production of collagen indicates that the cells produced collagen with different intrinsic mechanical properties in order to resist the effect of mechanical straining more effectively. Increasing the strain even further resulted in equal collagen production and collagen fraction compared to the 4% strained sample. However, a decrease in mechanical properties was observed, which possibly indicates that damage was introduced to the immature collagen fibers within the engineered construct. The upregulation of crosslink formation by mechanical loading is potentially explained by an increased TGF-β secretion due to mechanical loading. TGF-β upregulates lysyl hydroxylase 2 (LH2) expression, which in its turn facilitates HP crosslink formation.50 In contrast to previous studies,30,36 dynamic mechanical loading did not result in an increase of the mechanical properties. Similar to Mol et al.38 the tangent stiffness of the constructs, created using fibrin as a cell carrier, became high in a relatively short period of time. Mol et al.38 showed that the effect of mechanical loading only came to expression after 4 weeks of culture.
The microstructure of the strained engineered cardiovascular constructs showed a striking difference in cell and collagen orientation between the superficial layers and the deeper layers, whereas in the unstrained constructs this typical orientation was almost absent (Fig. 5). An oblique and perpendicular cell orientation has also been observed in studies where two dimensional substrates seeded with cells were subjected to strain.15,23,27,39 Similar to the superficial layers of the engineered construct, a relatively low amount of ECM was present in the 2D cultures. However, cells embedded in a 3D environment subjected to strain aligned parallel to the direction of strain.7,18,22,46 This corresponds to the alignment of cells deeper into the engineered constructs (Fig. 5). The orientation response of cells has been attributed to a strain avoidance mechanism (present in superficial layer of the construct) and cell contact guidance (present in deeper layers of the construct).7,39 Potentially the orientation response is determined by the amount of collagen surrounding the cells. In addition, in the strained samples the characteristic waviness of collagen seemed to be more present. The waviness might be related to the appearance of the non-linear behavior in engineered cardiovascular tissues (i.e., uncrimping of collagen fibers).17 As can be observed from the histology, the amount of collagen and cells in the layer of the construct close to the silicone is much lower. When the layer is observed with two photon microscopy (data not shown), much more scaffold compared to the superficial layer is observed. In addition, the limited amount of collagen is both randomly oriented and oriented in the direction of the scaffold fibers (contact guidance). Furthermore, only a very limited amount of cells could be detected, most likely due to the limited supply of nutrients.
In this study an adapted form of the Flexercell straining system was developed, which allows simultaneous straining of individual engineered cardiovascular constructs at various strain magnitudes during one experiment. The used system and the used analyses techniques are valuable tools to obtain an improved understanding of the effects of mechanical loading on tissue formation. This will help us to elucidate the effect of mechanical strain on tissue remodeling and ultimately optimize loading protocols for TE.
Acknowledgment
The authors wish to thank Jessica Snabel and Ruud Bank of TNO Leiden for analyzing our samples for collagen content and crosslink fraction.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Bader A., Schilling T., Teebken O. E., Brandes G., Herden T., Steinhoff G., Haverich A. (1998) Tissue engineering of heart valves–human endothelial cell seeding of detergent acellularized porcine valves. Eur. J. Cardiothorac. Surg. 14(3):279–284 [DOI] [PubMed]
- 2.Balestrini J. L., Billiar K. L. (2006) Equibiaxial cyclic stretch stimulates fibroblasts to rapidly remodel fibrin. J. Biomech. 39(16):2983–2990 [DOI] [PubMed]
- 3.Balguid, A., M. P. Rubbens, A. Mol, R. A. Bank, A. J. J. C. Bogers, J. P. van Kats, B. A. J. M. de Mol, F. P. T. Baaijens, C. V. C. Bouten. The role of collagen cross-links in biomechanical behavior of human aortic heart valve leaflets: relevance for tissue engineering. Tissue Eng. 13, 1501–1511, 2007 [DOI] [PubMed]
- 4.Banes A. J., Gilbert J., Taylor D., Monbureau O. (1985) A new vacuum-operated stress-providing instrument that applies static or variable duration cyclic tension or compression to cells in vitro. J. Cell Sci. 75:35–42 [DOI] [PubMed]
- 5.Bank R. A., Beekman B., Verzijl N., de Roos J. A., Sakkee A. N., TeKoppele J. M. (1997) Sensitive fluorimetric quantitation of pyridinium and pentosidine crosslinks in biological samples in a single high-performance liquid chromatographic run. J. Chromatogr. B Biomed. Sci. Appl. 703(1–2):37–44 [DOI] [PubMed]
- 6.Bank R. A., Jansen E. J., Beekman B., te Koppele J. M. (1996) Amino acid analysis by reverse-phase high-performance liquid chromatography: improved derivatization and detection conditions with 9-fluorenylmethyl chloroformate. Anal. Biochem. 240(2):167–176 [DOI] [PubMed]
- 7.Barocas V. H., Tranquillo R. T. (1997) An anisotropic biphasic theory of tissue-equivalent mechanics: the interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. J. Biomech. Eng. 119(2):137–145 [DOI] [PubMed]
- 8.Billiar K. L., Sacks M. S. (2000) Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp: part I—experimental results. J. Biomech. Eng. 122(1):23–30 [DOI] [PubMed]
- 9.Boerboom R. A., Driessen N. J. B., Bouten C. V. C., Huyghe J. M. R. J., Baaijens F. P. T. (2003) Finite element model of mechanically induced collagen fiber synthesis and degradation in the aortic valve. Ann. Biomed. Eng. 31(9):1040–1053 [DOI] [PubMed]
- 10.Boerboom R. A., Nash Krahn K., Megens R. A., van Zandvoort M. A. M. J., Merkx M., Bouten C. V. C. (2007) High resolution imaging of collagen organisation and synthesis using a versatile collagen specific probe. J. Struct. Biol. 159(3):392–399 [DOI] [PubMed]
- 11.Brown T. D. (2000) Techniques for mechanical stimulation of cells in vitro: a review. J. Biomech. 33(1):3–14 [DOI] [PubMed]
- 12.Campbell J. H., Efendy J. L., Campbell G. R. (1999) Novel vascular graft grown within recipient’s own peritoneal cavity. Circ. Res. 85(12):1173–1178 [DOI] [PubMed]
- 13.Cesarone C. F., Bolognesi C., Santi L. (1979) Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal. Biochem. 100(1):188–197 [DOI] [PubMed]
- 14.Dahl S. L. M., Rucker R. B., Niklason L. E. (2005) Effects of copper and cross-linking on the extracellular matrix of tissue-engineered arteries. Cell Transplant. 14(10):861–868 [PubMed]
- 15.Dartsch P. C., Hammerle H. (1986) Orientation response of arterial smooth muscle cells to mechanical stimulation. Eur. J. Cell Biol. 41(2):339–346 [PubMed]
- 16.Driessen N. J., Boerboom R. A., Huyghe J. M. R. J., Bouten C. V. C., Baaijens F. P. T. (2003) Computational analyses of mechanically induced collagen fiber remodeling in the aortic heart valve. J. Biomech. Eng. 125(4):549–557 [DOI] [PubMed]
- 17.Driessen N. J. B., Mol A., Bouten C. V. C., Baaijens F. P. T. (2007) Modeling the mechanics of tissue-engineered human heart valve leaflets. J. Biomech. 40(2):325–334 [DOI] [PubMed]
- 18.Eastwood M., Mudera V. C., McGrouther D. A., Brown R. A. (1998) Effect of precise mechanical loading on fibroblast populated collagen lattices: morphological changes. Cell Motil. Cytoskeleton 40(1):13–21 [DOI] [PubMed]
- 19.Engelmayr G. C. Jr., Rabkin E., Sutherland F. W. H., Schoen F. J., Mayer J. E. Jr., Sacks M. S. (2005) The independent role of cyclic flexure in the early in vitro development of an engineered heart valve tissue. Biomaterials 26(2):175–187 [DOI] [PubMed]
- 20.Farndale R. W., Buttle D. J., Barrett A. J. (1986) Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta. 883(2):173–177 [DOI] [PubMed]
- 21.Garvin J., Qi J., Maloney M., Banes A. J. (2003) Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 9(5):967–979 [DOI] [PubMed]
- 22.Grenier G., Remy-Zolghadri M., Larouche D., Gauvin R., Baker K., Bergeron F., Dupuis D., Langelier E., Rancourt D., Auger F. A., Germain L. (2005) Tissue reorganization in response to mechanical load increases functionality. Tissue Eng. 11(1–2):90–100 [DOI] [PubMed]
- 23.Hamilton D. W., Maul T. M., Vorp D. A. (2004) Characterization of the response of bone marrow-derived progenitor cells to cyclic strain: implications for vascular tissue-engineering applications. Tissue Eng. 10(3–4):361–369 [DOI] [PubMed]
- 24.Hinz B., Gabbiani G. (2003) Mechanisms of force generation and transmission by myofibroblasts. Curr. Opin. Biotechnol. 14(5):538–546 [DOI] [PubMed]
- 25.Hoerstrup S. P., Sodian R., Daebritz S., Wang J., Bacha E. A., Martin D. P., Moran A. M., Guleserian K. J., Sperling J. S., Kaushal S., Vacanti J. P., Schoen F. J., Mayer J. E. Jr. (2000) Functional living trileaflet heart valves grown in vitro. Circulation. 102(Suppl 3):44–49 [DOI] [PubMed]
- 26.Hoerstrup S. P., Zund G., Sodian R., Schnell A. M., Grunenfelder J., Turina M. I. (2001) Tissue engineering of small caliber vascular grafts. Eur. J. Cardiothorac. Surg. 20(1):164–169 [DOI] [PubMed]
- 27.Iba T., Sumpio B. E. (1991) Morphological response of human endothelial cells subjected to cyclic strain in vitro. Microvasc. Res. 42(3):245–254 [DOI] [PubMed]
- 28.Isenberg B. C., Tranquillo R. T. (2003) Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann. Biomed. Eng. 31(8):937–949 [DOI] [PubMed]
- 29.Kim B. S., Mooney D. J. (2000) Scaffolds for engineering smooth muscle under cyclic mechanical strain conditions. J. Biomech. Eng. 122(3):210–215 [DOI] [PubMed]
- 30.Kim B. S., Nikolovski J., Bonadio J., Mooney D. J. (1999) Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nature Biotech. 17(10):979–983 [DOI] [PubMed]
- 31.Kolpakov V., Rekhter M. D., Gordon D., Wang W. H., Kulik T. J. (1995) Effect of mechanical forces on growth and matrix protein synthesis in the in vitro pulmonary artery. Analysis of the role of individual cell types. Circ. Res. 77(4):823–831 [DOI] [PubMed]
- 32.Krahn K. N., Bouten C. V. C., van Tuijl S., van Zandvoort M. A. M. J., Merkx M. (2006) Fluorescently labeled collagen binding proteins allow specific visualization of collagen in tissues and live cell culture. Anal. Biochem. 350(2):177–185 [DOI] [PubMed]
- 33.Lee A. A., Delhaas T., McCulloch A. D., Villarreal F. J. (1999) Differential responses of adult cardiac fibroblasts to in vitro biaxial strain patterns. J. Mol. Cell Cardiol. 31(10):1833–1843 [DOI] [PubMed]
- 34.L’Heureux N., Paquet S., Labbe R., Germain L., Auger F. A. (1998) A completely biological tissue-engineered human blood vessel. FASEB J. 12(1):47–56 [DOI] [PubMed]
- 35.Mayer J. E. Jr. (1995) Uses of homograft conduits for right ventricle to pulmonary artery connections in the neonatal period. Semin. Thorac. Cardiovasc. Surg. 7(3):130–132 [PubMed]
- 36.Mol A., Bouten C. V. C., Zund G., Gunter C. I., Visjager J. F., Turina M. I., Baaijens F. P. T., Hoerstrup S. P. (2003) The relevance of large strains in functional tissue engineering of heart valves. Thorac. Cardiovasc. Surg. 51(2):78–83 [DOI] [PubMed]
- 37.Mol A., Driessen N. J. B., Rutten M. C. M., Hoerstrup S. P., Bouten C. V. C., Baaijens F. P. T. (2005) Tissue engineering of human heart valve leaflets: a novel bioreactor for a strain-based conditioning approach. Ann. Biomed. Eng. 33(12):1778–1788 [DOI] [PubMed]
- 38.Mol A., Rutten M. C. M., Driessen N. J. B., Bouten C. V. C., Zund G., Baaijens F. P. T., Hoerstrup S. P. (2006) Autologous human tissue-engineered heart valves: prospects for systemic application. Circulation 114(Suppl. 1):I152–I158 [DOI] [PubMed]
- 39.Neidlinger-Wilke C., Grood E. S., Brand R. A., Claes L. (2001) Cell alignment is induced by cyclic changes in cell length: studies of cells grown in cyclically stretched substrates. J. Orthop. Res. 19(2):286–293 [DOI] [PubMed]
- 40.Neuman R. E., Logan M. A. (1950) The determination of hydroxyproline. J. Biol. Chem. 184(1):299–306 [PubMed]
- 41.Niklason L. E., Gao J., Abbott W. M., Hirschi K. K., Houser S., Marini R., Langer R. (1999) Functional arteries grown in vitro. Science 284(5413):489–493 [DOI] [PubMed]
- 42.Sarasa-Renedo A., Chiquet M. (2005) Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand. J. Med. Sci. Sports. 15(4):223–230 [DOI] [PubMed]
- 43.Schenke-Layland K., Opitz F., Gross M., Doring C., Halbhuber K. J., Schirrmeister F., Wahlers Th., Stock U. A. (2003) Complete dynamic repopulation of decellularized heart valves by application of defined physical signals—an in vitro study. Cardiovasc. Res. 60(3):497–509 [DOI] [PubMed]
- 44.Schnell A. M., Hoerstrup S. P., Zund G., Kolb S., Sodian R., Visjager J. F., Grunenfelder J., Suter A., Turina M. (2001) Optimal cell source for cardiovascular tissue engineering: venous vs. aortic human myofibroblasts. Thorac. Cardiovasc. Surg. 49(4):221–225 [DOI] [PubMed]
- 45.Schoen F. J., and R. J. Levy. Founder’s Award, 25th Annual Meeting of the Society for Biomaterials, perspectives. Providence, RI, April 28–May 2, 1999. Tissue heart valves: current challenges and future research perspectives. J. Biomed. Mater. Res. 47(4):439–465, 1999 [DOI] [PubMed]
- 46.Seliktar D., Black R. A., Vito R. P., Nerem R. M. (2000) Dynamic mechanical conditioning of collagen–gel blood vessel constructs induces remodeling in vitro. Ann. Biomed. Eng. 28(4):351–362 [DOI] [PubMed]
- 47.Seliktar D., Nerem R. M., Galis Z. S. (2003) Mechanical strain-stimulated remodeling of tissue-engineered blood vessel constructs. Tissue Eng. 9(4):657–666 [DOI] [PubMed]
- 48.Stegemann, J. P., R. M. Nerem (2003) Altered response of vascular smooth muscle cells to exogenous biochemical stimulation in two- and three-dimensional culture. Exp. Cell Res. 283(2):146–155 [DOI] [PubMed]
- 49.Thom T., Haase N., Rosamond W., Howard V. J., Rumsfeld J., Manolio T., Zheng Z., Flegal K., O’Donnell C., Kittner S., Lloyd-Jones D., Goff D. C. Jr., Hong Y., Adams R., Friday G., Furie K., Gorelick P., Kissela B., Marler J., Meigs J., Roger V., Sidney S., Sorlie P., Steinberger J., Wasserthiel-Smoller S., Wilson M., Wolf P. (2006) Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 113(6):e85–e151 [DOI] [PubMed]
- 50.van der Slot A. J., Zuurmond A. M., van den Bogaerdt A. J., Ulrich M. M., Middelkoop E., Boers W., Karel Ronday H., De Groot J., Bank R. A., Huizinga T. W. (2004) Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biol. 23(4):251–257 [DOI] [PubMed]
- 51.Webb A. R., Yang J., Ameer G. A. (2004) Biodegradable polyester elastomers in tissue engineering. Expert Opin. Biol. Ther. 4(6):801–812 [DOI] [PubMed]