Abstract
Hepcidin is encoded as an 84 amino acid prepropeptide containing a typical N-terminal 24 amino acid endoplasmic reticulum targeting signal sequence, and a 35 amino acid proregion (pro) with a consensus furin cleavage site immediately followed by the C-terminal 25 amino acid bioactive iron-regulatory hormone (mature peptide). We performed pulse-chase studies of posttranslational processing of hepcidin in human hepatoma HepG2 cells and in primary human hepatocytes induced with bone morphogenic protein (BMP-9). In some experiments, the cells were treated with the furin protease inhibitor decanoyl-Arg-Val-Lys-Arg-chloromethylketone (CMK) or furin siRNA. In the absence of furin inhibitor, hepcidin was found to be processed in less than 1 hour and secreted as a 3 kD form reactive with anti-mature but not anti-pro antibody. In the presence of furin inhibitors or furin siRNA, a 6 kD form reactive with both anti-pro and anti-mature antibody was rapidly secreted into the medium. Processing was not affected by inhibitors of the hypoxia inducible factor (HIF) pathway, or by treatment with 30 μM holo- or apo-transferrin.
Conclusion
The hepatic prohormone convertase furin mediates the posttranslational processing of hepcidin. The proteolytic cleavage of prohepcidin to hepcidin is not regulated by iron-transferrin or the HIF pathway.
Keywords: Liver, iron regulation, pro, hepcidin, pulse, chase study, metabolic protein labeling
Hepcidin, a 25 amino acid peptide iron-regulatory hormone, is produced in the liver and secreted into blood plasma. It plays an essential role in iron homeostasis. Hepcidin regulates iron storage and uptake by binding ferroportin, the sole known iron exporter, and causing its internalization and degradation, thus preventing the release of iron from iron-storing and transporting cells [1]. By this mechanism, hepcidin inhibits intestinal iron absorption, the release of iron from storage in hepatocytes and the release of iron from macrophages involved in recycling senescent cells, thereby controlling the concentration of iron in plasma. Hepcidin is also responsible for the hypoferremia of inflammation [2], presumed to be a host defense mechanism that sequesters iron from infectious microorganisms. In accordance with its roles in iron homeostasis and host defense, hepcidin synthesis increases after dietary or parenteral iron loading and during inflammation, and is reduced by erythropoietic activity and hypoxia [2–5].
Human hepcidin is encoded as an 84 amino acid prepropeptide containing a typical N-terminal 24 amino acid endoplasmic reticulum targeting signal sequence, and a consensus furin cleavage site immediately preceding the C-terminal 25 amino acid bioactive peptide [3;6]. Hepcidin structure and function is conserved between fish and mammals: hepcidin gene sequences of bony fish and mammals encode similar prepropeptides, hepcidins from humans and bass have nearly identical structural conformations [7;8], and zebrafish hepcidin is fully active in a mammalian bioassay system [9]. Furin is a proprotein convertase that activates hormones by cleavage of the inactive protein precursor at the consensus site –RX(K/R)R- and is a member of a family of prohormone convertases with similar cleavage specificities [10]. The furin consensus site in prohepcidin is conserved in mammals and fish [11], indicating its potential importance in hepcidin processing. Hepcidin rapidly responds to iron supply and demand, as well as to inflammation and erythropoietic activity, and its concentrations in plasma and urine are likely to yield important diagnostic and prognostic information. It has been difficult to make antibodies to this peptide, most likely because of its well conserved conformation, its small size and tightly-folded structure. Several groups have instead assayed the hepcidin precursor in serum under the assumption that the concentrations of the precursor correlate with those of mature hepcidin [12–17]. However, no studies have reported how hepcidin is processed in the cell and whether the mechanisms of processing support a stable ratio of hepcidin precursor and mature peptide. In this study, we metabolically radiolabeled proteins in a human hepatocyte cell line and in primary human hepatocytes and selectively immunoprecipitated hepcidin and its precursors to analyze hepcidin processing. In addition, we examined the role of the furin proprotein convertase in the processing of hepcidin.
Materials and Methods
Materials
Furin inhibitor decanoyl-arg-val-lys-arg-carboxymethylketone (decanoyl-RVKR-CMK) was purchased from Bachem (Torrance, CA). The furin shortcut siRNA mix and TransPass R1 transfection reagent were purchased from New England Biolabs (Ipswich, MA). Prolyl-hydroxylase inhibitors dimethyloxylglycine (DMOG) and 2,4-diethylpyridinedicarboxylate (DPD) were from Cayman Chemical Corporation (Ann Arbor, Michigan). Apo-and holotransferrin was purchased from Celliance (Norcross, Georgia). Prestained low molecular weight markers were from Amersham Biosciences (GE Healthcare, Piscataway, NJ)
Cell labeling
The human cell line HepG2 clone 4246 (modified for increased hepcidin expression by infection with a hepcidin containing lentivirus [18]) was used to metabolically label hepcidin. HepG2 cells were maintained at 37C in 5% CO2 in IMDM (Invitrogen-Gibco, Carlsbad CA) with 10% FCS (Hyclone; Logan, UT), 50 μg/ml gentamicin and 10 μg/ml ciprofloxacin. Prior to radiolabeling, cells were depleted of the intracellular cysteine and methionine by incubation for 1–2 hr in cysteine and methionine-free RPMI (MP Biomedicals, Solon, OH) containing dialyzed 5% FCS (to deplete free amino acids). Cells, in T25 vented flasks, were labeled by the addition of 100 μCi 35SCys-Met (Easy Tag Express Protein Labeling Mix; Perkin Elmer/NEN Boston, MA). For “chase” experiments the cell layer was washed and incubated in non-radioactive RPMI medium for the indicated chase times. In addition to 35S labeling, some cells were labeled by addition of 25 μCi 14C-amino acid mixture (Amersham Biosciences, GE Healthcare, Buckinghamshire, UK).
Primary human hepatocytes (provided by Dr. Steven Strom, Liver Tissue Procurement and Distribution System, University of Pittsburgh, PA) were received approximately 3–5 days after plating and grown in T25 flasks. Cells were allowed to incubate at 37C in 5% CO2 for 1–3 hours prior to addition of 10 ng/ml BMP-9 (R&D Systems, Minneapolis, MN) to increase hepcidin synthesis [19]. After overnight incubation, cells were depleted of amino acids in cys-/met- RPMI media containing BMP-9 for 2–3 hours prior to radiolabeling.
In some experiments, furin inhibitor decanoyl-RVKR-CMK was added to the cys-/met- RPMI medium and maintained during the radiolabeling procedure. In other experiments, furin siRNA was used according to the manufacturer’s instructions to deplete furin mRNA. Prior to radiolabeling, the cells were transfected twice over a two day period with a 4 hour recovery in fresh medium between the two transfections. To determine if hypoxia affected hepcidin processing though the HIF pathway, broad spectrum prolyl-hydroxylase inhibitors DMOG and DPD were added at concentrations of 500 and 10 μM respectively, 24 hr prior to and during the radiolabeling procedure. In other experiments, Apo- or holotransferrin (30 μM) was added 24 hour prior to, and throughout radiolabeling to determine if iron-transferrin could modify hepcidin expression through altered processing.
Immunoprecipitation of radiolabeled hepcidin
Radiolabeled cells were washed with PBS then extracted by vigorous pipetting with 1 ml ice cold NETT buffer (20 mM Tris-HCl pH 7.4, 150 mM NaCl, 10 mM EDTA, 1% Triton X-100) containing protease inhibitor cocktail (P2714, Sigma, St. Louis, MO). Extracts were incubated on ice for 30 min then cell debris were removed by centrifugation. A volume of 30 μl rabbit polyclonal antiserum directed to the propiece (aa; 25–59)
SVFPQQTGQLAELQPQDRAGARASWMPMFQRRRRR (made by ResGen, Invitrogen) or to the mature synthetic refolded peptide (aa; 60–84; produced in our lab [20]) was added to a 1/5 volume of cleared cell lysate or culture media and incubated on ice for 30–60 minutes. Protein A-agarose (Roche, Mannheim, Germany) was added as a 50% slurry in PBS in a volume of 40 μl and mixed overnight at 4C. The agarose was washed with PBS and the immunoprecipitate eluted by incubation in 30–40 μl 3X sample loading buffer (170 mM Tris-HCL pH 8.8, 21% [wt/vol] glycerol, 6% SDS [wt/vol], 120 mM ditiothreitol) at 4C overnight then boiling for 20 min.
SDS-Tricine polyacrylamide gel electrophoresis (PAGE) and fluorography
Radiolabeled hepcidin was separated on a 16.5% SDS-Tricine polyacrylamide gel [21] with a 4% polyacrylamide stacking layer, stained with Coomassie blue, destained in 25% methanol/0.4% formaldehyde then soaked in liquid scintillant (1M sodium salicylate, 4% ethylene glycol, 35% ethanol). After drying, the gels were exposed to Kodak BioMax MS X-Ray film (Eastman Kodak, Rochester, NY) at −80C.
Results
Biosynthesis
The hepcidin cDNA sequence suggests that the peptide is initially synthesized as an 84 amino acid (aa) precursor (Figure 1) with a putative 24 aa signal sequence, a 35 aa pro-region and a 25 aa mature peptide. Because the mature peptide contains 8 cysteine residues and all three regions contain at least one methionine, a mixture of 35S-labeled cysteine and methionine was chosen to metabolically label this peptide. An autoradiogram representative of three separate pulse chase experiments in HepG2 cells is shown in Figure 2. We noted two initially synthesized forms of hepcidin, form 1 and 2, which were recognized by both anti-pro and anti-hepcidin antibodies and may correspond to preprohepcidin and prohepcidin (sizes 9.4 and 6.9 kD) respectively. Form 1 is rapidly cleaved to form 2 as evidenced by its disappearance from the cell lysate after a 15 min chase, best seen in Panel C. In addition, form 2 is converted to form 3 recognized only by anti-mature antibody and therefore identified as the mature form (expected size 2.8 kD). Form 3 is rapidly secreted from the cell as seen by its decreasing band intensity in cell lysate but increasing intensity in culture media over the 45 min chase period. A form likely corresponding to the cleaved propiece can be seen in panel A (corresponding to the 4.2 kD 35aa propiece) whereas in panel B, a cleaved form of the propiece can be seen in the culture medium in the EQ lane (marked by * and p). As compared to the cellular form, the propiece form detected in the culture supernatant appears to be further cleaved.
Figure 1. Amino acid sequence of hepcidin precursor.
Underlined residues were radiolabeled with 35-S labeled amino acids. The boxed region denotes the furin consensus cleavage site. The molecular weight of the hepcidin precursor is shown.
Figure 2. Pulse-chase study of hepcidin processing in Hep-G2 cells treated with and without furin proproteinase inhibitor.
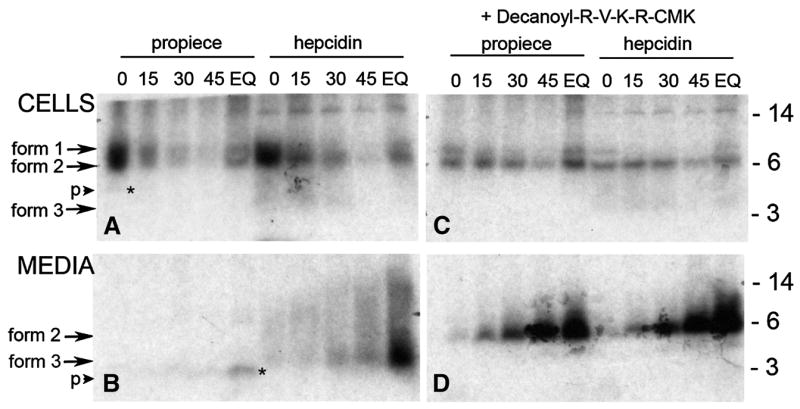
Cells were labeled with 35S-met-cys for 1 hr then subjected to cold chase for the times in minutes indicated above the lane. Lane EQ was labeled for 3 hours without cold chase prior to processing to incorporate radioactivity into all peptide forms. Cell lysates (top panel) or the corresponding culture media (lower panel) were immunoprecipitated with either anti-pro (propiece) or anti-mature (hepcidin) antibody and analyzed on SDS-tricine PAGE. The autoradiograms of the gels are shown with the molecular weight standards indicated on the right side of the figure. Three forms of hepcidin are seen as marked by arrows. A cleaved form of the propiece is indicated by the small arrow (p) and the asterisk (panels B and D). In the right panel, cells were treated with furin inhibitor (decanoyl-RVKR-CMK) during the amino acid depletion step (1 hr) and during the radiolabeling procedure.
Furin Inhibition
Guided by the presence of a consensus furin cleavage site (indicated by the boxed region in Figure 1), we next examined the involvement of furin in hepcidin processing. Panels C and D (Figure 2) show an autoradiogram of immunoprecipitated proteins from cells treated with the furin inhibitor decanoyl-RVKR-CMK. The addition of the inhibitor completely blocked the conversion of form 2 to form 3 but not cleavage of form 1 to form 2. Form 2 was secreted into the culture media and was not further cleaved as is seen by the increasing intensity of the form 2 band immunoprecipitated with either the anti-pro or anti-mature hepcidin antibody. Prohepcidin generated in the presence of the inhibitor accumulated to a greater extent than the mature peptide indicating that the propeptide maybe more stable or interacts less with other proteins in the medium. Like cells treated with the CMK furin inhibitor, cells transfected with furin siRNA also accumulated increased amounts of form 2 indicating a partial loss of prohepcidin convertase activity (Figure 3, Panels C and D). Processing of the propiece also appears to be altered in furin-inhibited cells. This can be seen in Figure 3 where the banding pattern is different in the propiece immunoprecipitated from culture medium (compare panel B and D; EQ lanes) where there are two processed forms in the control, panel B and only one in panel D (marked by *). Because there is complete inhibition of processing from form 2 to form 3 with the furin inhibitor decanoyl-RVKR-CMK and partial inhibition with furin siRNA, we conclude that form 3 recognized by anti-mature hepcidin antibody is mature hepcidin (25aa) and that it is generated after cleavage of the proprotein by furin at the furin consensus site (Figure 1). In addition, hepcidin or prohepcidin is not stored in the cell but rapidly released after synthesis regardless of its cleavage by furin.
Figure 3. Pulse-chase study of hepcidin processing in HepG2 cells transfected with furin siRNA.
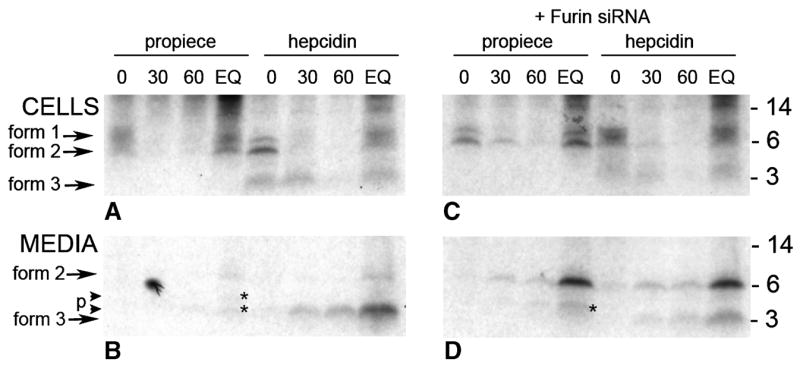
Cells were transfected twice over a 48 hr period with either blank vector (panels A and B) or vector containing furin siRNA (panels C and D). Cells were labeled with 35S-met-cys for 1 hr then subjected to cold chase for the times indicated. Lane EQ was labeled for 3 hr. Cell lysates (top panel) or the corresponding culture media (lower panel) were immunoprecipitated with either anti-pro (propiece) or anti-mature (hepcidin) antibody and analyzed on SDS-tricine PAGE. The autoradiogram of the gels are shown with the corresponding time of cold chase indicated above the lane (minutes). Molecular weight standards are indicated on the right panels. Three forms of hepcidin are seen as indicated by the arrows. Cleaved forms of the propiece are indicated by the small arrows (p) and the asterisks (panels B and D).
Effect of transferrin and prolyl-hydroxylase inhibitors
We next examined whether the processing of prohepcidin to hepcidin is affected by the physiologic hepcidin regulators, iron and hypoxia. We therefore tested the effect of holo- and apotransferrin on hepcidin processing, and similarly examined the effects of prolyl-hydroxylase inhibitors that interfere with the hypoxia-regulated HIF (hypoxia-inducible factor) pathway. As seen in Figure 4, there is no difference in the radiolabeled protein patterns of immunoprecipitate from control cells and media compared to those treated with 30 μM apo- or holo-transferrin. In addition, there was no difference in hepcidin processing in cells treated with the hypoxia-mimicking prolyl-hydroxylase inhibitors DMOG and 2,4-DPD, nor was there any difference in the timing of hepcidin processing (data not shown).
Figure 4. Hepcidin processing in HepG2 cells treated with apo- and holo-transferrin and with prolyl-hydroxylase inhibitors.
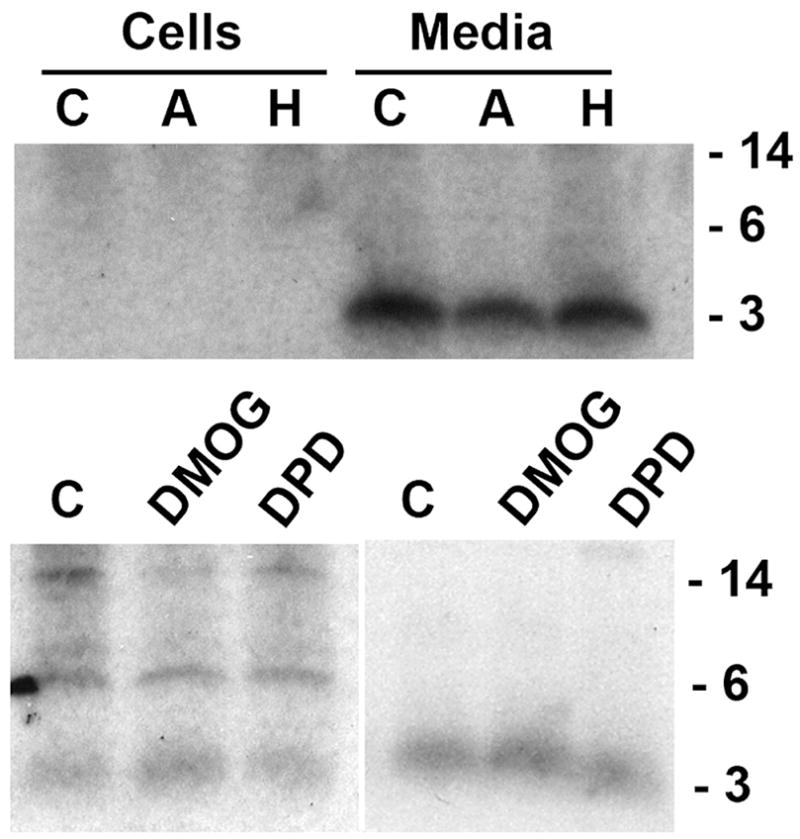
Cells were labeled with 35S-met-cys for 3 hr. Cell lysates or the corresponding culture media were immunoprecipitated with anti-mature (hepcidin) antibody and analyzed on SDS-tricine PAGE. The autoradiograms of the gels are shown with the molecular weight standards indicated on the right side of the figure. In the upper panel, cells were treated with solvent control (C), or 30 μM apo-transferrin (A) or holo-transferrin (H); in the lower panel, cells were treated with solvent control (C) or 500 μM DMOG or 10 μM 2,4-DPD. All treatments were for 18 hr prior to and during radiolabeling.
Hepcidin processing in primary human hepatocytes
Primary human hepatocytes were treated with BMP-9 to induce the expression of hepcidin [19] as otherwise the hepcidin peptide concentrations were too low to detect by metabolic radiolabeling. BMP-9 was added approximately 20 hr prior to and during amino acid depletion and radiolabeling with 35S-met/cys and 14C labeled amino acids, without or with the CMK furin inhibitor during the depletion and the radiolabeling period. As in HepG2 cells, hepcidin is first made as a proprotein (form 2), then rapidly cleaved (form 3) and secreted from the cell (Figure 5). The furin inhibitor CMK completely inhibited processing to the mature peptide but did not inhibit secretion from the cell. Immunoprecipitation with anti-pro (panel B) detected prohepcidin in the cell and a cleaved product released to the culture medium during the first hour of radiolabeling. However the propiece is not detected at either 15 and 45 minutes of cold chase. A small amount of the prohepcidin as well as the cleaved propiece is seen in the EQ lane (4 hour radiolabel). Furin inhibition prevented processing to the mature form but did not prevent release of the prohepcidin from the cell. However there is a 3–5 kD form of the pro-piece seen in the culture medium in the EQ lane of furin inhibitor-treated cells indicating that prohepcidin can also be cleaved by a process not involving furin.
Figure 5. Pulse-chase study of hepcidin processing in primary human hepatocytes.
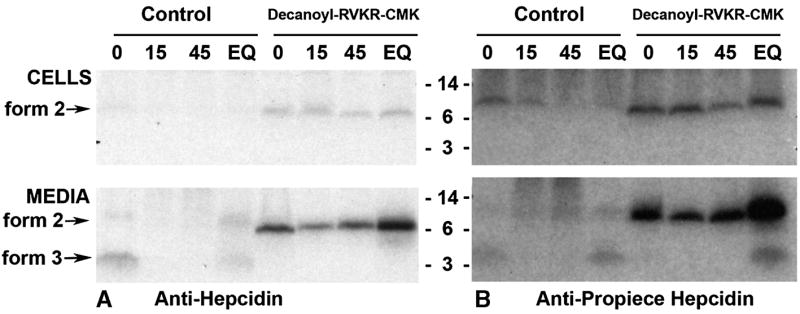
Cells were treated for 18 hr with 10 ng/ml BMP-9 prior to, and during the depletion and radiolabeling steps to increase hepcidin synthesis. Cells were labeled with 35S-met-cys and 14C-amino acids for 1 hr then subjected to cold chase for the times indicated above the lanes, in minutes. Cell lysates and conditioned media in lane EQ are from hepatocytes labeled for 4 hr prior to processing. Cell lysates (top panel) or the corresponding media (lower panel) were immunoprecipitated with anti-mature hepcidin antibody (panel A) or anti-pro-peptide (panel B) and analyzed on SDS-tricine PAGE. Autoradiograms of the gels are shown with the molecular weight standards indicated. In the right half of the autoradiogram, cells were treated with furin inhibitor (decanoyl-RVKR-CMK) during the amino acid depletion step (1 hr) prior to and during the radiolabeling procedure.
Discussion
Hepcidin is principally synthesized in the liver, by hepatocytes. We metabolically radiolabeled hepcidin and immunoprecipitated the labeled protein with polyclonal antibodies to mature hepcidin (aa 60–84) or to the pro-region (aa 25–59). In this work we showed that hepcidin is initially synthesized as a larger precursor protein, undergoing two cleavages (the signal sequence then the pro-region) and rapidly secreted from the cell as can be seen by its presence in the culture medium after one hour of radiolabeling (Figure 2; ‘chase’ time zero). Inhibition of furin proprotein convertase with the chemical inhibitor decanoyl-RVKR-CMK or inhibition of furin synthesis by siRNA blocks the second cleavage of the hepcidin precursor but does not inhibit its release from the cell, indicating that furin is the principal enzyme involved in hepcidin maturation. As the inhibition of processing by furin siRNA was not complete, the contribution of other furin-like prohormone convertases to hepcidin maturation cannot be ruled out. Relatively small amounts of mature hepcidin were detected intracellularly indicating that it is not stored in secretory granules. Similar analysis of the processing of cysteine-rich human defensin peptides [22] found in azurophil granules of neutrophils showed much slower processing on the order of 4–24 hours as compared to hepcidin, which takes less than an hour to be processed and released after synthesis. A recent report [23] immunolocalized hepcidin in HepG2 and RIM5F cells in a cytoplasmic punctuate staining pattern but the specific compartment was not further identified, and could represent hepcidin in transit through the cellular synthetic and secretory machinery.
Several groups have used the prohepcidin ELISA assay (DRG International Inc., USA) to analyze hepcidin production in serum assuming that prohepcidin is secreted and is proportional to levels of mature hepcidin. This ELISA uses an antibody directed to the hepcidin pro-region (aa 25–59). Our study indicates that this antibody will recognize both the pro-region and pro-hepcidin (aa 25–84). However we found that prohepcidin is only transiently present in the cell lysate of HepG2 cells (Figure 2 panel A; time zero). Thereafter the cleaved pro-piece can be seen in the culture supernatant but the size is variable, probably due to progressive proteolysis (Figure 2 and 3). In HepG2 cells, secretion of pro-hepcidin is detectable only in the presence of furin inhibitor (Figure 2 and 3). However, in primary human hepatocytes induced with BMP-9, prohepcidin is transiently detected immediately after labeling (Figure 5) but not during the subsequent chase period. Thus it appears that there is no fixed relationship between hepcidin and prohepcidin release into the media. In contrast to serum and urinary hepcidin concentrations [4;14;20;24–27], serum prohepcidin levels have not reflected the expected hepcidin responses to physiologically relevant stimuli [17;28].
Inhibition of furin activity prevented the conversion of prohepcidin to hepcidin and also appeared to stabilize the prohepcidin peptide both in the cell and the media, as seen by the more intensely radiolabeled bands in immunoprecipitate from inhibitor-treated cells compared to the control. Retention of the pro-region appears to slow the secretion of the peptide from the cell and once secreted it could delay degradation or inhibit hepcidin binding to a serum protein thereby leaving more peptide available for immunoprecipitation. Defensins, which are also amphipathic cysteine-rich cationic peptides, are resistant to proteolytic degradation. However, when these peptides are released into serum they are bound by α2-macroglobulin which both inhibits defensin activity and may metabolize the peptide by the endocytic pathway [29]. Like the proregion of neutrophil defensins [30], the proregion of hepcidin may facilitate peptide transit through subcellular compartments or prevent the potentially toxic effect of the mature cationic amphipathic peptide on the cellular machinery.
In summary, we have shown that hepcidin is initially synthesized as a larger precursor protein, undergoing two cleavages (the signal sequence then the pro-region) and rapidly secreted from the cell as can be seen by its presence in the culture medium after one hour of radiolabeling. Mature hepcidin is generated by the removal of the proregion by the prohormone convertase furin and possibly other members of the furin family. Secretion from the cell does not require the second cleavage as enzymatic inhibition of furin prevents cleavage but not secretion from the cell. Without furin inhibition the pro-region can be released from the primary human hepatocytes but it appears to be present at low levels compared to mature hepcidin. Although hepcidin synthesis in hepatocytes is regulated by iron, erythropoietic activity and inflammation, the processing of hepcidin uses the generic prohormone convertase furin and appears to be constitutive.
Acknowledgments
We are grateful for the funding of this study by the National Institutes of Health NIH RO1 DK 065029 and The Will Rogers Fund to T.G.
Abbreviations
- DMOG
dimethyloxylglycine
- DPD
2,4-diethylpyridinedicarboxylate
- BMP
bone morphogenic protein
- HIF
hypoxia inducible factor
- EQ
equilibrium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 2.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loreal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 4.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 7.Hunter HN, Fulton DB, Ganz T, Vogel HJ. The solution structure of human hepcidin, a peptide hormone with antimicrobial activity that is involved in iron uptake and hereditary hemochromatosis. J Biol Chem. 2002;277:37597–37603. doi: 10.1074/jbc.M205305200. [DOI] [PubMed] [Google Scholar]
- 8.Lauth X, Babon JJ, Stannard JA, Singh S, Nizet V, Carlberg JM, Ostland VE, Pennington MW, Norton RS, Westerman ME. Bass hepcidin synthesis, solution structure, antimicrobial activities and synergism, and in vivo hepatic response to bacterial infections. J Biol Chem. 2005;280:9272–9282. doi: 10.1074/jbc.M411154200. [DOI] [PubMed] [Google Scholar]
- 9.Nemeth E, Preza GC, Jung CL, Kaplan J, Waring AJ, Ganz T. The N-terminus of hepcidin is essential for its interaction with ferroportin: structure-function study. Blood. 2006;107:328–333. doi: 10.1182/blood-2005-05-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- 11.Shike H, Lauth X, Westerman ME, Ostland VE, Carlberg JM, Van Olst JC, Shimizu C, Bulet P, Burns JC. Bass hepcidin is a novel antimicrobial peptide induced by bacterial challenge. Eur J Biochem. 2002;269:2232–2237. doi: 10.1046/j.1432-1033.2002.02881.x. [DOI] [PubMed] [Google Scholar]
- 12.Gehrke SG, Kulaksiz H, Herrmann T, Riedel HD, Bents K, Veltkamp C, Stremmel W. Expression of hepcidin in hereditary hemochromatosis: evidence for a regulation in response to serum transferrin saturation and non-transferrin-bound iron. Blood. 2003 doi: 10.1182/blood-2002-11-3610. [DOI] [PubMed] [Google Scholar]
- 13.Kattamis A, Papassotiriou I, Palaiologou D, Apostolakou F, Galani A, Ladis V, Sakellaropoulos N, Papanikolaou G. The effects of erythropoetic activity and iron burden on hepcidin expression in patients with thalassemia major. Haematologica. 2006;91:809–812. [PubMed] [Google Scholar]
- 14.Kemna E, Pickkers P, Nemeth E, van der HH, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 15.Laskowska-Klita T, Chelchowska M, Swiatek E, Ambroszkiewicz J, Leibschang J. Serum pro-hepcidin and iron markers during uncomplicated pregnancy. Eur J Obstet Gynecol Reprod Biol. 2006 doi: 10.1016/j.ejogrb.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Tiker F, Celik B, Tarcan A, Kilicdag H, Ozbek N, Gurakan B. Serum pro-hepcidin levels and relationships with iron parameters in healthy preterm and term newborns. Pediatr Hematol Oncol. 2006;23:293–297. doi: 10.1080/08880010600629213. [DOI] [PubMed] [Google Scholar]
- 17.Hadley KB, Johnson LK, Hunt JR. Iron absorption by healthy women is not associated with either serum or urinary prohepcidin. Am J Clin Nutr. 2006;84:150–155. doi: 10.1093/ajcn/84.1.150. [DOI] [PubMed] [Google Scholar]
- 18.Rivera S, Liu L, Nemeth E, Gabayan V, Sorensen OE, Ganz T. Hepcidin excess induces the sequestration of iron and exacerbates tumor-associated anemia. Blood. 2005;105:1797–1802. doi: 10.1182/blood-2004-08-3375. [DOI] [PubMed] [Google Scholar]
- 19.Truksa J, Peng H, Lee P, Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci USA. 2006;103:10289–10293. doi: 10.1073/pnas.0603124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 21.Schaegger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 22.Valore EV, Ganz T. Posttranslational processing of defensins in immature human myeloid cells. Blood. 1992;79:1538–1544. [PubMed] [Google Scholar]
- 23.Fein E, Merle U, Ehehalt R, Herrmann T, Kulaksiz H. Regulation of hepcidin in HepG2 and RINm5F cells. Peptides. 2007;28:951–957. doi: 10.1016/j.peptides.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Detivaud L, Nemeth E, Boudjema K, Turlin B, Troadec MB, Leroyer P, Ropert M, Jacquelinet S, Courselaud B, Ganz T, Brissot P, Loreal O. Hepcidin levels in humans are correlated with hepatic iron stores, hemoglobin levels, and hepatic function. Blood. 2005;106:746–748. doi: 10.1182/blood-2004-12-4855. [DOI] [PubMed] [Google Scholar]
- 25.Kemna EH, Tjalsma H, Podust VN, Swinkels DW. Mass spectrometry-based hepcidin measurements in serum and urine: analytical aspects and clinical implications. Clin Chem. 2007;53:620–628. doi: 10.1373/clinchem.2006.079186. [DOI] [PubMed] [Google Scholar]
- 26.Papanikolaou G, Tzilianos M, Christakis JI, Bogdanos D, Tsimirika K, MacFarlane J, Goldberg YP, Sakellaropoulos N, Ganz T, Nemeth E. Hepcidin in iron overload disorders. Blood. 2005;105:4103–4105. doi: 10.1182/blood-2004-12-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomosugi N, Kawabata H, Wakatabe R, Higuchi M, Yamaya H, Umehara H, Ishikawa I. Detection of serum hepcidin in renal failure and inflammation by using ProteinChip System. Blood. 2006;108:1381–1387. doi: 10.1182/blood-2005-10-4043. [DOI] [PubMed] [Google Scholar]
- 28.Roe MA, Spinks C, Heath AL, Harvey LJ, Foxall R, Wimperis J, Wolf C, Fairweather-Tait SJ. Serum prohepcidin concentration: no association with iron absorption in healthy men; and no relationship with iron status in men carrying HFE mutations, hereditary haemochromatosis patients undergoing phlebotomy treatment, or pregnant women. Br J Nutr. 2007;97:544–549. doi: 10.1017/S0007114507336829. [DOI] [PubMed] [Google Scholar]
- 29.Panyutich A, Ganz T. Activated alpha 2-macroglobulin is a principal defensin-binding protein. Am J Respir Cell Mol Biol. 1991;5:101–106. doi: 10.1165/ajrcmb/5.2.101. [DOI] [PubMed] [Google Scholar]
- 30.Valore EV, Martin E, Harwig SS, Ganz T. Intramolecular inhibition of human defensin HNP-1 by its propiece. J Clin Invest. 1996;97:1624–1629. doi: 10.1172/JCI118588. [DOI] [PMC free article] [PubMed] [Google Scholar]



