Abstract
Objective
To test the hypothesis that the sensory-motor characteristics of the reflexes evoked upon stimulation with air and water infusions differ, we studied the effect of pharyngeal stimulation on the pharyngeal-upper esophageal sphincter (UES) interactions in healthy neonates
Study design
Pharyngo-UES-esophageal manometry was recorded in 10 neonates at 39 ± 4 wk postmenstrual age. Pharyngeal infusions (n=155) of air (0.1–2.0 ml) and sterile water (0.1–0.5 ml) were given. Two types of reflexes were recognized: Pharyngeal reflexive swallowing (PRS) and pharyngo-UES-contractile reflex (PUCR). Frequency occurrence, distribution of reflexes, threshold volume, response time, and stimulus-response relationship were evaluated.
Results
The reflex response rate for air was 30% and was 76% for water (P<0.001). The frequency occurrence of PRS was greater than PUCR with air and water (P<0.05), although the stimulation thresholds and response latency were similar. Graded volumes of water but not air resulted in an increased frequency of PRS (P<0.01).
Conclusions
PRS is the most frequent response, and characteristics of the reflexes are distinct between air vs. water stimuli. These methods have implications for the evaluation of swallowing in infants.
Keywords: Pharyngeal reflexive swallowing, upper esophageal sphincter, pharyngo-UES-contractile reflex, neonates
Swallowing activity appears in the fetal life around 11 weeks gestation,1 and sucking and swallowing skills develop progressively during fetal and neonatal maturation.2, 3 The survival rate among preterm neonates is increasing, and one of the reasons for prolonged hospitalization is a high prevalence of swallowing difficulty.4
Previous studies in infants and children have addressed the preparatory and oral phases of swallow,1, 5, 6 pharyngeal phase of swallow and relationship with sleep and breathing,7–9 and upper esophageal sphincter (UES) motor function.10–12 The integrated relationship between the swallowing phases has not been systematically investigated in neonates. Using manometric methods, we characterized the UES contractile and relaxation properties during spontaneous swallow induced primary peristalsis sequences across the age spectrum, from healthy premature infants to adult volunteers.13 However, UES responses induced upon pharyngeal stimulation have not been described in neonates.
The pharynx is the site of constant stimulation during breathing, during bolus oral feeding, or during gastro-esophago-pharyngeal reflux events. The neonatal aerodigestive tract is a common site manipulated acutely (as in apparent life threatening events), or chronically (as in assisted respiration or assisted enteral nutrition). Therefore, the rationale for this investigation was to evaluate the immediate responses resulting from pharyngeal stimulation in the population that received neonatal ICU care. The primary objectives of our study were to define the frequency of occurrence and to elucidate the characteristics of pharyngeal – UES reflex interactions elicited upon pharyngeal provocation to orally feeding in prematurely born healthy human neonates. We tested the hypothesis that pharyngeal stimulation with air and water infusions result in distinct neuromotor responses.
METHODS
Participants
Eligible subjects were orally feeding healthy prematurely born neonates with appropriate growth at birth and at study. Gestational age (GA) among infants was determined by maternal history and obstetric data. Postmenstrual age (PMA) was calculated by adding chronological age to GA. All infants were examined by the principal investigator (SRJ), as well as the attending neonatologist, and were deemed healthy at study. None of the infants had a presumed or proven clinical diagnosis of GER, and none received prokinetic agents at study or at discharge. Infants with congenital birth defects, neurological abnormalities, perinatal asphyxia, gastrointestinal abnormalities and recognizable chromosomal disorders were excluded. The subjects had normal head ultrasound evaluation and were of appropriate growth for age at birth and at testing. Subjects received appropriate respiratory assistance because of premature birth, and none had a respiratory, cardiac or neurological disease diagnosis at the time of evaluation.
This protocol was approved by the IRB at Columbus Children’s Research Institute, and HIPAA guidelines were complied. Informed consent was obtained from parents. Vital signs, including heart rate, respiratory rate, and oxygen saturation measured by pulse oximetry, were simultaneously monitored to ensure patient safety.
Pneumohydraulic Micromanometry methods
Subjects underwent pharyngo-esophageal manometry using a specially designed Dentsleeve manometry catheter capable of recording motility from the pharyngeal port, UES sleeve, and 3 esophageal ports. The pharyngeal infusion port was located 0.5 cm above the pharyngeal recording port. The catheter assembly was attached to the pneumohydraulic continuous micromanometric water perfusion system adapted for neonates, as described.14–16 Water was perfused at a rate of 0.02 ml per min per esophageal port, 0.04 ml per min for the UES sleeve and 0.01 ml per min for the pharyngeal recording ports using the Dentsleeve perfusion pump (Dentsleeve Pty. Ltd, Ontario, Canada). The response rate was 220 mmHg per sec for manometric ports and 850 mmHg per sec for UES sleeve. These adjustments in perfusion rates were intended to maintain patency of perfusion ports and maintain sleeve performance, in addition to minimizing water load. Becton Dickinson pressure transducers (DTX™ Plus TNF-R; Becton Dickinson, Franklin Lakes, New Jersey), and UPS 2020 amplifiers (Medical Measurement Systems, Dover, NH) were used to record the pressure signals. Concurrently, submental EMG was also synchronized with manometric signals to confirm pharyngeal phase of swallow. EMG was measured by UPS 2020 (Medical Measurement Systems, Dover, NH). Input impedance was 2M2 Ohm differential. The output was the averaged signal that represented the swallow.
Manometry technique
We have described this technique before. 13–16 In brief, the manometric channels were calibrated at the level of mid axillary line. The catheter was placed by transnasal route in unsedated supine infants with the head in the mid line. The positioning of the pharyngeal and UES channels was ascertained by the method of station pull through technique in 0.5 cm intervals, such that UES sleeve straddled the high-pressure zone at final placement. Subjects were allowed to adapt for about 30 minutes before the pharyngeal infusion protocol.
Pharyngeal Infusion Protocol
As the neonatal pharynx is a site of frequent stimulation with air and liquids, both air and sterile water were tested. First, graded volumes of air (0.1, 0.3, 0.5, 1.0, and 2.0 mL) were infused during a period of pharyngo-esophageal quiescence to determine threshold volume and dose-response relationship. Each volume was given in the same order after allowing a period of 40–60 seconds to elapse in between, so as to ensure clearance and eliminate residual effects if any. Similarly, graded volumes of sterile water (0.1, 0.3, and 0.5 mL) were infused. Each volume was given at least twice to test consistency in responses. In pilot studies with water infusions, we noted consistent responses with 0.5 mL and absence of responses with 0.01 ml; hence we did not test 1.0 mL volumes for safety reasons and 0.01 mL volumes for lack of responses. To evaluate the response by chance alone, sham infusions (0.0 mL) related responses were recorded by placing an event marker under identical testing conditions. All infusions were administered abruptly by the same investigator (SRJ) to minimize variability.
A priori definitions and data analysis
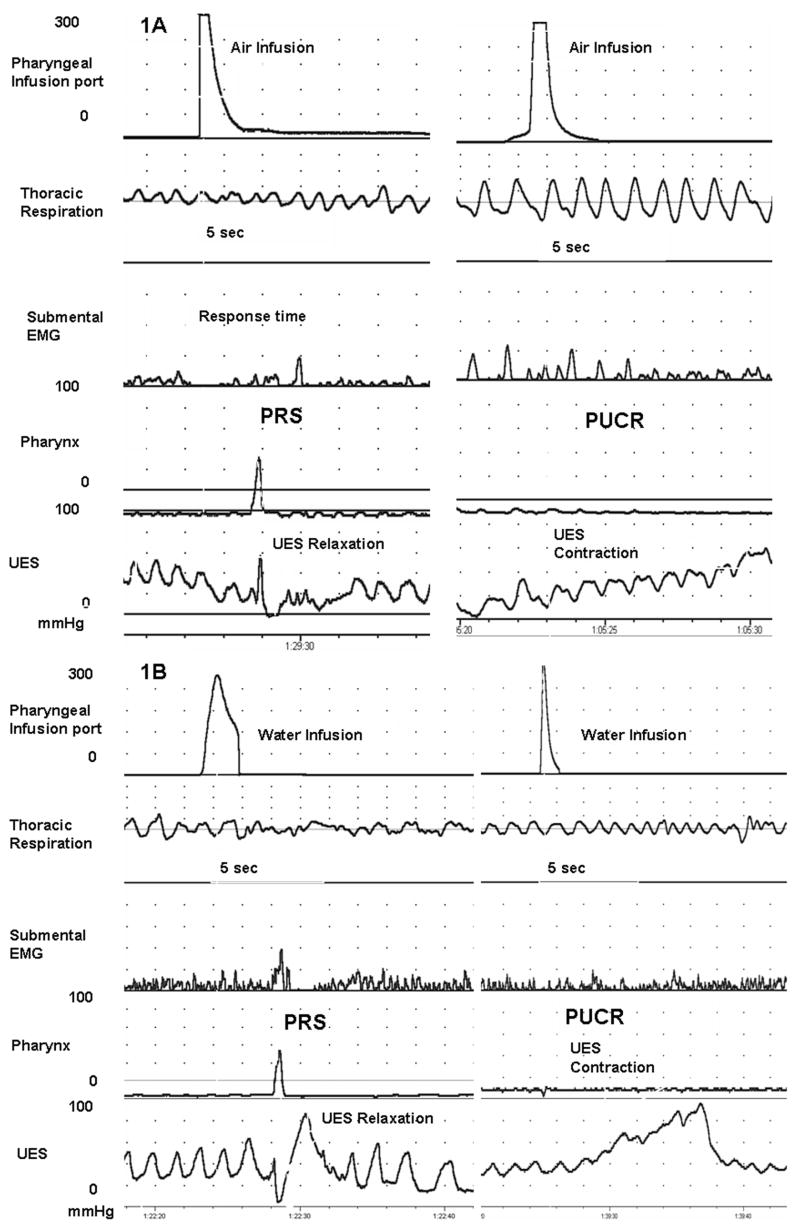
Pharyngeal Reflexive Swallow (PRS) was defined as the occurrence of pharyngeal swallow within 5 seconds of pharyngeal infusion (Figure 1). PRS was identified by the presence of pharyngeal waveform along with submental EMG signal, the relaxation of UES and propagation of the waveform into the esophageal body. Pharyngo-UES-contractile reflex (PUCR) was defined as an increase in UES pressure greater than 4 mmHg within 5 seconds of pharyngeal infusion (Figure 1).
Figure 1.
Examples of pharyngeal reflexive swallowing (PRS) and pharyngo-UES-contractile reflex (PUCR) evoked upon pharyngeal air (Figure 1A) and water (Figure 1B) infusions are shown in a representative neonate. PRS is characterized by the occurrence of pharyngeal waveform, submental EMG signal, and UES relaxation. PUCR is characterized by an increase in UES pressure after the infusion.
Pharyngo-UES-esophageal manometry waveforms were evaluated with each stimulus and compared with baseline motor activity by three observers (SRJ, AG or ES). The agreement rate for change in pharyngo-UES motor activity was 1.0 between the observers. Presence of UES relaxation or contraction with stimulus was further considered in the analysis. The agreement rate between two observers (SRJ and AG) was computed for frequency of PRS and PUCR. The proportion of agreement observed for PRS was 1.00 (0.11 SE, Cohen’s Kappa), and for PUCR was 0.94 (0.17 SE, Cohen’s Kappa).
The following sensory variables were analyzed: 1) Threshold volume, defined as the least infusion volume resulting in either response at least 50 % of the times. Mean threshold volume for each infusion medium was also calculated for each reflex. 2) Response time, defined as the time taken for the onset of the reflex response from the peak stimulus (Figure 1,A).
The following motor characteristics were analyzed: 1) Frequency occurrence of PRS or PUCR based on the a priori definition, 2) percentage distribution of PRS or PUCR or lack of a response to each stimulus mode, and 3) stimulus volume-response relationship for PRS and PUCR. Resting UES pressure was measured as the mean of 5 readings taken at end-expiration prior to each infusion. Change in UES pressure was calculated as the difference between resting UESP and maximum pressure increase after the infusion.
Statistical analysis
Descriptive data are given as mean ± SD. Nonparametric tests (signed-rank tests) were performed when averages per patient were used for the analyses. When all the individual data were used, GEE models with logit link function with repeated measurements were performed to study the media effect (water/air) on the binary outcome variables while controlling for different infusion volumes. Linear mixed effects models with repeated measures were used to study the effect of media on continuous outcome variables while controlling for different infusion volumes. Square root transformation was used when normality assumption was not met. P-value< 0.05 was considered significant. STATA v. 9.0. StataCorp. College Station, TX and SAS v9.1. SAS Institute Inc., Cary, NC were used for statistical analysis.
RESULTS
Participant characteristics
Ten (5F:5M) orally feeding healthy neonates (30 ± 5 wk gestation) that were of appropriate growth were tested at 39 ± 4 wk PMA (weight 2.6 ± 1.2 kg). The APGAR scores (median) at 1 and 5 min were 6 and 8 respectively. Infants tolerated the study without changes in cardio-respiratory measures (heart rate, respiratory rate and oxygen saturation). At discharge, all subjects received full oral feeds.
No pharyngo-UES-esophageal motor activity was noted within 10 sec after sham stimulus, compared to the baseline prior to the sham stimulus. (Figure 1, A and B)
Distribution and frequency occurrence of responses to stimuli
A total of 161 infusions were given across 10 subjects, and 155 infusions (106 air, 49 water) were analyzable (96.3%). The cumulative response rate was 30% with air infusions (32 out of 106) and 76% with water infusions (37 out of 49) (air vs. water, GEE, P = 0.033).
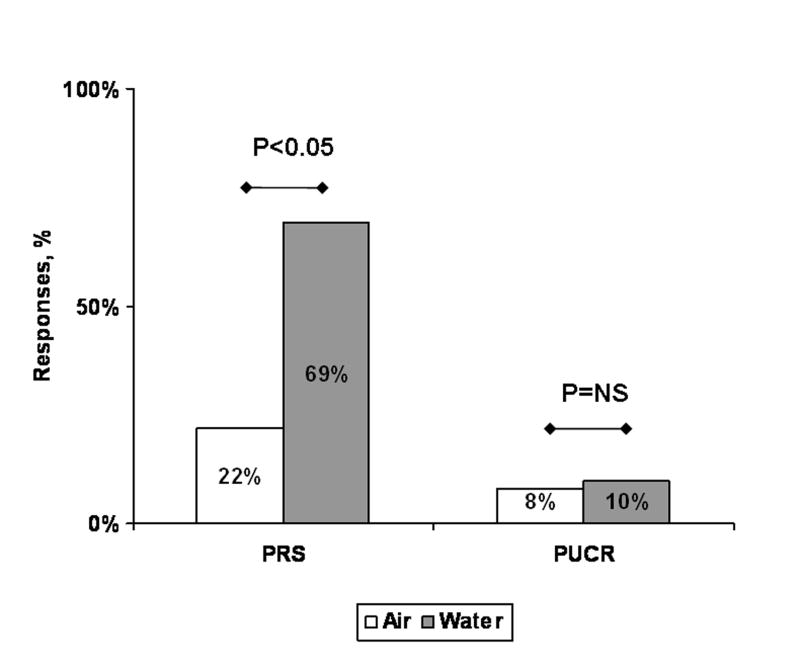
Upon further analysis of PRS and PUCR as individual responses, PRS was the most frequent response with both air and water. The frequency of PRS was also greater with water infusions (vs. air infusions, GEE, P = 0.045, Figure 2). PRS responses were solitary at 0.1 ml water infusion, and multiple PRS events were noted with volume increments (Figure 3). No statistically significant difference in PUCR response was found between water and air infusions (GEE, P=0.136).
Figure 2.
Frequency and distribution of PRS and PUCR with air and water infusions. Between air and water, the frequency of PRS was greater (P < 0.05, GEE) with water than with air stimuli. Frequency of PUCR was similar between water and air stimuli. Within the same media, the frequency occurrence of PRS was greater than PUCR with air and water stimuli.
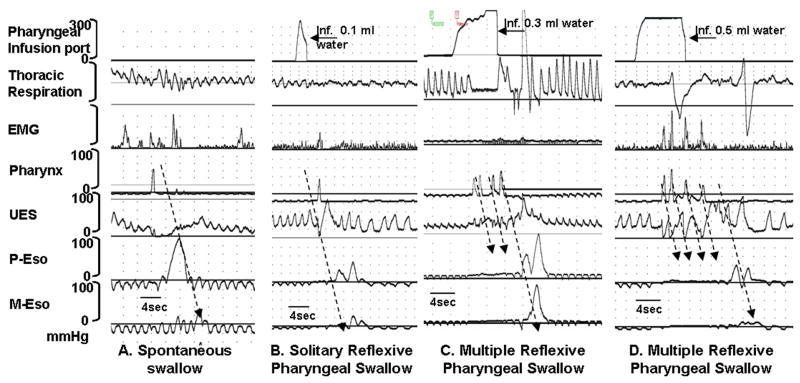
Figure 3.
Examples of A) spontaneous swallow, B) solitary PRS, and C and D) multiple swallow sequences. Each swallow is associated with submental EMG signal and UES relaxation. Recordings from P-Eso, M-Eso and D-Eso represent proximal-, middle-, and distal esophageal motility respectively. In figures A and B, note the propagation of peristaltic waveforms with solitary swallows. In figures C and D, multiple succeeding swallows inhibit the propagation of previous swallow. Only the terminal pharyngeal swallow resulted in a fully propagated sequence.
Threshold volumes and Response latency
The threshold volumes to evoke either response were significantly different: 0.4 ± 0.3 mL for air and 0.2 ± 0.1 mL for water (signed-rank test, P = 0.002). Water was a reliable stimulus and yielded consistent response with less variability. The response times to evoke PRS for air and water were not significantly different: 1.9 ± 1.3 sec and 1.5 ± 1.0 sec for air and water, respectively (P= 0.138). The response times to evoke PUCR were marginally different: 0.6 ± 1.2 and 3.0 ± 1.7 sec, for air and water, respectively (signed-rank test, P =0.063).
Stimulus Volume - Response relationship for PRS with air and water infusions
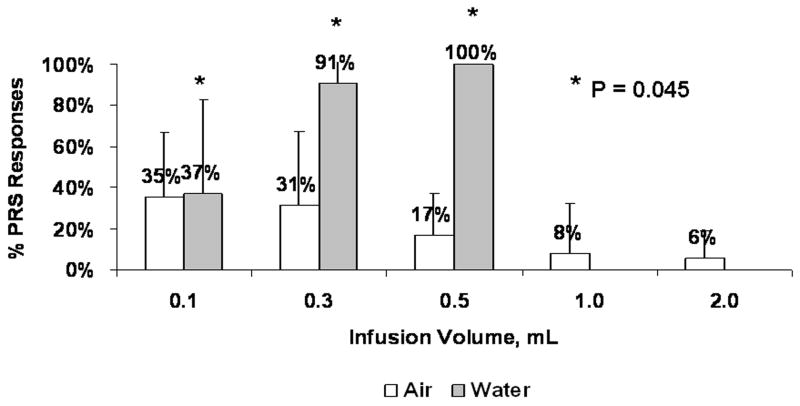
The stimulus volume - response relationship for PRS with air and water infusions is shown in Figure 4. Comparison between the PRS recruitment frequency (%) at identical doses of air and water (0.1 ml, 0.3 ml, and 0.5ml) was performed. At 0.3 ml volumes, water infusions resulted in a 3-fold increase in PRS (31 ± 36 and 91 ± 19, for air and water, respectively; GEE, P = 0.010). At 0.5 ml volumes, water infusions resulted in a 6-fold increase in PRS compared to 0.1 ml (17 ± 20 and 100 ± 0 for air and water, respectively). Water stimuli resulted in multiple swallows (Figure 3) in a dose-dependent manner. At 0.1 ml, 0.3ml, and 0.5 ml the frequency (mean ± SD) of pharyngo-UES swallows per infusion were 2 ± 1, 7 ± 5, and 5 ± 2 respectively (GEE, P = 0.0011; square root transformation was used).
Figure 4.
Stimulus Volume-PRS response relationship with air and water infusions. Note progressive increase in swallow frequency with graded volume increments of water, but not with air (P = 0.045).
Stimulus-Response relationship for PUCR with air and water
With respect to the frequency of PUCR with graded volumes of air or water infusions, the comparisons within and between air and water were similar and not significant.
DISCUSSION
The unique relationship between pharynx and UES elicited upon pharyngeal provocation in healthy neonates using novel micromanometry methods are reported. The major findings are: 1) occurrence of PRS as a chief response to air and water stimuli, 2) use of liquid as a reliable medium to evoke PRS, 3) significantly greater occurrence and distribution of PRS with increments in water volumes than in air, 4) inconsistent occurrence and distribution of PUCR with both air and water, 5) different recruitment rates of reflexes between air and water at identical volumes, 6) presence of multiple deglutition sequences with increment in volumes of water, and 7) defining a novel, safe and reliable method to investigate sensory-motor aspects of neonatal swallow physiology.
The aerodigestive protective mechanisms in human adult and animal models in response to pharyngeal infusions have been characterized. 17–21 During the propagation of deglutition sequences, the airway is protected from anterograde aspiration by the pharyngeal, UES and glottal reflexes. 18, 22, 23 In adult studies, PUCR was the most frequent response, which was associated with closure of laryngeal vestibule. Such mechanisms may protect the airway from inadvertent entry of material into larynx as in GER events. The differences in voluntary, instructional, spontaneous and reflexive swallowing have been characterized in adults at the aerodigestive tract and brain level.23 Such characteristics have not been well defined in neonates. Neonates advance with their feeding skills during development. 3, 13 Although sucking-swallowing characteristics have been described in neonates, 3, 5, 6 methods to evaluate pharyngeal and UES phase of swallowing were lacking in neonates. In this report, we defined the sensory motor aspects of pharyngeal-UES interactions in healthy neonates as a prelude to applications in neonates at risk for dysphagia.
Air stimuli have resulted in inconsistent responses, and may be related to adaptation of the pharyngeal airway to constant movement of airflow with respiration, rather than to trigger swallowing with each breath. Alternatively, as air stimuli increased in intensity, there was a decrease in frequency of PRS. This may be a defensive reflex mechanism to prevent aerophagia. The neonatal pharynx can be exposed to high air flow rates delivered with continuous positive airway pressure systems. Aerophagia and gastric distention are common clinical problems in neonates, and have been associated with variety of events including GER, belching, hiccups, feeding problems and spontaneous bowel perforation. 24, 25 Therefore, infrequency of air swallows at higher volumes observed in our study may be a mechanism to prevent aerophagia and related problems.
With water stimuli, solitary swallow sequences at lower volumes and multiple swallow sequences at higher volumes were evident. Therefore, higher liquid volumes recruit more swallow sequences to facilitate complete pharyngeal clearance. This phenomenon is a potential protective reflex mechanism to prevent aspiration at higher pharyngeal liquid volumes. Furthermore, water stimuli were more sensitive and consistent in evoking responses at a lesser volume than air stimuli. Interestingly, the response times were similar, supporting similar afferent-efferent neuromotor pathways with either stimulus.
Unlike in adults 17–18, PRS rather than PUCR, was the principal dynamic defensive response in neonates. The reasons for this disparity may be explained by structural differences: 26 1) the presence of a smaller pharyngo-UES segment, 2) absence of an oropharynx, and 3) a relatively elevated and anteriorly located larynx.
Alternatively, the frequent occurrence of PRS in neonates may be due to functional differences in pharyngo-UES interactions compared to adults. 13, 17–19 Neonates lack volitional swallowing and frequently inhibit UES contractile tone resulting in relaxation of cricopharyngeus muscle. In contrast, in adults, there may be an increase in cholinergic tone secondary to vagal neural output manifesting as an increase in resting UES pressure. 13
Mechano- or osmo- receptor stimulation of the pharynx may have stimulated the vagal-glossopharyngeal afferents, finally activating the vagal nuclei and efferents. 15, 27–31 The exact nature of mechano- or osmo- receptor stimulation is not well understood during development. In this study, the effects of sensory stimulation culminated in the UES relaxation (PRS) or UES contraction (PUCR).
Our findings may provide the physiological basis behind the safe pharyngo-UES clearance mechanisms in healthy neonates. The deglutition sequence is an important primary method of aerodigestive clearance in neonates, and may be the reason for frequent swallowing and auto resuscitation.8–9 We noted an increased frequency of multiple swallows with a larger pharyngeal liquid bolus where the succeeding swallow inhibited the esophageal propagation of the previous swallow. These aerodigestive defensive reflex responses may occur due to the presence of pharyngeal stimulus (refluxate entry into pharynx or bolus presence with feeding), and may complement the peristaltic reflexes or UES contractile reflex evoked upon esophageal provocation. 15, 29, 30, 32
There are potential translational implications of this study. Our findings may be used during a FEES (flexible endoscopic evaluation of swallow) procedure in an infant with swallowing problems. Smaller volumes of air infusions into the pharynx via the endoscope would trigger less swallows (PRS) for evaluation. Conversely, the endoscope could potentially be used to infuse graded water infusions to induce more swallows (PRS). If these responses are not observed, it could indicate a defect in the evolving neurocircuitry responsible for normal swallowing in infants. These methods may aid in the evaluation of swallowing physiology in neonates at crib side. Our data can be a reference for future studies in this vulnerable population. Pacing of swallowing skills may be dependent on bolus volume presented to pharynx, and modification of oral feeding strategies may be appropriate in slowing down the bolus flow.33 Characterization of pharyngeal-UES motor defects is a necessary step to improve the swallowing skills in infants at risk of dysphagia.
Acknowledgments
GRANT SUPPORT: This study was supported in part by NIH grant RO1 DK 068158 (SRJ)
ABBREVIATIONS
- PRS
pharyngeal reflexive swallowing
- UES
upper esophageal sphincter
- PUCR
Pharyngo-UES-contractile reflex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bu’Lock F, Woolridge MW, Bairn JD. Development of coordination of sucking, swallowing, and breathing: ultrasound study of term and preterm infants. Dev Med Child Neurol. 1990;32:669–678. doi: 10.1111/j.1469-8749.1990.tb08427.x. [DOI] [PubMed] [Google Scholar]
- 2.Miller JL, Sonies BC, Macedonia C. Emergence of oropharyngeal, laryngeal and swallowing activity in the developing fetal upper aerodigestive tract: an ultrasound evaluation. Early Hum Dev. 2003;71:61–87. doi: 10.1016/s0378-3782(02)00110-x. [DOI] [PubMed] [Google Scholar]
- 3.Gewolb IH, Vice FL, Schweitzer-Kenney EL, Taciak VL, Bosma JF. Developmental patterns of rhythmic suck and swallow in preterm infants. Developmental Medicine and Child Neurology. 2001;43:22–27. doi: 10.1017/s0012162201000044. [DOI] [PubMed] [Google Scholar]
- 4.Mercado-Deane MG, Burton EM, Harlow SA, Glover AS, Deane DA, Guill MF, Hudson V. Swallowing dysfunction in infants less than 1 year of age. Pediatr Radiol. 2001;31:423–8. doi: 10.1007/s002470100456. [DOI] [PubMed] [Google Scholar]
- 5.Lau C, Alagurusamy R, Schanler RJ, Smith EO, Shulman RJ. Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta Paediatr. 2000;89:846–52. [PubMed] [Google Scholar]
- 6.Lau C, Smith EO, Schanler RJ. Coordination of suck-swallow and swallow respiration in preterm infants. Acta Paediatr. 2003;92:721–7. [PubMed] [Google Scholar]
- 7.Pickens DL, Schefft GL, Thach BT. Pharyngeal fluid clearance and aspiration preventive mechanisms in sleeping infants. J Appl Physiol. 1989;66:1164–1171. doi: 10.1152/jappl.1989.66.3.1164. [DOI] [PubMed] [Google Scholar]
- 8.Thach BT. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am J Medicine. 2001;111:69S–77S. doi: 10.1016/s0002-9343(01)00860-9. [DOI] [PubMed] [Google Scholar]
- 9.Page M, Jeffery HE. Airway protection in sleeping infants in response to pharyngeal fluid stimulation in the supine position. Pediatr Res. 1998;44:691–8. doi: 10.1203/00006450-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Omari T, Snel A, Barnett C, Davidson G, Haslam R, Dent J. Measurement of upper esophageal sphincter tone and relaxation during swallowing in premature infants. Am J Physiol. 1999;277:G862–6. doi: 10.1152/ajpgi.1999.277.4.G862. [DOI] [PubMed] [Google Scholar]
- 11.Willing J, Davidson GP, Dent J, Cook I. Effect of gastro-esophageal reflux on upper esophageal sphincter motility in children. Gut. 1993;34:904–10. doi: 10.1136/gut.34.7.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jadcherla SR, Shaker R. Esophageal and UES motor function in babies. Am J Medicine. 2001;111:64–68. doi: 10.1016/s0002-9343(01)00848-8. [DOI] [PubMed] [Google Scholar]
- 13.Jadcherla SR, Duong HQ, Hofmann C, Hoffmann R, Shaker R. Characteristics of upper esophageal sphincter and esophageal body during maturation in healthy human neonates compared with adults. Neurogastroenterol Motil. 2005;17:663–70. doi: 10.1111/j.1365-2982.2005.00706.x. [DOI] [PubMed] [Google Scholar]
- 14.Jadcherla SR. Manometric evaluation of esophageal-protective reflexes in infants and children. Am J Medicine. 2003;115 :157–160. doi: 10.1016/s0002-9343(03)00215-8. [DOI] [PubMed] [Google Scholar]
- 15.Jadcherla SR, Duong HD, Jadcherla SR, Duong HQ, Hoffmann RG, Shaker R. Esophageal body and upper esophageal sphincter motor responses to esophageal provocation during maturation in preterm newborns. J Pediatr. 2003;143:31–8. doi: 10.1016/S0022-3476(03)00242-7. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Jadcherla SR. The relationship between somatic growth and In Vivo esophageal segmental and sphincteric growth in human neonates. J Pediatr Gastroenterol and Nutr. 2006;43:35–41. doi: 10.1097/01.mpg.0000226368.24332.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaker R, Hogan WJ. Reflex-mediated enhancement of airway protective mechanisms. Am J Med. 2000;108:8–14. doi: 10.1016/s0002-9343(99)00289-2. [DOI] [PubMed] [Google Scholar]
- 18.Shaker R, Ren J, Xie P, Lang IM, Bardan E, Sui Z. Characterization of the pharyngo-UES contractile reflex in humans. Am J Physiol. 1997;273:G854–8. doi: 10.1152/ajpgi.1997.273.4.G854. [DOI] [PubMed] [Google Scholar]
- 19.Dua K, Bardan E, Ren J, Sui Z, Shaker R. Effect of chronic and acute cigarette smoking on the pharyngoglottal closure reflex. Gut. 2002;51:771–5. doi: 10.1136/gut.51.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang IM, Shaker R. An overview of the upper esophageal sphincter. Curr Gastroenterol Rep. 2000;2:185–90. doi: 10.1007/s11894-000-0059-z. [DOI] [PubMed] [Google Scholar]
- 21.Lang IM, Shaker R. Anatomy and physiology of the upper esophageal sphincter. Am J Med. 1997;103:50S–55. doi: 10.1016/s0002-9343(97)00323-9. [DOI] [PubMed] [Google Scholar]
- 22.Kendall KA. Oropharyngeal swallowing variability. Laryngoscope. 2002;112:547–51. doi: 10.1097/00005537-200203000-00025. [DOI] [PubMed] [Google Scholar]
- 23.Kahrilas PJ, Dodds WJ, Dent J, Logemann JA, Shaker R. UES function during deglutition. Gastroenterology. 1988;96:52–62. doi: 10.1016/0016-5085(88)90290-9. [DOI] [PubMed] [Google Scholar]
- 24.Wilson SL, Thach BT, Brouillette RT, Abu-Osba YK. Coordination of breathing and swallowing in human infants. J Appl Physiol: Respirat Environ Exercise Physiol. 1981;50:851–858. doi: 10.1152/jappl.1981.50.4.851. [DOI] [PubMed] [Google Scholar]
- 25.Hwang JB, Choi WJ, Kim JS, Lee SY, Jung CH, Lee YH, Kam S. Clinical features of pathologic childhood aerophagia: Early recognition and essential diagnostic criteria. JPGN. 2005;41:612–616. doi: 10.1097/01.mpg.0000179856.68968.e0. [DOI] [PubMed] [Google Scholar]
- 26.Arvedson JC, Lefton-Greif MA. Pediatric Videofluoroscopic Swallow Studies: A Professional Manual with Caregiver Guidelines. The psychological corporation; San Antonio, TX: 1998. [Google Scholar]
- 27.Goyal RK, Padmanabhan R, Sang Q. Neural circuits in swallowing and abdominal vagal afferent-mediated lower esophageal sphincter relaxation. Am J Med. 2001;111:95–105. doi: 10.1016/s0002-9343(01)00863-4. [DOI] [PubMed] [Google Scholar]
- 28.Broussard DL, Altschuler SM. Central integration of swallow and airway-protective reflexes. Am J Med. 2000;108:62–67. doi: 10.1016/s0002-9343(99)00340-x. [DOI] [PubMed] [Google Scholar]
- 29.Sengupta JN, Kauvar D, Goyal RK. Characteristics of vagal esophageal tension-sensitive afferent fibers in the opossum. J Neurophysiol. 1989;61:1001–1010. doi: 10.1152/jn.1989.61.5.1001. [DOI] [PubMed] [Google Scholar]
- 30.Longhi EH, Jordan PH. Necessity of a bolus for propagation of primary peristalsis in the canine esophagus. Am J Physiol. 1971;220:609–612. doi: 10.1152/ajplegacy.1971.220.3.609. [DOI] [PubMed] [Google Scholar]
- 31.Lang IM, Medda BK, Shaker R. Mechanisms of reflexes induced by esophageal distention. Am J Physiol (GI and Liver Physiol) 2001;281:G1246–G1263. doi: 10.1152/ajpgi.2001.281.5.G1246. [DOI] [PubMed] [Google Scholar]
- 32.Jadcherla SR, Hoffmann RG, Shaker R. Effect of maturation on the magnitude of mechanosensitive and chemosensitive reflexes in the premature human esophagus. J Pediatr. 2006;141:77–82. doi: 10.1016/j.jpeds.2006.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arvedson JC. Management of pediatric dysphagia. Otolaryngol Clin North Am. 1998 Jun;31:453–76. doi: 10.1016/s0030-6665(05)70064-5. [DOI] [PubMed] [Google Scholar]