Abstract
Introduction
a heparin preparation with low antithrombin activity and different disaccharide composition than mammalian heparin was isolated from the body of the ascidian Styela plicata (Chordata-Tunicata). The disaccharide composition and the effect of the invertebrate glycan on venous and arterial models of thrombosis was investigated.
Methods and Results
High performance liquid chromatography of the products formed by a mixture of heparin-lyases showed that the ascidian heparin is composed mainly by ΔUA(2SO4)-1→4-β-D-GlcN(SO4) (47.5%), ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(6SO4) (38.3%) disaccharides. Smaller amounts of the disaccharides ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4)(6SO4) (2.8%) and ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4) (8.0%). The invertebrate heparin has an aPTT activity of 18 IU/mg and an antithrombin-mediated anti-thrombin and anti-factor Xa activities 10-fold lower than that of mammalian heparin. In a venous model of thrombosis in the vena cava, S.plicata heparin inhibits only 80% of thrombosis at a dose 10-fold higher than that of the mammalian heparin that inhibits 100% of thrombosis. However, in an arterio-shunt model of arterial thrombosis, both S.plicata and mammalian heparin possess equivalent antithrombotic activity. It is also shown that at equivalent doses, ascidian heparin has a lower bleeding effect than mammalian heparin.
Conclusion
the antithrombin-mediated anticoagulant activity of heparin polymers is not directly related to antithrombotic potency in the arterio-venous shunt. The results of the present work suggest that heparin preparations obtained from the body of S.plicata may have a safer therapeutic action in the treatment of arterial thrombosis than mammalian heparin.
Keywords: Heparin, dermatan sulfate, antithrombotic effect, arterio-venous model, venous model, ascidian
Introduction
Heparin is an anticoagulant glycosaminoglycan (GAG) used in the clinical practice to prevent thrombo-embolic events [1,2]. The anticoagulant activity of heparin is due to the presence of various antithrombin (AT)-binding sequences including the well know pentasaccharide [GlcNAc(6SO4)-GlcA-GlcNS(3SO4)-IdoA(2SO4)-GlcNS(6SO4)] [5–12]. In the presence of heparin, the rates of inhibition of thrombin, factor IXa, and factor Xa by AT are increased ~ 1,000-fold so that inhibition is essentially instantaneous [13].
Currently, commercial heparin preparations are obtained from mammalian sources, either from porcine or bovine intestine or bovine lung. Non-animal sources of heparin for pharmaceutical use are currently not available. However, the occurrence of heparin is not restricted to mammals. Several heparin and heparin-like polymers have been described in invertebrate animals, such as mollusks [14–20], crustaceans [19–23] and ascidians [24–26].
Ascidians, also known as tunicates, are marine invertebrates that belong to the phylum Chordate, subphylum Urochordate. The characteristic feature of this group is the presence of an external tunic, which protects and supports the body of the animal. Different types of sulfated polysaccharides and GAGs have been reported in ascidians. The sulfated polysaccharides are restricted to the tunic, occurring mainly as a high molecular weight sulfated L-galactan [27–30]. No GAGs are present in the tunic, but occur in high amounts in different tissues of the body [31–35]. In the ascidian Styela plicata, an oversulfated dermatan sulfate composed mainly by IdoA(2SO4)-GalNAc(4SO4) disaccharide units and a heparin composed mainly by ΔUA(2SO4)-GlcN(SO4)(6SO4) disaccharide units have been previously detected in the intestine, heart, pharynx and mantle [35]. In the intestine and pharynx, heparin occurs in intracellular granules of epithelial cells that form a layer separating the organ from the external environment [24]. Heparin with a similar disaccharide composition has also been detected in intracellular granules of accessory cells, namely test cells, that surround the oocyte of S.plicata [24].
Recently, we reported the occurrence of heparin inside intracellular granules of a basophil-like cell, which circulates in the hemolymph of S.plicata. Disaccharide analysis of this heparin indicated that the polymer is composed mainly by ΔUA(2SO4)-1→4-β-D-GlcN(SO4) (39.7%) and ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(6SO4) (38.2%). Small amounts of the 3-O-sulfated disaccharides ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4) (9.8%) and ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4)(6SO4) (3.8%) were also detected [submitted for publication].
Analysis of the anticoagulant activity of the heparins obtained from intestine, heart, pharynx and mantle of S.plicata, in addition to that from the test cells, indicated that these polymers inhibit thrombin activity by antithrombin in a concentration at least 10-fold higher than that required for mammalian heparin to achieve the same inhibitory effect [24,35]. However, in the case of the heparin obtained from the circulating basophil-like cell, the anti-thrombin activity is equivalent to that of mammalian heparin [submitted for publication]. Overall, these data show that heparin polymers with apparently distinct disaccharide composition and different anticoagulant activities occur in different types of cells of S.plicata. Therefore, it would be interesting to investigate the composition and the anticoagulant/antithrombotic activity, as well as the bleeding effect of the resulting heparin preparation obtained from the whole body of the ascidian.
The study of the relationship between structure and anticoagulant activity of new heparin polymers and their effect on thrombosis models in animals that mimic different pathophysiological conditions may contribute to the discovery of new antithrombotic agents that are effective in specific thrombotic conditions.
Our results show that the AT-mediated anti-thrombin and anti-factor Xa activities of the heparin preparation obtained from the body of S.plicata are 10-fold lower than that of mammalian heparin and this lower anticoagulant activity correlates directly with a lower antithrombotic effect of the ascidian heparin in a venous model of thrombosis. However, in an arterial model, both S.plicata and mammalian heparin possess equivalent antithrombotic activity. It is also shown that at equivalent doses, ascidian heparin has a lower bleeding effect than mammalian heparin.
Material and Methods
Materials
Heparan sulfate from human aorta was extracted and purified as described previously [36]. Chondroitin 4-sulfate from whale cartilage, chondroitin 6-sulfate from shark cartilage, dermatan sulfate and heparin from porcine intestinal mucosa (180 Units/mg), twice-crystallized papain (15 units/mg protein) and the standard disaccharides α-ΔUA-1→4-GlcN(SO4), α-ΔUA-1→4-GlcNAc(6SO4), α-ΔUA(2SO4)-1→4-GlcNAc, α-ΔUA(2SO4)-1→4-GlcN(SO4), α-ΔUA(2SO4)-1→4-GlcNAc(6SO4), α-ΔUA-1→4-GlcN(SO4)(6SO4) and α-ΔUA(2SO4)-1→4-GlcN(SO4)(6SO4) were purchased from Sigma (St. Louis, MO, USA); chondroitin AC lyase (EC 4.2.2.5) from Arthrobacter aurenses, chondroitin ABC lyase (EC 4.2.2.4) from Proteus vulgaris, heparan sulfate lyase (EC 4.2.2.8) and heparin lyase (EC 4.2.2.7) from Flavobacterium heparinum were from Seikagaku America Inc. (Rockville, MD, USA). For HPLC-SAX experiments, the enzymes lyases from Flavobacterium heparinum Heparinase I (EC 4.2.2.7), Heparinase II (no EC number), Heparinase III (EC 4.2.2.8) were obtained from Grampian Enzymes (Aberdeen). Agarose (standard low Mr) was obtained from Bio-Rad (Richmond, CA, USA); Toluidine blue was from Fisher Scientific (NJ, USA) and 1,9-dimethylmethylene blue from Serva Feinbiochimica (Heidelberg, Germany); human antithrombin, human factor Xa, and thrombin were from Hematologic Technologies Inc. (VT, USA) or from Hyphen Biomed (France); thrombin chromogenic substrate tosyl-Gly-Pro-Arg-p-nitroanilide acetate (Chromozyn TH) and factor Xa chromogenic substrate N-methoxycarbonyl-D-norleucyl-glycyl-L-arginine-4-nitranillide-acetate were from Roche/Amersham-Biosciences from Brazil.
Extraction of the sulfated polysaccharides
Adult specimens of the ascidian Styela plicata were collected in Rio de Janeiro at Guanabara Bay. Animals were maintained in an aerated aquarium until use. The body of the ascidians was separated from the tunic, which was discarded. The body was cut in small pieces, immersed in acetone and kept for 24 h at 4°C. The dried body (1 g) was suspended in 20 ml of 0.1 M sodium acetate buffer (pH 5.5), containing 100 mg papain, 5 mM EDTA, and 5 mM cysteine and incubated at 60°C during 24 h. The incubation mixture was then centrifuged (2000 × g for 10 min at room temperature), the supernatant separated and the precipitate was incubated with papain twice-again, as described above. The polysaccharides were then precipitated from the clear supernatant of the three extractions with 2 vol. of 95% ethanol and maintained at 4°C for 24 h. The precipitates formed were collected by centrifugation (2000 × g for 10 min at room temperature) and freeze dried.
Electrophoretic analysis
Agarose gel- the crude glycans from the body or the purified heparin from S.plicata (1.5 μg as uronic acid), before or after incubation with specific GAG lyases, were analyzed by agarose gel electrophoresis, as described previously [31]. Briefly, the glycans, and a mixture of standard GAGs, containing chondroitin sulfate, dermatan sulfate and heparan sulfate (1.5 μg as uronic acid of each) were applied to a 0.5% agarose gel in 0.05 M 1,3-diaminopropane/acetate (pH 9.0), and run for 1 h at 110 mV. After electrophoresis the glycans were fixed with aqueous 0.1 % cetylmethylammonium bromide solution and stained with 0.1% toluidine blue in acetic acid/ethanol/water (0.1:5:5, v/v). Polyacrylamide gel- the molecular mass of the purified heparin from S.plicata was estimated by polyacrylamide gel electrophoresis. Samples (~ 10 μg) were applied to a 1-mm-thick 6% polyacrylamide slab gel, and after electrophoresis at 100 V for ~1 h in 0.06 M sodium barbital (pH 8.6), the gel was stained with 0.1% toluidine blue in 1% acetic acid. After staining, the gel was washed overnight in 1% acetic acid. The molecular mass markers used were: dextran 500 (average MW 500,000), chondroitin 4-sulfate from whale cartilage (average MW 34,000); chondroitin 6-sulfate from shark cartilage (average MW 64,000), porcine intestinal mucosa heparin (average MW 18,000) and porcine intestinal dermatan sulfate (average MW 40,000).
Purification of the S.plicata heparin
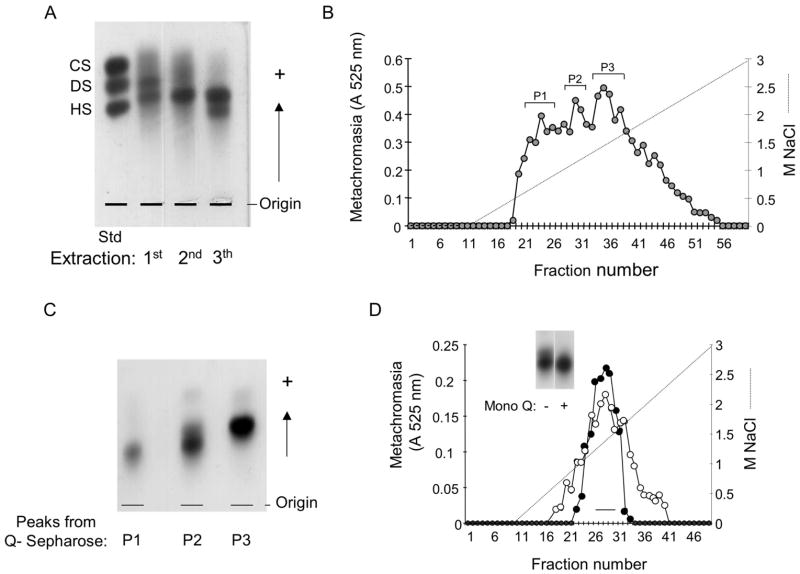
The glycans obtained in the third extraction of the tissues of S.plicata (~8 mg), containing a mixture of heparin and dermatan sulfate were applied to a Q-sepharose-FPLC column, equilibrated with 20 mM Tris/HCl buffer (pH 8.0). The glycans were eluted by a linear gradient of 0–3.0 M NaCl (150 ml) at a flow rate of 2.0 ml/min. Fractions of 1.5 ml were collected and checked by metachromatic assay using 1,9-dimethyl-methylene blue [37]. Fractions eluted with different NaCl concentrations were pooled as peaks and denominated P1, P2 and P3, as indicated in Figure 1 B. The peaks were dialyzed against distilled water and lyophilized.
Figure 1. Purification of the S.plicata heparin.
A. Agarose gel electrophoresis of the crude glycans isolated from the body of S.plicata. The body of the ascidian was submitted to 3 proteolytic digestions and the glycans (~1.5 μg, as uronic acid) obtained in each extraction were applied to a 0.5% agarose gel in 0.05 M 1,3-diaminopropane/acetate (pH 9.0), along with a mixture of standard glycosaminoglycans, containing chondroitin sulfate (CS), dermatan sulfate (DS) and heparan sulfate (HS). B. Ion-exchange chromatography on Q-sepharose. The glycans from the 3rd extraction (~8 mg) were fractionated on a Q-sepharose column, as described in Material and Methods. Fractions eluted with different NaCl concentrations were pooled, and denominated P1, P2 and P3. C. Agarose gel electrophoresis of the fractions from the Q-sepharose column. The glycans pooled in P1, P2 and P3 (~1.5 μg, as uronic acid) were applied to a 0.5% agarose gel in 0.05 M 1,3-diaminopropane/acetate (pH 9.0) and submitted to an electophoretic run as described in Material and Methods. D. Ion-exchange chromatography on a Mono Q column. About 2 mg of P2 from the Q-sepharose column of mammalian (-●-) or S.plicata heparin (-○-) were applied to a Mono Q column and the chromatography developed as described in Material and Methods. The fractions eluted at ~ 1.2 M NaCl were pooled as indicated by the horizontal bar and analized by agarose gel electrophoresis (insert), as described earlier.
The glycans (~2 mg) obtained in fraction P2 from the Q-sepharose column were applied to a Mono Q-FPLC column, equilibrated with 20 mM Tris/HCl buffer (pH 8.0). The glycans were eluted by a linear gradient of 0–3.0 M NaCl (45 ml) at a flow rate of 0.5 ml/min. Fractions of 0.5 ml were collected and checked by metachromatic assay, as described above. The glycans eluted at the center of the peak with ~ 1.2 M NaCl were pooled as indicated in Figure 1 D, dialyzed against distilled water and lyophilized.
Enzymatic treatment
Chondroitin lyases- the purified S.plicata heparin (~50 μg) was incubated with 0.01 U of chondroitin AC or ABC lyase in 0.1 ml 50 mM Tris-HCl buffer (pH 8.0), containing 5 mM EDTA and 15 mM sodium acetate. After incubation at 37°C for 12 h, the mixtures were analyzed by agarose gel electrophoresis, as described earlier.
Heparan sulfate and heparin lyases- about 50 μg (as dry weight of each) of the purified S.plicata heparin was incubated with 0.005 U of either heparan sulfate lyase or heparin lyase in 100 μL of 100 mM sodium acetate buffer (pH 7.0), containing 10 mM calcium acetate for 17 h at 37°C. At the end of the incubation period, the mixtures were analyzed by agarose gel electrophoresis, as described earlier.
CTA-SAX chromatography of S.plicata heparin digests- preparation of the digests: exhaustive digestion of the S.plicata heparin (0.1 mg) was performed at room temperature for 48 h with a mixture of 2.5 mIU of heparinase I, 2.5 mIU of heparinase II and 2.5 mIU of heparinase III in a total volume of 30 μl of 100 mM sodium acetate buffer, pH 7.0, containing 2 mM Ca(OAc)2 and 2 mg/ml BSA. The columns used for the chromatography of the heparin digests were dynamically coated with CTA, as described earlier [38]. The eluting salt was ammonium methane sulfonate, prepared by neutralization until pH 2.5 of methane sulfonic acid by aqueous ammonia solutions. The solvent B of CTA-SAX was 2 M ammonium methane sulfonate at pH 2.5. The solvent A was water (quality Milli-Q) adjusted to pH 3 by addition of methane sulfonic acid. A linear gradient starting from 1% B to 100% B within 74 min was used at a flow rate of 0.22 ml/min. After 74 min, the percentage of B remained at 100%. After each run, a reconditioning step of 18 min was used. Column temperature was 40°C.
Anticoagulant action measured by aPTT (Activated Partial Thromboplastin Time)- activated partial thromboplastin clotting time assays were carried out as following: normal human plasma (100 μl) was incubated with 10 μl of a solution of mammalian or S.plicata heparin (0.01–100 μg) at 37°C for 1 min. Then 100 μl of APTT reagent (Celite – Biolab) were added and incubated at 37°C. After 2 min of incubation 100 μl of 0.25 M CaCl2 were added to the mixtures and the clotting time recorded in a coagulometer (Amelung KC4A).
Inhibition of thrombin or factor Xa by antithrombin in the presence of mammalian or ascidian heparin- these effects were evaluated by the assay of amydolytic activity of thrombin, using chromogenic substrate, as described [24]. Incubations were performed in disposable UV semi-microcuvettes. The final concentrations of reactants included 50 nM antithrombin, 15 nM thrombin, 20 nM factor Xa and 0–10 μg/ml heparin in 100 μl of 0.02 M Tris/HCl, 0.15 M NaCl, and 1.0 mg/ml polyethylene glycol (pH 7.4) (TS/PEG buffer). Thrombin or factor Xa was added last to initiate the reaction. After 60-s incubation at room temperature, 500 μl of 100 μM chromogenic substrate Chromogenix TH or N-methoxycarbonyl-D-norleucyl-glycyl-L-arginine-4-nitranillide-acetate in TS/PEG buffer was added and the absorbance at 405 nm was recorded for 100 s. The rate of change of absorbance was proportional to the thrombin or factor Xa activity remaining in the incubation. No inhibition occurred in control experiments in which thrombin or factor Xa was incubated with antithrombin in the absence of heparin. Nor did inhibition occur when thrombin or factor Xa was incubated with heparin alone over the range of concentrations tested.
Animal procedures
All animal work was carried out in accordance with the Brazilian Animal Protection Law and we followed the institutional guidelines for animal care and experimentation.
Venous thrombosis- the antithrombotic activity of mammalian or S.plicata heparin was investigated in rats with brain thromboplastin as the thrombogenic stimulus [39]. Briefly, Wistar rats (both sexes, ~300 g body weight) were anaesthetized by intramuscular injection of 100 mg/kg body weight of ketamine (gently donated by Cristalia, São Paulo, Brazil) and 16 mg/kg body weight of xylasin (Bayer AS, São Paulo, Brazil). The abdomen of each animal was opened and the vena cava was carefully dissected. A segment of 0.7 cm was prepared beginning just below the branch of the right renal vein up to after the left renal vein, which was ligated. Mammalian heparin (Sigma-Aldrich, 180 units/mg) or S.plicata heparin at the doses of 0.25–2.0 mg/kg body weight were administered intravenously 2.0 cm below the distal loose suture and allowed to circulate for 5 min. Then, brain thromboplastin (Biolab-Merieux AS, Rio de Janeiro, Brazil) (5 mg/kg body weight) was slowly injected intravenously 1.0 cm below the distal loose suture and the venous segment was initially clamped at the proximal site and then at the distal suture. After 20 min of stasis, the thrombus formed in the occluded segment was washed with 5 % sodium citrate, dried for 1 h at 60°C and weighted. At least 5 animals were used per group. Mean thrombus weight was obtained by the average weight from each group. The results are expressed as percentage of thrombosis and 100% of thrombosis represents the average thrombus weight in the absence of any amount of the polysaccharide.
Arterial-venous shunt- the effect of mammalian or S.plicata heparin on arterial thrombosis was investigated by the arterio-venous shunt thrombosis model adapted from the technique of Vogel [39]. Under anesthesia, the jugular vein and the carotid artery of the opposite side were exposed, and the silk thread side of the shunt tubing was inserted into the jugular vein and ligated. After three minutes, the glycan dissolved in physiologic saline, was injected into the tail vein (1 ml/kg), the glass tube side of the shunt tubing was cannulated into the carotid artery. After five minutes of the glycan administration, the blood was allowed to flow into the shunt tubing. After 5 minutes of perfusion, the silk thread was pulled out of the tube, gently washed in 3.8% sodium citrate, dried at room temperature overnight, and weighed. The weight gain was considered to represent clot accretion onto the thread. The results are expressed as % of thrombosis as described in the previous paragraph. At least 5 animals were used per group.
Ex-vivo anticoagulant action measured by aPTT (activated Partial Thromboplastin Time)- to determine the effect of S.plicata heparin on coagulation, Wistar rats (both sexes, ~ 300g body weight) were anaesthetized with an intramuscular injection of 100 mg/Kg of ketamine (Cristália, São Paulo, Brazil) and 16 mg/Kg of xylazine (Bayer AS, São Paulo, Brazil), supplemented as needed. The right carotid artery was isolated and cannulated with a 22-gauge catheter (Jelco, Johnson & Johnson Medical Inc., USA) for blood collection and injection of the heparin samples. Blood samples (~ 500μl) were collected into 2.8% sodium citrate (9:1, v/v) before and 5, 10, 15, 30 and 60 min after infusion of 2.0 mg/Kg of the ascidian heparin, for analysis of aPTT. At least 4 animals were used per group.
Bleeding effect- for evaluation of the bleeding effect, Wistar rats (both sexes, ~ 300g body weight) were anaesthetized with a combination of xylazine and ketamine, as described above. A cannula was inserted into the right carotid artery for administration of heparin samples (0.5 or 5.0 mg/Kg). The polysaccharide was then allowed to circulate for 5 min and the rat-tail was cut 3 mm from the tip. The tail was carefully immersed in 40 ml of distillated water at room temperature. Blood loss was determined 60 min later by measurement of the hemoglobin content in water solution using a spectrophotometric method, as described by Herbert et al, (40). The volume of blood was deduced from a standard curve based on absorbance at 540 nm. At least 4 animals were used per group. Hexuronic acid- the hexuronic acid content of the glycans from the various tissues was estimated by the carbazole reaction [41].
Statistical analysis- results are expressed as mean ± standard deviation. The difference between two groups was tested using the T-test.
Results
Isolation and characterization of S.plicata heparin
The body of the ascidian S.plicata was separated from the tunic and subjected to three consecutive proteolytic digestions with papain. The polysaccharides obtained in each extraction were precipitated with ethanol and analyzed by agarose gel electrophoresis. Metachromatic bands with different migration patterns, representing distinct glycans were observed in each extraction (Figure 1 A). A band migrating between standard heparan sulfate (HS) and dermatan sulfate (DS) is present in the material obtained in the first, second and third extractions. This band corresponds to a DS polymer, which was characterized previously [33]. Another band migrating as standard DS is present only in the first extraction and represents a non-identified sulfated polysaccharide. The material obtained in the third extraction contained, in addition to the DS band, a band migrating approximately as standard HS. The material obtained in the third extraction, containing a mixture of DS and a HS-migrating polymer was applied to a Q-sepharose column, as described under Material and Methods. Three peaks, named P1, P2 and P3, eluted from the column with different NaCl concentrations (Figure 1 B). The material recovered in P1 was very scarce and was discarded. P3 eluted from the ion-exchange column free of contaminating material, as indicated by a homogeneous metachromatic band on agarose gel (Figure 1 C). P2 is enriched with the HS-migrating glycan, but is slightly contaminated with DS. A further purification of the HS-migrating glycan was achieved by another ion-exchange chromatography on a Mono Q column. The HS-glycan was eluted from the column at the same NaCl concentration (~1.3 M) required to elute mammalian heparin (Figure 1 D). The fractions eluted at the center of the peak were pooled and analyzed by agarose gel (Figure 1 D, insert). A homogeneous metachromatic band free of contaminating DS was obtained and used in further experiments. After the purification steps, about 2.6 mg of the HS-migrating material was recovered from 1 g of the dry body of S.plicata.
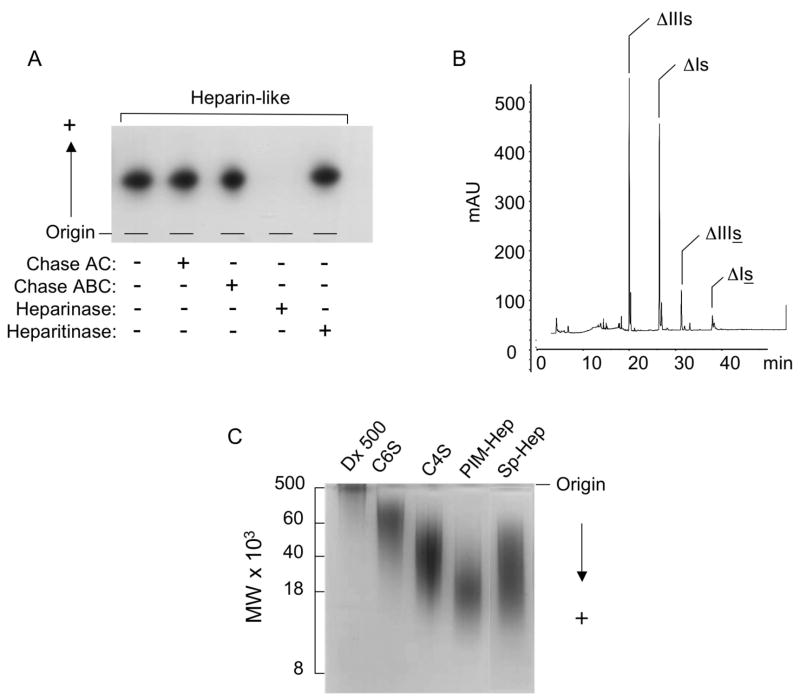
In order to obtain information about the nature of the HS-migrating glycan, the purified polymer from the Mono Q column was incubated with chondroitinase AC-/ABC-lyase and heparin-/heparan sulfate-lyase, and the products were analyzed by agarose gel electrophoresis (Figure 2 A). The HS-migrating glycan was resistant to the action of chondroitin AC/ABC lyases, as well as heparan sulfate lyase, but was completely degraded by heparin lyase, indicating that this is a heparin-like glycosaminoglycan. CTA-SAX HPLC analysis of the products formed by the action of heparin-lyase (I, II and III) on the purified S.plicata heparin revealed that it is formed mainly by the disaccharides ΔUA(2SO4)-1→4-β-D-GlcN(SO4) (47.5%) and ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(6SO4) (38.3%). Smaller amounts of the disaccharides ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4)(6SO4) (2.8%) and ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4) (8.0%) were also present (Figure 2 B and Table 1).
Figure 2. Characterization of the S.plicata heparin.
A. Agarose gel electrophoresis. The purified glycan from the Mono Q column (~50 μg) was analyzed by agarose gel electrophoresis, before (−) or after (+) incubation with chondroitin AC (Chase AC) or ABC (Chase ABC) lyases, or heparin (heparanse) and heparan sulfate lyases (heparitinase), as described in Materials and Methods. B. HPLC analysis. The disaccharides formed by exhaustive action of heparinase I, II, III on the purified S.plicata heparin were applied to a CTA-SAX HPLC column. The column was eluted with a gradient of NaCl as described in Material and Methods. The eluant was monitored for UV absorbance at 232 nm. The assigned peaks correspond to the disaccharides: ΔIIIs, ΔUA(2SO4)-1→4-β-D-GlcN(SO4); ΔIs, ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(6SO4); ΔIs, ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4)(6SO4); ΔIIIs, ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4). C. Polyacrylamide gel electrophoresis. The purified S.plicata heparin (Sp-Hep) (~ 10 μg dry weight) and the molecular weight markers dextran 500 (Dx 500, average MW 500,000), chondroitin 4-sulfate from whale cartilage (C4S, average MW 34,000); chondroitin 6-sulfate from shark cartilage (C6S, average MW 64,000), porcine intestinal mucosa heparin (PIM, average MW 18,000) and porcine intestinal dermatan sulfate (average MW 40,000) (~ 10 μg as dry weight of each) were applied to a 1-mm-thick 6% polyacrylamide slab gel, as described in Material and Methods.
Table 1.
Disaccharide composition of ascidian and mammalian heparins.
| Disaccharide | % of the disaccharides
|
||
|---|---|---|---|
| Test cella | Whole bodyb | PIHa,c | |
| ΔUA-1→4-β-D-GlcN(6SO4) | <1 | 1.4 | <5 |
| ΔUA-1→4-β-D-GlcN(SO4)(6SO4) | 25 | 2.0 | 9-11 |
| ΔUA(2SO4)-1→4-β-D-GlcN(SO4) | <1 | 47.5 | 6-8 |
| ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(6SO4) | 75 | 38.3 | 60-70 |
| ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4) | <1 | 8.0 | <1 |
| ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4)(6SO4) | <1 | 2.8 | <1 |
Data from reference 24.
This work.
PIH, porcine intestinal heparin (data not published).
The assigned peaks correspond to the disaccharides: ΔIIIs, ΔUA(2SO4)-1→4-β-D-GlcN(SO4); ΔIs, ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(6SO4); ΔIs ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4)(6SO4); ΔIIIs, ΔUA(2SO4)-1→4-β-D-GlcN(SO4) (3SO4).
An estimative of the molecular weight of the purified S.plicata heparin was carried out by polyacrylamide gel electrophoresis, where its migration was compared with those of know molecular weight standard GAGs. As shown in Figure 2 C, S.plicata heparin migrated as a very broad band in the range of ~55,000 to ~12,000 Da, indicating an average molecular weight of ~ 34,000 Da, which is higher than that of porcine intestinal mucosa heparin (~18,000 Da).
Anticoagulant properties of S.plicata heparin
The anticoagulant properties of the S.plicata heparin was evaluated by the aPTT assay using human plasma and by measuring the inhibition of thrombin or factor Xa by AT in the presence of increasing concentrations of the invertebrate glycan (Figure 3). Using a parallel standard curve based on the aPTT activity of a heparin containing 180 units/mg, we estimate the anticoagulant activity of the ascidian heparin as ~ 18 units/mg (Figure 3 A). Similar to mammalian heparin, the invertebrate counterpart inhibited thrombin (Figure 3 B) and factor Xa (Figure 3 C) in the presence of AT. However, total inhibition of thrombin or factor Xa by the ascidian glycan was achieved in a much higher dose. Thus, the IC50 for thrombin inhibition by AT was ~ 0.5 and 5.0 μg/ml for mammalian and S.plicata heparin, respectively; for factor Xa inhibition by AT, the IC50 was ~ 1 and 11 μg/ml for mammalian and ascidian heparin, respectively.
Figure 3. Anticoagulant properties of S.plicata heparin.
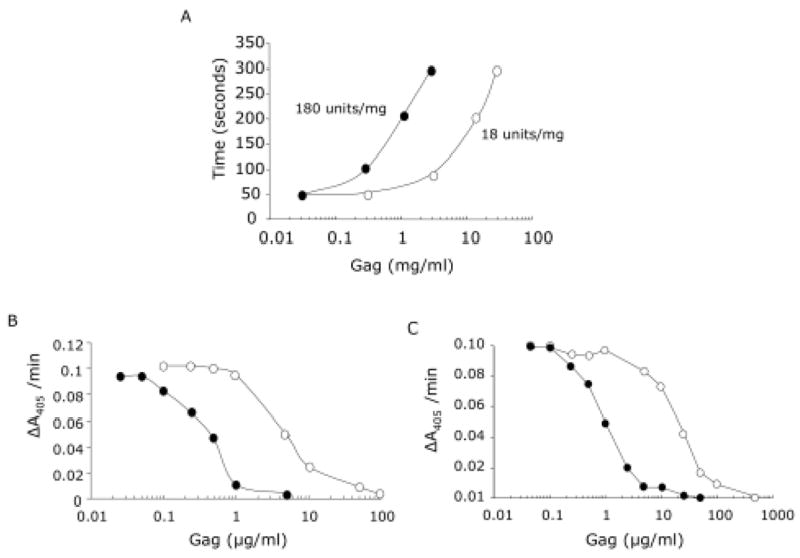
A. aPTT- the activated Partial Thromboplastin Time values were determined in citrate-anticoagulated human plasma in the presence of increasing concentrations mammalian (-●-) or S.plicata (-○-) heparins. B and C, inhibition of thrombin (B) or factor Xa (C) activity by AT in the presence mammalian (-●-) or S.plicata (-○-) heparins. AT was incubated with thrombin (15 nm) or factor Xa (20 nm) in the presence of various concentrations of heparin. After 60 seconds, the remaining thrombin activity was determined with a chromogenic substrate (ΔA405/min).
Antithrombotic effect of S.plicata heparin
The effect of mammalian and S.plicata heparin on thrombosis in vivo was investigated using two experimental models in rats, namely venous thrombosis model in the vena cava and arterio-venous shunt. A single intravascular injection of mammalian heparin, given 5 min before the thrombogenic stimulus with rabbit brain thromboplastin caused a dose-dependent inhibition of thrombus formation in the venous model (Figure 4 A). At the dose of 0.25 mg/Kg body weight, a 50% reduction of thrombosis was observed and with 0.5 mg/Kg body weight, a reduction of >95% in thrombosis was observed in all animals tested. S.plicata heparin was ineffective to inhibit venous thrombosis up to the dose of 0.5 mg/kg body weight and total inhibition was not achieved even at a dose 4-fold higher than that required for mammalian heparin to completely inhibit thrombosis. At the dose of 1.0 mg/kg body weight, ascidian heparin inhibited 80% of thrombosis. A 2-fold increment in the dose did not improve the antithrombotic effect of the invertebrate glycan (Figure 4 A).
Figure 4. Antithrombotic and anticoagulant properties of S.plicata heparin in vivo.
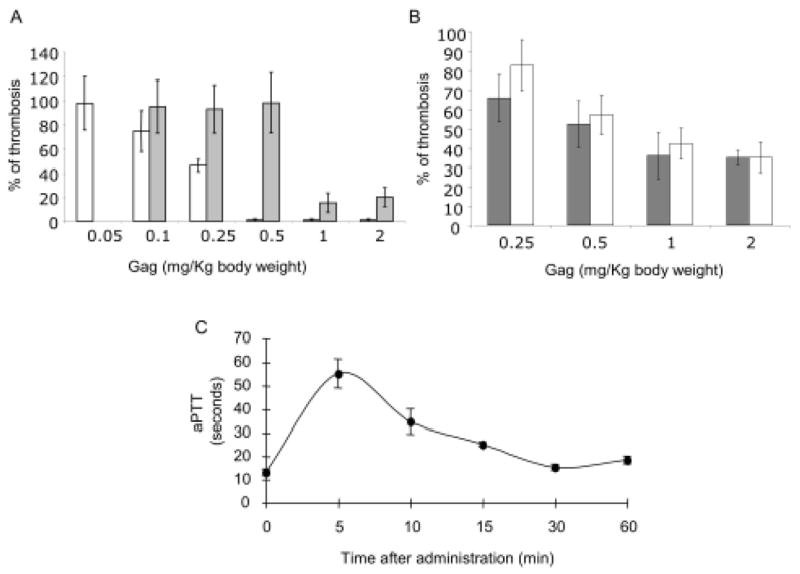
The antithrombotic activity of the S.plicata heparin was investigated using a venous thrombosis in the vena cava (A) or an arterio-venous shunt (B) models, as described in Material and Methods. Mammalian (open bars) or S.plicata heparin (closed bars) at various doses were injected via intravascular route. Mean thrombus weight was obtained from each group and then expressed as percentage of thrombosis (Mean ± SD, N=5). 100% of thrombosis represents the average thrombus weight in the absence of any amount of the polysaccharide. C. Anticoagulant effect of S.plicata heparin in vivo. Blood samples (~ 500 μl) were collected into 2.8% sodium citrate (9:1, v/v) before and at different periods of time after intravascular administration of 2.0 mg/Kg body weight of S.plicata heparin. Activated partial thromboplastin time (aPTT) (mean ± SD, n=4) was determined on the ex vivo rat plasma, as described in Material and Methods.
In contrast to the effect observed in the venous thrombosis, intravascular injection of S.plicata heparin inhibited thrombus formation in the arterio-venous shunt at the same extent as mammalian heparin (Figure 4 B). The same inhibitory effect was achieved by approximately equivalent doses of ascidian and mammalian heparin. Both heparins were not able to totally inhibit thrombosis up to the dose of 2 mg/kg body weight.
To investigate the effect of S.plicata heparin on coagulation in vivo, 2 mg/Kg of the ascidian heparin was injected by intravascular route as a bolus in the carotid artery of anesthetized Wistar rats. Blood was collected at different times after administration of the glycan for aPTT analysis. As shown in Figure 4 C, the aPTT increased up to ~ 4-fold during the first 5 min, following injection of the S.plicata heparin. After 5 min the aPTT started to decrease reaching control values in 30 min.
Bleeding effect
The hemorrhagic effect of mammalian and S.plicata heparins was assessed based on blood loss from a rat cut-tail, after intravascular administration. At the dose of 5 mg/Kg body weight, which is 2.5-fold higher than that required for maximum antithrombotic effect, S.plicata heparin did not modify the blood loss compared with rats receiving saline (Table 2). The blood loss increased ~ 3-fold in rats receiving the same dose of porcine heparin (Table 2). The dose of mammalian heparin to achieve 100% inhibition of thrombosis (0.5 mg/kg) did not increase the blood loss.
Table 2.
Bleeding effect of mammalian and S.plicata heparin.
| Blood loss (μl)a | |
|---|---|
| Saline | 50 ± 5.5 |
| Heparin from: | |
| Mammal (0.5 mg/kg) | 37 ± 11 |
| Mammal (5.0 mg/kg) | 146 ± 16 |
| S.plicata (5.0 mg/kg) | 57 ± 12 |
The volume of blood was deduced from a standard curve of blood based on absorbance at 540 nm, as described by Herbert et al. (40).
Discussion
Structure and anticoagulant properties of S.plicata heparin
The heparin preparation isolated from the body of S.plicata is composed by heparin polymers with different disaccharide composition that are synthesized by different types of cells, namely, test cell [24,25], pharynx and intestine epithelial cells [24,35], basophil-like cell [submitted for publication]. The mixture of these heparin polymers gives rise to a heparin preparation that accounts for ~ 0.26 % (2.6 mg/g of dry body) of the total weight of the ascidian body. This recover is similar to that of the heparin obtained from the mollusk Tapes phylippinarum (0.21 %) [42] in comparison to ~ 0.022 % of pig intestinal mucosa heparin [43].
Disaccharide analysis indicated that S.plicata heparin is composed mainly by the di-sulfated disaccharide (ΔUA(2SO4)-1→4-β-D-GlcN(SO4) (47.5%), followed by the tri-sulfated disaccharide ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(6SO4) (38.3%). Smaller, but significant concentrations of the 3-O-sulfated-containing disaccharides ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4) (8.0%) and ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4)(6SO4) (2.8%) are also present in the ascidian heparin. The disaccharide ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4)(6SO4) was previously identified in depolymerized porcine mucosa heparin [38]. The ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4) tentative structure was attributed according to several analytical indications: the UV maximum at 232 nm is characteristic of the hexuronic acid bearing 2-O sulfate, the selective detection signal at 202–247 nm shows absence of N-acetyl and a characteristic minimum of absorbance due to the presence of 3-O sulfated moiety (for complete a method description, see also [38]. LC-MS experiment assigns a molecular weight of 577 Da (data not shown). Thus, the chromatographic retention is clearly not compatible with the only other possible alternative structure ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(6SO4). Therefore, the disaccharide structure is unequivocally attributed to ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4).
The disaccharide composition of the S.plicata heparin differs from that of porcine intestinal mucosa heparin, which is composed mainly by ΔUA(2SO4)-1→4-β-D-GlcN(SO4)(3SO4) (~70%) disaccharide units, followed by much lower concentrations of other disaccharides (Table 1). In mammalian heparin, disaccharides containing 3-O-sulfated glucosamine correspond to ~3.0% of the total disaccharides. These residues are found mainly in the AT-binding sequence of heparin and are an essential requirement for the potent anticoagulant activity of heparin [44]. Although these residues occur in higher proportion in S.plicata heparin, corresponding to ~11% of the total disaccharides, the ascidian glycan is about 10-fold less anticoagulant than mammalian heparin. This may indicates that most of the 3-O-containing disaccharides occur outside the AT-binding sequence, which would be present in a smaller number in the chains of the ascidian heparin. Heparins enriched in 3-O-sulfated glucosamine, have been reported in the mollusks Anomalocardia brasiliana, Tivela mactroides and Tapes phylippinarum [15,42]. Different from S.plicata heparin, the presence of 3-O-sulfated glucosamine sequences in mollusk heparins is associated with a high anticoagulant activity (320 – 350 IU/mg) [14,42].
The average molecular weight of the ascidian heparin, estimated by polyacrylamide gel electropphoresis, is ~34.000 Da, which is much higher than mammalian (~18,000 Da) or mollusks heparins (~22,500 Da) [15].
Effect of S.plicata heparin in thrombosis models
The antithrombotic effect of S.plicata heparin was evaluated in two models of thrombosis. One of the models, widely used for venous thrombosis, is based on the evaluation of thrombus formation induced by two major factors, activation of coagulation and stasis [39]. The resultant venous thrombus is composed of fibrin and red blood cells. In the arterio-venous shunt model, thrombosis is initiated by platelet adherence to a silk thread anchored in the shunt, and both activation of platelets and coagulation contribute to thrombus formation [39]. In the venous model, S.plicata heparin was much less potent to inhibit thrombosis than mammalian heparin, and complete thrombus inhibition was not achieved, even at a concentration 4-fold higher than that required for mammalian heparin to inhibit 100% thrombosis. This is in accordance to the lower anticoagulant activity of the invertebrate heparin, since in venous thrombosis coagulation is the preponderant mechanism for thrombus formation. In the arterial model of the arterio-venous shunt, where platelet aggregation is the preponderant factor for thrombus formation, S.plicata heparin inhibited thrombosis at the same potency as mammalian heparin, despite the higher AT activity of the mammalian glycan. These results suggest that heparin also inhibits arterial thrombosis by inhibiting platelet aggregation, and this mechanism is independent of the AT activity of polymer. In this aspect, S.plicata and mammalian heparin would have equivalent potency. It has been reported that thrombin-induced platelet activation is inhibited by high- and low-molecular-weight heparin and this inhibitory activity is directly related to the molecular weight of the heparin preparation [45].
It is interesting to notice that significant antithrombotic effect of heparin in the arterial model is obtained at much higher doses, when compared to venous thrombosis. At higher doses mammalian heparin is 2-fold more hemorrhagic than the invertebrate counterpart (Table 2). Therefore, heparin preparations obtained from the body of S.plicata would have a safer therapeutic action in the treatment of arterial thrombosis than mammalian heparin.
Acknowledgments
This work was supported by grants from CNPq, FAPERJ and the NIH Fogarty International Center (R03 TW05775). Mauro S.G. Pavão is a research fellow from CNPq.
Abbreviations: The abbreviations used are
- ΔUA
α-Δ4,5 unsaturated hexuronic acid
- ΔUA(2SO4)
α-Δ4,5 unsaturated hexuronic acid 2-sulfate
- α-L-IdoA
α-L-iduronic acid
- α-L-IdoA-2(SO4)
α-L-iduronic acid 2-sulfate
- GlcN(SO4)
GlcN(SO4)(6SO4) and GlcN(SO4)(3SO4)(6SO4), derivatives of D-glucosamine, bearing a sulfate ester at position N, and at both positions N and 6, and at positions N, 3 and 6, respectively
- GlcNAc
N-acetyl-D-glucosamine
- GlcNAc(6SO4)
N-acetyl-D-Glucosamine 6-sulfate
- HPLC
high-performance liquid chromatography
- FPLC
fast protein liquid chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leyvraz PF, Richard J, Buchamann F. Adjusted versus fixed-dose subcutaneous heparin in the prevention of deep vein thrombosis after total hip replacement. N Engl J Med. 1983;309:954–8. doi: 10.1056/NEJM198310203091605. [DOI] [PubMed] [Google Scholar]
- 2.Poller L. Therapeutic ranges in anticoagulant administration. Brit Med J. 1985;290:1683–9. doi: 10.1136/bmj.290.6483.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mant MJ, O’Brien BD, Thong KL, Hammond GW, Birtwhistle RV, Grace GC. Haemorrhagic complications of heparin therapy. Lancet. 1997;1:1133–5. doi: 10.1016/s0140-6736(77)92388-1. [DOI] [PubMed] [Google Scholar]
- 4.Salzman EW, Deykin D, Shapiro RM, Rosemberg RD. Management of heparin therapy-controlled prospective trial. N Engl J Med. 1975;292:1046–50. doi: 10.1056/NEJM197505152922002. [DOI] [PubMed] [Google Scholar]
- 5.Lindahl U, Backstrom G, Thunberg L, Leder IG. Evidence for a 3-O-sulfated D-glucosamine residue in the antithrombin-binding sequence of heparin. Proc Natl Acad Sci USA. 1980;77:6551–5. doi: 10.1073/pnas.77.11.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casu B, Oreste P, Torri G, Zoppetti G, Choay J, Lormeau JC, Petitou M, Sinay P. The structure of heparin oligosaccharide fragments with high anti-(factor Xa) activity containing the minimal antithrombin III-binding sequence. Chemical and 13C nuclear-magnetic-resonance studies. Biochem J. 1981;197:599–609. doi: 10.1042/bj1970599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ototani N, Kikuchi M, Yosizawa Z. Structure and biological activity of finback-whale (Balaenoptera physalus L.) heparin octasaccharide. Chemical, carbon-13 nuclear-magnetic-resonance, enzymic and biological studies. Biochem J. 1982;205:23–30. doi: 10.1042/bj2050023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thunberg L, Backstrom G, Lindahl U. Further characterization of the antithrombin-binding sequence in heparin. Carbohydr Res. 1982;100:393–410. doi: 10.1016/s0008-6215(00)81050-2. [DOI] [PubMed] [Google Scholar]
- 9.Bjork I, Lindahl U. Mechanism of the anticoagulant action of heparin. Mol Cell Biochem. 1982;48:161–82. doi: 10.1007/BF00421226. [DOI] [PubMed] [Google Scholar]
- 10.Lindahl U, Thunberg L, Backstrom G, Riesenfeld J, Nordling K, Bjork I. Extension and structural variability of the antithrombin-binding sequence in heparin. J Biol Chem. 1984;259:12368–76. [PubMed] [Google Scholar]
- 11.Atha DH, Stephens AW, Rosenberg RD. Evaluation of critical groups required for the binding of heparin to antithrombin. Proc Natl Acad Sci USA. 1984;81:1030–4. doi: 10.1073/pnas.81.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atha DH, Lormeau JC, Petitou M, Rosenberg RD, Choay J. Contribution of monosaccharide residues in heparin binding to antithrombin III. Biochemistry. 1985;24:6723–29. doi: 10.1021/bi00344a063. [DOI] [PubMed] [Google Scholar]
- 13.Jordan RE, Oosta GM, Gardner WT, Rosenberg RD. The binding of low molecular weight heparin to hemostatic enzymes. J Biol Chem. 1980;255:10073–80. [PubMed] [Google Scholar]
- 14.Dietrich CP, de Paiva JF, Moraes CT, Takahashi HK, Porcionatto MA, Nader HB. Isolation and characterization of a heparin with high anticoagulant activity from Anomalocardia brasiliana. Biochim Biophys Acta. 1985;843:1–7. doi: 10.1016/0304-4165(85)90041-8. [DOI] [PubMed] [Google Scholar]
- 15.Pejler G, Danielsson A, Bjork I, Lindahl U, Nader HB, Dietrich CP. Structure and antithrombin-binding properties of heparin isolated from the clams Anomalocardia brasiliana and Tivela mactroides. J Biol Chem. 1987;262:11413–21. [PubMed] [Google Scholar]
- 16.Dietrich CP, Nader HB, de Paiva JF, Santos EA, Holme KR, Perlin AS. Heparin in mollusks: chemical, enzymatic degradation and 13C and 1H n.m.r. spectroscopical evidence for the maintenance of the structure through evolution. Int J Biol Macromol. 1989;11:361–6. doi: 10.1016/0141-8130(89)90008-1. [DOI] [PubMed] [Google Scholar]
- 17.Nader HB, Chavante SF, dos-Santos EA, Oliveira TW, de-Paiva JF, Jeronimo SM, Medeiros GF, de-Abreu LR, Leite EL, de-Sousa-Filho JF, et al. Heparan sulfates and heparins: similar compounds performing the same functions in vertebrates and invertebrates? Braz J Med Biol Res. 1999;32:529–38. doi: 10.1590/s0100-879x1999000500005. [DOI] [PubMed] [Google Scholar]
- 18.Arumugam M, Shanmugam A. Extraction of heparin and heparin-like substance from marine mesogastropod mollusc Turritella attenuata (Lamarck, 1779) Indian J Exp Biol. 2004;42:529–32. [PubMed] [Google Scholar]
- 19.Hovingh P, Linker A. An unusual heparan sulfate isolated from lobsters (Homarus americanus) J Biol Chem. 1982;257:9840–44. [PubMed] [Google Scholar]
- 20.Dietrich CP, Paiva JF, Castro RA, Chavante SF, Jeske W, Fareed J, Gorin PA, Mendes A, Nader HB. Structural features and anticoagulant activities of a novel natural low molecular weight heparin from the shrimp Penaeus brasiliensis. Biochim Biophys Acta. 1999;1428:273–83. doi: 10.1016/s0304-4165(99)00087-2. [DOI] [PubMed] [Google Scholar]
- 21.Chavante SF, Santos EA, Oliveira FW, Guerrini M, Torri G, Casu B, Dietrich CP, Nader HB. A novel heparan sulphate with high degree of N-sulphation and high heparin cofactor-II activity from the brine shrimp Artemia franciscana. Int J Biol Macromol. 2000;27:49–57. doi: 10.1016/s0141-8130(99)00114-2. [DOI] [PubMed] [Google Scholar]
- 22.Tirumalai R, Subramoniam T. Carbohydrate components of lipovitellin of the sand crab Emerita asiatica. Mol Reprod Dev. 2001;58:54–62. doi: 10.1002/1098-2795(200101)58:1<54::AID-MRD8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 23.Demir M, Iqbal O, Dietrich CP, Hoppensteadt DA, Ahmad S, Daud AN, Fareed J. Anticoagulant and antiprotease effects of a novel heparinlike compound from shrimp (Penaeus brasiliensis) and its neutralization by heparinase I. Clin Appl Thromb Hemost. 2001;7:44–52. doi: 10.1177/107602960100700110. [DOI] [PubMed] [Google Scholar]
- 24.Cavalcante MC, Allodi S, Valente AP, Straus AH, Takahashi HK, Mourao PA, Pavao MS. Occurrence of heparin in the invertebrate styela plicata (Tunicata) is restricted to cell layers facing the outside environment. An ancient role in defense? J Biol Chem. 2000;275:36189–86. doi: 10.1074/jbc.M005830200. [DOI] [PubMed] [Google Scholar]
- 25.Cavalcante MC, de Andrade LR, Du Bocage Santos-Pinto C, Straus AH, Takahashi HK, Allodi S, Pavao MS. Colocalization of heparin and histamine in the intracellular granules of test cells from the invertebrate Styela plicata (Chordata-Tunicata) J Struct Biol. 2002;137:313–21. doi: 10.1016/s1047-8477(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 26.Pavao MS. Structure and anticoagulant properties of sulfated glycosaminoglycans from primitive Chordates. An Acad Bras Cienc. 2002;74:105–112. doi: 10.1590/s0001-37652002000100007. [DOI] [PubMed] [Google Scholar]
- 27.Pavao MS, Albano RM, Lawson AM, Mourao PA. Structural heterogeneity among unique sulfated L-galactans from different species of ascidians (tunicates) J Biol Chem. 1989;264:9972–79. [PubMed] [Google Scholar]
- 28.Pavao MS, Mourao PA, Mulloy B. Structure of a unique sulfated alpha-L-galactofucan from the tunicate Clavelina. Carbohydr Res. 1990;208:153–61. doi: 10.1016/0008-6215(90)80095-k. [DOI] [PubMed] [Google Scholar]
- 29.Albano RM, Pavao MS, Mourao PA, Mulloy B. Structural studies of a sulfated L-galactan from Styela plicata (Tunicate): analysis of the Smith-degraded polysaccharide. Carbohydr Res. 1990;208:163–174. doi: 10.1016/0008-6215(90)80096-l. [DOI] [PubMed] [Google Scholar]
- 30.Pavao MS, Rodrigues MA, Mourao PA. Acidic polysaccharides of the ascidian Styela plicata. Biosynthetic studies on the sulfated L-galactans of the tunic, and preliminary characterization of a dermatan sulfate-like polymer in body tissues. Biochim Biophys Acta. 1994;1199:229–37. doi: 10.1016/0304-4165(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 31.Pavao MS, Mourao PA, Mulloy B, Tollefsen DM. A unique dermatan sulfate-like glycosaminoglycan from ascidian. Its structure and the effect of its unusual sulfation pattern on anticoagulant activity. J Biol Chem. 1995;270:31027–36. doi: 10.1074/jbc.270.52.31027. [DOI] [PubMed] [Google Scholar]
- 32.Pavao MS. Unique sulfated polysaccharides from ascidians (Chordata, Tunicata) Braz J Med Biol Res. 1996;29:1227–33. [PubMed] [Google Scholar]
- 33.Pavao MS, Aiello KR, Werneck CC, Silva LC, Valente AP, Mulloy B, Colwell NS, Tollefsen DM, Mourao PA. Highly sulfated dermatan sulfates from Ascidians. Structure versus anticoagulant activity of these glycosaminoglycans. J Biol Chem. 1998;273:27848–57. doi: 10.1074/jbc.273.43.27848. [DOI] [PubMed] [Google Scholar]
- 34.Cavalcante MC, Mourao PA, Pavao MS. Isolation and characterization of a highly sulfated heparan sulfate from ascidian test cells. Biochim Biophys Acta. 1999;1428:77–87. doi: 10.1016/s0304-4165(99)00046-x. [DOI] [PubMed] [Google Scholar]
- 35.Gandra M, Cavalcante M, Pavao M. Anticoagulant sulfated glycosaminoglycans in the tissues of the primitive chordate Styela plicata (Tunicata) Glycobiology. 2000;10:1333–40. doi: 10.1093/glycob/10.12.1333. [DOI] [PubMed] [Google Scholar]
- 36.Cardoso LE, Mourao PA. Glycosaminoglycan fractions from human arteries presenting diverse susceptibilities to atherosclerosis have different binding affinities to plasma LDL. Arterioscler Thromb. 1994;14:115–24. doi: 10.1161/01.atv.14.1.115. [DOI] [PubMed] [Google Scholar]
- 37.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–77. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 38.Mourier PA, Viskov C. Chromatographic analysis and sequencing approach of heparin oligosaccharides using cetyltrimethylammonium dynamically coated stationary phases. Anal Biochem. 2004;332:299–313. doi: 10.1016/j.ab.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Vogel GM, Meuleman DG, Bourgondien FG, Hobbelen PM. Comparison of two experimental thrombosis models in rats effects of four glycosaminoglycans. Thromb Res. 1989;54:399–410. doi: 10.1016/0049-3848(89)90210-7. [DOI] [PubMed] [Google Scholar]
- 40.Herbert JM, Herault JP, Bernat A, van Amsterdam RG, Vogel GM, Lormeau JC, Petitou M, Meuleman DG. Biochemical and pharmacological properties of SANORG 32701. Comparison with the “synthetic pentasaccharide’ (SR 90107/ORG 31540) and standard heparin. Circ Res. 1996;79:590–600. doi: 10.1161/01.res.79.3.590. [DOI] [PubMed] [Google Scholar]
- 41.Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 42.Cesaretti M, Luppi E, Maccari F, Volpi N. Isolation and characterization of a heparin with high anticoagulant activity from the clam Tapes phylippinarum: evidence for the presence of a high content of antithrombin III binding site. Glycobiology. 2004;14:1275–1284. doi: 10.1093/glycob/cwh128. [DOI] [PubMed] [Google Scholar]
- 43.Linhardt RJ, Ampofo SA, Fareed J, Hoppensteadt D, Mulliken JB, Folkman J. Isolation and characterization of human heparin. Biochemistry. 1992;31:12441–5. doi: 10.1021/bi00164a020. [DOI] [PubMed] [Google Scholar]
- 44.Oscarsson LG, Pejler G, Lindahl U. Location of the antithrombin-binding sequence in the heparin chain. J Biol Chem. 1989;264:296–304. [PubMed] [Google Scholar]
- 45.De Candia E, De Cristofaro R, Landolfi R. Thrombin-induced platelet activation is inhibited by high- and low-molecular-weight heparin. Circulation. 1999;99:3308–14. doi: 10.1161/01.cir.99.25.3308. [DOI] [PubMed] [Google Scholar]