Abstract
The formation of the nervous system is initiated when ectodermal cells adopt the neural fate. Studies in Xenopus demonstrate that inhibition of BMP results in the formation of neural tissue. However, the molecular mechanism driving the expression of early neural genes in response to this inhibition is unknown. Moreover, controversy remains regarding the sufficiency of BMP inhibition for neural induction. To address these questions, we performed a detailed analysis of the regulation of the soxB1 gene, sox3, one of the earliest genes expressed in the neuroectoderm. Using ectodermal explant assays, we analyzed the role of BMP, Wnt and FGF signaling in the regulation of sox3 and the closely related soxB1 gene, sox2. Our results demonstrate that both sox3 and sox2 are induced in response to BMP antagonism, but by distinct mechanisms and that the activation of both genes is independent of FGF signaling. However, both require FGF for the maintenance of their expression. Finally, sox3 genomic elements were identified and characterized and an element required for BMP-mediated repression via Vent proteins was identified through the use of transgenesis and computational analysis. Interestingly, none of the elements required for sox3 expression were identified in the sox2 locus. Together our data indicate that two closely related genes have unique mechanisms of gene regulation at the onset of neural development.
Keywords: Neural induction, Xenopus laevis, gene regulation, neural induction, Sox2, Sox3, BMP, FGF, Vent
Introduction
The molecular mechanism of neural induction in vertebrates has yet to be defined as evidence supports both the neural default and the instructive signaling models. The neural default model states that neural tissue is formed when the epidermal-inducing factor BMP (bone morphogenetic protein) is absent (Hawley et al., 1995; Weinstein and Hemmati-Brivanlou, 1997; Wilson and Hemmati-Brivanlou, 1995). Indeed, Spemann’s organizer releases antagonists of BMP such as Noggin (Zimmerman et al., 1996), Chordin (Piccolo et al., 1996) and Follistatin (Fainsod et al., 1997), each capable of inhibiting BMP signaling. While this model stemmed from work in amphibians, studies in mouse and human embryonic stem cells suggest that BMP regulates ectodermal fate signaling in disparate species (Pera et al., 2004; Tropepe et al., 2001; Vallier et al., 2004; Ying et al., 2003). In contrast, the instructive signaling model, while not disputing a role for BMP inhibition in neural induction, posits that BMP inhibition may not be sufficient for neural induction. The observation in chick that the temporal expression of BMP inhibitors does not correspond to the timing of neural induction and that BMP antagonists alone were not capable of inducing neural tissue outside of the presumptive neural plate suggest that other signals may also play a role in neural induction (Linker and Stern, 2004; Streit et al., 2000; Streit et al., 1998; Streit and Stern, 1999). Two candidate signaling pathways are Wnt and FGF (Fibroblast Growth Factor).
In both frogs and chick, Wnt signaling affects the fate of ectodermal cells by altering the level of BMP expression and signaling. In Xenopus, activation of maternal Wnt signaling leads to the down-regulation of BMP expression thereby neuralizing ectodermal tissue non-cell autonomously (Baker et al., 1999; Domingos et al., 2001; Gomez-Skarmeta et al., 2001). Interestingly, recent work suggests that β-catenin with Tcf3 inhibits the expression of early neural markers in a cell-autonomous fashion in Xenopus (Heeg-Truesdell and Labonne, 2006), thus complementing studies in chick demonstrating that Wnt signaling promotes BMP signaling and epidermal formation by decreasing responsiveness to FGF (Wilson et al., 2001).
FGF signaling has multiple roles in neural development. In chick, FGF has the dual role of blocking BMP signaling and independently promoting neural differentiation (Streit et al., 2000; Wilson et al., 2000). In ascidians, FGF signaling is the predominant mechanism of neural induction with BMP antagonism only playing a role later in development (Bertrand et al., 2003; Darras and Nishida, 2001; Hudson et al., 2003; Lemaire et al., 2002). In contrast, in frogs and fish, FGF has an uncertain role in neural induction and its primary role may be inhibition of BMP signaling (Furthauer et al., 1997; Kuroda et al., 2005; Pera et al., 2003). While the exact role of FGF and Wnt in neural induction is still controversial, these studies demonstrate that a complex integration of these pathways is required to maintain and pattern neural tissue.
A response to neural induction is conserved across vertebrates: the expression of the early neural genes sox2 and sox3 in the neuroectoderm. Sox2 and Sox3 are members of the SoxB1 (Sox1, 2, &3) subgroup of HMG-box transcription factors. These genes share remarkable sequence similarity and are expressed in the neuroectoderm at the time of neural induction (Bowles et al., 2000; Hardcastle and Papalopulu, 2000; Mizuseki et al., 1998; Pevny et al., 1998; Rex et al., 1997; Uchikawa et al., 1999; Uwanogho et al., 1995; Wood and Episkopou, 1999). Sox2 is well studied in Xenopus (Kishi et al., 2000; Mizuseki et al., 1998) and in mouse (Avilion et al., 2003; Graham et al., 2003; Rizzoti et al., 2004). Evidence indicates that Sox2 is required for neural formation and the maintenance of a neural progenitor/stem cell population (Graham et al., 2003; Taranova et al., 2006). Furthermore, extensive analysis of SOX2 regulation in chick revealed multiple, highly conserved enhancer modules one of which drives expression in the neural plate (Uchikawa et al., 2003). Analysis of this 56 bp enhancer indicates that both FGF and Wnt signaling are required to directly initiate expression of SOX2 (Takemoto et al., 2006).
In contrast, little has been reported on the function and regulation of sox3. In mouse, functional studies have uncovered roles in sex determination and pituitary formation (Laumonnier et al., 2002; Rizzoti et al., 2004) and expression studies in mouse of mSox3 identified anterior and posterior neural enhancers which also drove expression in the CNS of tailbud Xenopus embryos (Brunelli et al., 2003). However, elements driving expression in the Xenopus gastrula were not uncovered.
In this study, we examine the regulation of the early neural gene sox3 as a means of identifying components of the neural induction pathway in Xenopus laevis. By comparing the regulatory mechanism of sox3 to that of its paralog sox2, we can begin to determine if there is a globally conserved mechanism for the induction of early neural genes. Here we report the following results: (1) FGF is required for the maintenance but not the induction of sox2 and sox3 in animal caps; (2) both genes are induced in response to inhibition of BMP, but different regulatory proteins are required; (3) while the sox2 regulatory domains are highly conserved among vertebrates, the sox3 regulatory regions are not and (4) the downstream effectors of BMP signaling, Vent1 and Vent2, restrict expression of sox3 to the neural ectoderm. Thus, these data support two models of neural induction with expression of sox3 driven by derepression from BMP signaling while the expression of sox2 requires instructive information.
Materials and Methods
Embryo culturing and manipulations
Xenopus laevis embryos were obtained using standard methods (Sive et al., 2000) and staged according to Nieuwkoop and Faber (1994). Animal ectodermal explants were isolated from stage 8–9 embryos and cultured in 0.75X Normal Amphibian Medium (NAM) with gentamycin with or without 3.1 μg/ml cycloheximide and 0.5 ug/ml BMP plus 0.5% BSA. Explants were collected between stages 10.5 and 17 based on sibling embryos.
Plasmid construction
Sox3 regulatory elements were cloned by inverse PCR. The PCR fragments were inserted into the plasmid pCR 2.1- TOPO using the TopoTA kit (Invitrogen Corporation) and sequenced. To generate sox3-GFP, a 1.552 kb fragment upstream of the ATG was fused to EGFP and the SV40 polyA in pCS2+ in which the CMV promoter was removed. In the constructs with a minimal promoter, a 90 bp minimal cytoskeletal actin promoter (Mohun et al., 1987) was cloned as a blunt- Hind||| fragment into the Sma|- Hind||| sites of pGL3 (Promega) in which luciferase was replaced with EGFP. Internal deletions and binding-site mutations of sox3-GFP were generated by DPN mutagenesis.
mRNA Synthesis and Microinjection
Synthetic capped mRNAs were made by in vitro transcription using mMessage mMachine kits (Ambion). For explant assays, 500 pg activinB mRNA (Thomsen et al., 1990) or 25 pg noggin mRNA (Geng et al., 2003; Knecht et al., 1995) was injected into the animal pole of a 1-cell embryo with or without 0.5 ng–1 ng of dominant negative Xfrizzled-8 mRNA (ΔXfz8, Sokol, 1996), 0.75–1.5 ng of inhibitory Xtcf-3 mRNA (ΔTcf3, Molenaar et al., 1996), or 50–100 pg of truncated Xfgfr1 mRNA (XFD, Amaya et al., 1993). 1–1.4 ng of vent or 0.8–1.4 ng of VPvent mRNA (Onichtchouk et al., 1998) with 250 pg of lacZ mRNA was injected into 1 of 2-cells or 0.1 ng of vent1, vent2, and/or .05 ng of both was injected into 1 of 32-cells and embryos were cultured until stage 12.5 and analyzed by whole-mount in situ hybridization (WISH) or RT-PCR.
Transgenesis
Transgenic embryos were generated as described by Kroll and Amaya (Kroll and Amaya, 1996) with the following modifications: 250 ng of linearized DNA, 5 μl sperm diluent buffer (SDB) and 3.5*104 nuclei/ml sperm nuclei are incubated with 5 μl of metaphase oocyte cytoplasm extract until nuclei decondense (approximately 8 minutes). This is diluted in SDB to 70 nuclei/μl and kept at 15°C until injected into oocytes at a flow rate of 0.6 μl/min over approximately 2 seconds. The total number of embryos, the transgenic efficiency and percentage of embryos with the same expression pattern are in Tables 1 and S1. The remaining embryos either showed no expression or patches of expression that are characterized as unintegrated expression.
TABLE 1.
Expression of GFP constructs during neural induction and neurulation
| Figure | Construct | Stage | N | Pan-ectoderm % | (n) | Neuroectoderm % | (n) |
|---|---|---|---|---|---|---|---|
| 4A–F | Sox3-GFP | 10–10.5 | 104 | 77 | (30) | 23 | (9) |
| 11–11.5 | 138 | 52 | (27) | 48 | (25) | ||
| 12 | 44 | 8 | (2) | 92 | (23) | ||
| 6C | p6 | 12 | 208 | 45 | (40) | 55 | (48) |
| p7 | 12 | 51 | 81 | (13) | 19 | (3) | |
| p8 | 12 | 45 | 45 | (10) | 55 | (12) | |
| p9 | 12 | 62 | 47 | (8) | 53 | (9) | |
| p10 | 12 | 36 | 73 | (16) | 27 | (6) | |
| p10 | 17–20 | 71 | 68 | (13) | 32 | (6) |
Whole-mount in situ hybridization
WISH was performed as described in (Harland, 1991; Hemmati-Brivanlou et al., 1990).
Bioinformatics
Xenopus laevis sox3 genomic sequences were processed through RepeatMasker to filter out interspersed repeat elements that can create false alignments (Smit and Green, unpublished data; www.repeatmasker.org). Repeatmasker identified the sequence from -790 to −429 bp as a portion of a PiggyBac-like DNA transposon sequence.
Pairwise alignments were performed using both local and global alignment algorithms, using the servers and tools freely available at the zPicture (Schwartz et al., 2000) and Vista (Frazer et al., 2004) sites, respectively. Sequence homology of consensus transcription factor binding elements was identified using rVISTA (www.dcode.org), MatInspector (http://www.genomatix.de/) and rankVista (www.genome.lbl.gov). To identify sequence conservation across multi-species, sox3 genomic sequences were aligned and visualized using ECR browser (www.dcode.org).
Results
Comparison of sox3 and sox2 expression in embryos and ectodermal explants
X. laevis sox3 and sox2 are both expressed in the presumptive neural ectoderm and CNS (Kishi et al., 2000; Penzel et al., 1997; (Nitta et al., 2006). To directly compare their temporal and spatial expression patterns, we analyzed embryos by RT-PCR (Fig. 1A) and whole mount in situ hybridization (WISH) (Fig. 1B). Xenopus sox3 is maternally and zygotically expressed and RT-PCR analysis demonstrates that sox3 levels peak at stage 10–11, prior to the peak of sox2 expression at stage 12–13 (Fig. 1A).WISH analysis further highlights the differences in expression of sox3 and sox2. Like SOX3 in chick, Xenopus sox3 is expressed initially throughout the animal ectoderm and is later restricted to the dorsal ectoderm (Penzel et al., 1997; Uwanogho et al., 1995). Sox3 expression is stronger on the dorsal side by stage 10 and restricted to the dorsal side by stage 11.5 (data not shown). In contrast, sox2 is first detectable by WISH at the onset of gastrulation (stage 10) in the dorsal ectoderm. As gastrulation proceeds expression remains restricted to the presumptive neural plate. During neurulation, both genes are expressed in the neural tube and the otic placodes (Fig 1B) (Schlosser and Ahrens, 2004).
Fig. 1. Comparison of sox3 and sox2 expression in whole embryos and ectodermal explants.

(A) Temporal expression of sox3 and sox2 in whole embryos examined by RT-PCR. ODC is used as a loading control. RT- is no reverse transcriptase and E is egg. (B) WISH analysis of sox3 and sox2 expression in cleavage (stage 6), blastula (stage 9), gastrula (stage 10–12.5) and neurula (stage 18) embryos. Stage 6–10 embryos are viewed from the animal pole with dorsal to the right, stage 10.5 and 12.5 embryos from the vegetal pole with dorsal to the top, and stage 18 embryos from dorsal side with anterior to the top. The arrowhead and asterisk mark the midline and otic placode, respectively. (C) Expression of sox3 and sox2 in animal ectodermal explants isolated from uninjected embryos and embryos injected with noggin mRNA (indicated by +).
To characterize the expression of sox2 and sox3 in response to BMP inhibition, we analyzed their expression in untreated and noggin-injected animal ectodermal explants (caps, Fig. 1C, Fig. S1). In untreated explants, sox3 and sox2 expression pattern differs; sox3 mRNA is present from stage 8.5 until stage 11.5, while sox2 is not expressed. Both are induced in caps in response to Noggin. Sox3 message is detectable through stage 17 and sox2 is expressed by stage 11.25 (data not shown) and maintained through stage 17. This demonstrates that both are induced in response to BMP inhibition.
FGF signaling is required for the maintenance of sox2 and sox3 in ectodermal explants
We also analyzed the effect of Wnt and FGF signaling on sox2 and sox3 expression in animal caps. We isolated animal caps from embryos expressing proteins that inhibit signaling of the canonical Wnt (ΔTcf3, ΔXfz8) (Molenaar et al., 1996; Sokol, 1996), or FGF pathways (XFD) (Amaya et al., 1991). Expression of these dominant negative proteins in embryos produced the expected results, (Hardcastle et al., 2000; Roel et al., 2002; Wallingford et al., 2001), however, they did not alter expression of sox2 or sox3 in untreated animal caps (data not shown). To determine if inhibition of Wnt or FGF signaling interferes with the ability of Noggin to induce expression of sox2 and 3, we analyzed expression in animal caps from embryos injected with noggin in combination with ΔTcf3, ΔXfz or XFD mRNA. Neither ΔTcf3 nor ΔXfz8 altered sox3 or sox2 expression in response to Noggin (Fig. 2). Notably, while no change was observed in gastrula stage ectodermal explants injected with both noggin and XFD mRNA, expression of both sox2 and sox3 was strongly reduced by neurula stage (st. 17). These data indicate that FGF signaling is required for the maintenance of early neural genes and that neither Wnt nor FGF signaling is required for the induction of sox2 or sox3 in animal caps.
Fig. 2. FGF is required for the maintenance but not the induction of sox2 and sox3 expression in ectodermal explants.

Expression of (A) sox3 and (B) sox2 in animal ectodermal explants as revealed by WISH. Explants were excised between stages 8–9 from untreated embryos or those injected at the 1-cell stage with mRNA for Noggin alone, Noggin plus ΔTCF3, Noggin plus ΔXfz8 or Noggin plus XFD mRNA. Explants were cultured until early gastrula (stage 10.5/11), late gastrula (stage 12.5) or neurula (stage 17) stage as determined by whole embryo controls.
The inhibition of sox3 by BMP and the induction of sox2 by Noggin require de novo protein synthesis
We have shown that sox2 and sox3 expression is induced by the inhibition of BMP in animal caps. To understand how inhibition of BMP is translated into a transcriptional response, we analyzed the effect of inhibiting protein synthesis on sox expression (Fig. 3). Animal caps from uninjected and noggin-injected embryos were cultured with the protein synthesis inhibitor cycloheximide (CHX), collected at stages 10.5, 12.5 and 17 and then assayed for sox3 (Fig 3A) or sox2 (Fig 3B) expression by WISH. To ensure that CHX-treated caps maintained high levels of BMP protein, we added exogenous BMP protein to the media. Protein synthesis was effectively blocked by CHX treatment as demonstrated by the absence of Xbra expression and elongation of the animal caps from embryos injected with activin mRNA (data not shown).
Fig. 3. Sox3 inhibition by BMP and sox2 induction by Noggin require de novo protein synthesis.

Expression of (A) sox3 and (B) sox2 in ectodermal explants revealed by WISH. Explants were excised at stage 8–9 from untreated embryos or embryos injected with noggin mRNA at the 1-cell stage. Explants were incubated with or without BMP protein and/or cycloheximide (CHX) as indicated at the top of each row. Explants were collected at early gastrula (stage 10.5), late gastrula (stage 12.5) or neurula (stage 17) as indicated to the left. The whole embryo control by which the stage was determined is in the column on the far right.
In CHX-incubated caps, sox3 mRNA was detectable from stage 10.5 through stage 17 in the absence of Noggin and the presence of exogenous BMP (Fig. 3A, columns 2 and 4). Therefore, a BMP-responsive protein must be synthesized to either degrade maternal sox3 message or to repress sox3 expression at stage 12.5. One possibility is that targets of BMP signaling (e.g. Vent2 or Msx1) must be synthesized to repress sox3. In contrast, sox2 is not expressed in uninjected caps treated with CHX (Fig. 3B, column 2) and is not induced by Noggin in the presence of CHX. Therefore, one interpretation is that the inhibition of sox3 by BMP signaling requires the synthesis of a repressor protein and it is derepressed in response to neural induction while sox2 expression requires the synthesis of an activator protein.
A 1.4 kb upstream regulatory fragment which is not conserved across species recapitulates sox3 expression
To determine the mechanism by which the BMP signaling pathway represses the induction of neural cell fates, we sought to identify cis-regulatory elements necessary for sox expression in early gastrulae. The regulation of SOX2 has been studied extensively in chick and the regulatory sequence is highly conserved across species (Uchikawa et al., 2003). Therefore, we chose to focus on the identification of modules required for sox3 regulation and compare them to those previously identified for sox2. Transgenic embryos containing a 1.5 kb sox3-GFP reporter construct (Fig. 4A–F) were generated and analyzed by WISH for GFP mRNA. WISH was used because the level of GFP protein does not accumulate to detectable levels by the gastrula stage and is difficult to detect due to the high level of background autofluorescence. As expected, there was variability in the expression pattern likely due to the site of integration and the transgene copy number. However, the majority of transgenic embryos expressed GFP in the patterns shown in Fig. 4A–F.
Fig. 4. A 1.55 kb Xlsox3 upstream regulatory region is partially conserved in X. tropicalis and mimics endogenous sox3 expression.
(A–F) WISH of transgenic embryos expressing the Xlsox3 –GFP reporter construct. (A) Vegetal view of a stage 10.5 and (B) stage 12 embryo with dorsal to the top. (C) Anterior view of a stage 21 embryo on top with a dorsal view of the same embryo directly below. (D) Lateral view of a stage 24 embryo with a dorsal view of the same embryo directly below. (E) Dorsal view of a stage 27 embryo. (F) Lateral view of the head of a stage 33 embryo. The line represents the sox3 upstream regulatory region and −1.55 represents the distance in kb from the ATG. The green box represents the EGFP coding region and the black box the SV40 polyA region. (G) Evolutionary conserved regions between X. laevis and X. tropicalis sox3. A diagram of the sox3 regulatory region used in (A–F) is at the top with a percent identity plot (pip) of Xlsox3 aligned to a homologous region of Xtsox3 below it. At the bottom is a pip of Xtsox3 aligned to Xlsox3. The blue box represents a 484 bp region in X. laevis which does not align to the Xtsox3 sequence. The gray box represents a 30 bp region which is conserved between mouse and Xlsox3. The X-axis of the pip is the length of the sequence in bases or kilobases as designated. The Y-axis spans 50–100% nucleotide identify with the light horizontal line in the center representing 75% nucleotide identity. The red regions under the curve represent evolutionary conserved blocks. Two large blocks of the Xlsox3 upstream regulatory region align with Xtsox3. In Xtsox3, the first block (farthest from the ATG) is split into two blocks.
The sox3-GFP pattern recapitulates the endogenous sox3 expression pattern with some differences. In stage 10.5 transgenic embryos, GFP is expressed throughout the ectoderm with higher levels in the dorsal ectoderm (Fig. 4A). By stage 12.5, GFP expression is restricted to the dorsal neuroectoderm (Fig. 4B). Unlike endogenous sox3, GFP is expressed in the ectoderm overlying the notochord and in the involuting marginal zone adjacent to the blastopore indicating that a module required for restriction of expression is missing. In early tailbud embryos (stage 21 and stage 24), sox3-GFP is expressed throughout the CNS and in the optic placodes but is missing from the otic placodes (Fig. 4C and D). In late tailbud embryos, like sox3, sox3-GFP is expressed in the CNS and developing eyes (Fig. 4E). In stage 33 embryos, expression is strongest in the lens epithelium, forebrain and midbrain (Fig. 4F). Additional regulatory sequences including the 5′ UTR and the 3′UTR were tested in transgenic gastrula and neurula embryos (data not shown) but they did not alter the expression pattern, thus demonstrating they are not required for expression. Strikingly, the 1.5 kb upstream regulatory fragment contains most of the information necessary to recapitulate the spatial and temporal expression of sox3. Importantly, the onset and restriction of expression to the neuroectoderm occur at the same time as endogenous sox3 allowing us to use this sox3-GFP construct to identify cis-elements that respond to neural induction and that are required for restriction of expression to the neuroectoderm.
The recent publication of X. tropicalis sox2 (Xtsox2) and sox3 (Xtsox3) genomic sequence (www.jgi.org), allowed comparison of the X. laevis and X. tropicalis sequences for evolutionary conserved regions (ECRs) and conserved transcription factor binding sites using zPicture and rVista (Loots and Ovcharenko, 2004; Ovcharenko et al., 2004). In sequences flanking Xtsox2, we identified 8 of the 11 ECRs shown to regulate SOX2 expression in chick (Fig. S2A) (Uchikawa et al., 2003). The ECRs downstream of Xtsox2 are in the same order as in cSOX2. However, the region containing N2, NOP-1 and N3 is inverted with respect to that region in avians. 56 bp of the N1 enhancer (N1c) was shown to respond to Henson’s node signals and to contain a Wnt and FGF responsive region that is required for expression in the posterior neural plate (Takemoto et al., 2006). Interestingly, while N1c is present in X. tropicalis with greater than 70% identity at approximately the same location (13 kb downstream of sox2), only one of the two Lef consensus binding sites required for response to Wnt is present and the FGF responsive element is missing indicating that the enhancer may not have the same role in Xenopus as it does in chicken (Fig. S2B).
We next compared the regulatory region of Xlsox3 with that of Xtsox3 and identified two highly conserved 500 bp blocks that flank a 484 bp nonhomologous region (Fig. 4G). While the flanking sequences of Xtsox3 and Xlsox3 are highly conserved, comparison of the ~ 30 kb of X. tropicalis sequence with that of mouse and human revealed only a few small blocks of homology between the three species. Furthermore, none of the sequences previously identified to be important for expression of mSox3 were present (Brunelli et al., 2003) and none of the cSOX2 ECRs was found in regions flanking Xtsox3. Therefore, even though sox2 and sox3 are both induced in the neuroectoderm in response to BMP inhibition and have overlapping expression in the CNS, they do not have obvious cis-modules in common.
Computational analysis revealed that the 1.5 kb fragment that is sufficient to drive expression of X. laevis sox3 is not conserved with species other than X. tropicalis. In fact, there is little conservation between the flanking regions of X. tropicalis, human and mouse sox3. Furthermore, the modules important for cSOX2 are not present in the Xtsox3 surrounding sequence and the modules conserved between Xtsox3 and Xlsox3 are not in regions surrounding Xtsox2. These analyses indicate that the modules that regulate sox2 are not conserved for sox3 and vice versa.
Identification of cis-regulatory regions required for the onset and restriction of sox3 expression
To identify cis-elements important for the onset and restriction of sox3 expression, numerous 5′ end- and internal deletions of sox3-GFP were constructed. These constructs referred to as p2-p17 were used to generate transgenic early and late gastrula (stage 10.5, 12–12.5, respectively) and/or neurula (stage 17–20) embryos. The average transgenic efficiency for sox3-GFP was 46% in gastrula and 57% in neurula embryos. The total number of embryos (N) and the number of transgenic embryos expressing GFP in gastrula and neurula embryos (n) is presented in Table S1 and Table 1 with representative embryos shown in Fig. 5 and 6, respectively.
Fig. 5. A 74 bp region is required for expression of sox3-GFP.
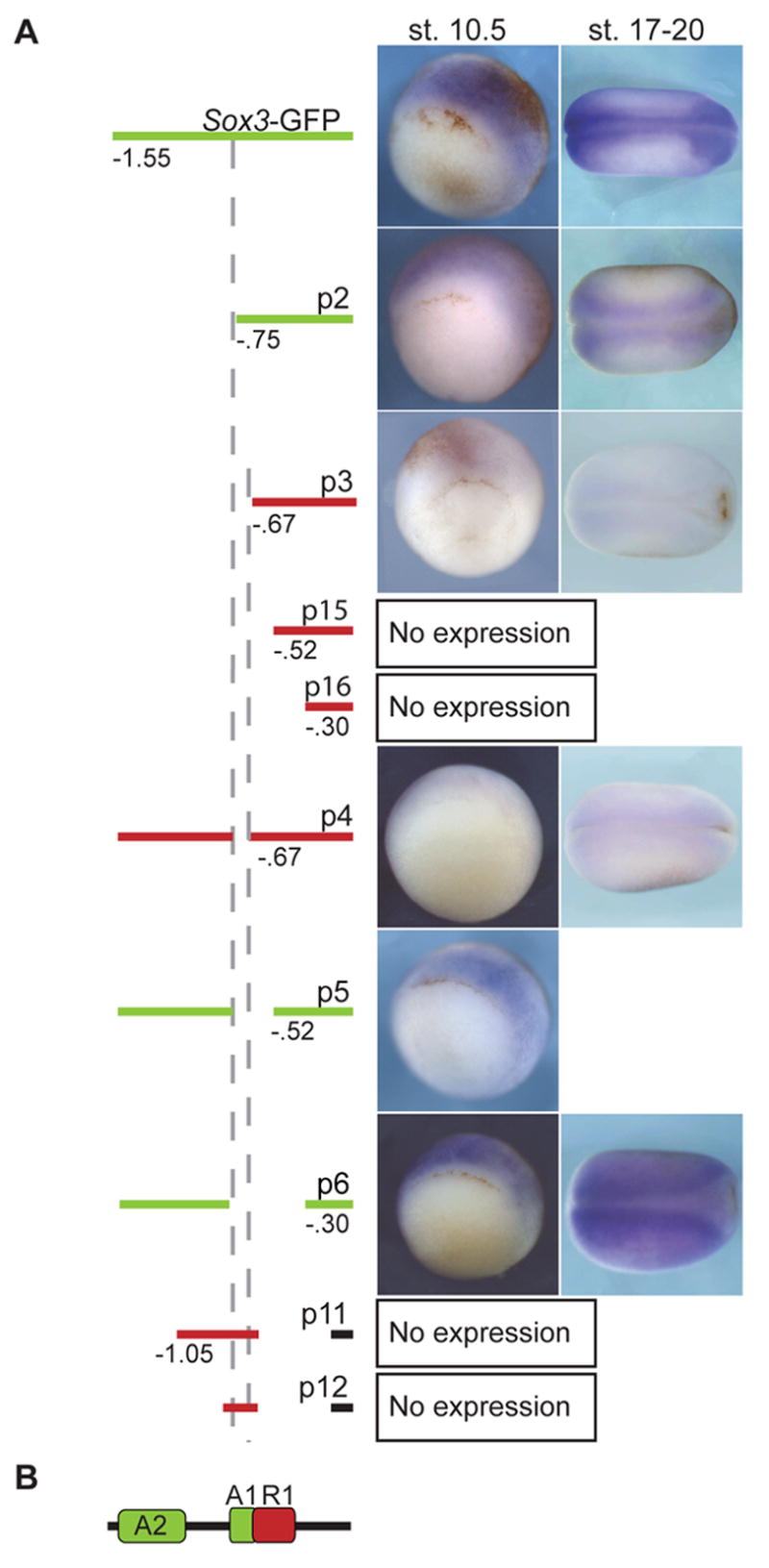
WISH for GFP expression in gastrula (stage 10.5) and neurula (stage 17–20) transgenic embryos containing sox3-GFP or deletions. (A) Diagrams of 5′ end and internal deletions of the sox3 upstream regulatory region are labeled p1-6, 11, 12, 15 and 16. The region included in the construct is indicated by a bar and deletions by the absence of this bar. A red bar denotes no expression of GFP, while a green bar marks those constructs that drive GFP expression. Numbers indicate the deletion end points with the left dashed vertical line marking 746 bp upstream of the ATG and the right dashed line marking 672 bp upstream. (B) A model summarizing the data. The region between −672 bp and −746 bp (green A2, activator 2) is required for expression except when R1 (repressor module) is deleted. In the absence of A2 and R1, a region between −746 bp and −1.55 kb (A1) is required for expression. The total numbers of embryos with the expression pattern shown and the relative levels of expression are in Table S1.
Fig. 6. Identification of transcription factor binding sites required for regulation of sox3-GFP expression.

(A) Sequence of regulatory elements and putative transcription factor binding sites. The boxed region is the putative activator module, A2, which is deleted in p4. The remaining sequence is the putative repressor module, R1 which is deleted in p7. The entire region in A plus sequences up to −299 are deleted in p6. Putative forkhead (FKHD) binding sites are labeled in green and putative Vent1 and Vent2 sites are in red. (B) WISH of transgenic embryos (stage 17–20) expressing sox3-GFP constructs. Embryos are a dorsal view with anterior to the right. A diagram of the upstream regulatory region shown in A is on the left. The numbers in the lower right hand corner refer to the number of embryos with the expression pattern shown over the total number of embryos. (C) WISH of transgenic embryos (stage 12) expressing sox3-GFP constructs. Embryos are a vegetal view with dorsal to the top. An inverted triangle indicates a deletion and an X indicates that a site has been mutated. The total numbers of embryos with the expression pattern shown are in Table 1. Putative TF sites from panel A are represented schematically in the same colors: green circle, Fkhd; red hexagon, Vent1 and Vent2.
Deletion analysis revealed that a regulatory fragment extending to −746 bp upstream of the ATG (p2) is sufficient to drive expression in the ectoderm of early gastrula embryos and in the CNS of neurula embryos (Fig. 5). With a slightly larger 5′ end-deletion which extends to −672 bp, fewer embryos express sox3-GFP and those that do maintain a much lower level of expression (p3, Fig. 5A). As expected, constructs with regulatory regions extending to −520 bp and −299 bp also showed little expression (data not shown). These results indicate that the region between −746 and −672 bp is necessary for expression. To confirm this, an internal deletion of this region (p4) was generated and tested in transgenic gastrula and neurula embryos. The majority of the p4 embryos showed no expression with only a few expressing low levels of GFP (Fig. 5A, Table S1). This demonstrates that p4, like p3, drives only very weak GFP expression and this is due to the loss of an enhancer (A1) between −746 and −672 bp (Fig. 5B).
To determine if A1 is sufficient to drive expression in the ectoderm, fragments extending from −746 to −630 and −1047 to −630 bp were linked to the CSKA minimal promoter and GFP and used to generate transgenic embryos (Fig. 5A, p11 and p12). These transgenic embryos were assayed for expression by WISH but only ~ 10% expressed GFP weakly in the ectoderm similar to that seen for P3 and p4 (Table S1). Therefore, the region deleted in p3 and p4 (−746 to −672 bp) is necessary but not sufficient for expression.
Larger internal deletions revealed a module required for repression of expression. While the deletion between −746 and −672 (p4) resulted in a loss of expression, a larger deletion from −746 to −520 (p5) resulted in expression in the ectoderm of gastrula embryos. Furthermore, an even larger deletion from −746 to −299 (p6) resulted in robust expression throughout the ectoderm in the gastrula and neurula embryos (Fig. 5A). One interpretation of these data is that a repressor module (R1) lies between −672 bp and −299 bp and removal of this repressor region abrogates the need for the A1 enhancer (Fig. 5B). This indicates that a second enhancer module (A2) is present between −746 and −1550 bp and is required for full expression.
Identification of transcription factor sites required for the regulation of sox3-GFP
The upstream regulatory region of sox3 was analyzed to identify transcription factor sites conserved with X. tropicalis. Within A2 is an Elk-1 (Ets-like) consensus motif conserved between X. laevis (−1495 bp) and X. tropicalis (−1274 bp) (Dalton and Treisman, 1992; Rao and Reddy, 1992; Treisman et al., 1992) and a 30 bp direct repeat. However, removal of the Elk site and direct repeat via a 200 bp 5′ deletion did not reduce expression levels (p17, 53% expression in ectoderm, Table S1). A1 (green box, Fig. 6A) contains two adjacent forkhead binding sites (FKHD, green text, Fig. 6A) with core sequences identical to the 7–bp consensus site RTAAAYA (Kaufmann et al., 1995). These sites were mutated in sox3-GFP and tested in transgenic embryos (Fig. 6B). Mutation of the distal FKHD site (p13) led to a decrease in expression in neurula embryos (Fig. 6B). Only 13% of the embryos expressed GFP at low levels in the anterior CNS compared to 31% of sox3-GFP embryos expressing high levels of GFP throughout the CNS. Mutation of the proximal FKHD site (p14) had no effect; p14 embryos expressed high levels of GFP throughout the CNS. Thus, the distal FKHD site is required for expression of sox3-GFP in the CNS.
To further characterize the putative repressor module, R1 (−672 and −463 bp) was deleted leaving A1 intact to generate construct p7 (Fig. 6C). Both p7 and p6 (A1 and R1 deleted) are expressed throughout the ectoderm and are never restricted to the neuroectoderm. While 92% of stage 12, sox3-GFP embryos express GFP in only the dorsal ectoderm, 81% of p7 embryos express GFP throughout the ectodermal tissue (Table 1). This supports the hypothesis that the R1 is required for inhibition of sox3 expression in non-neural ectoderm.
Computational analysis of R1 revealed one Vent1 consensus motif (Friedle et al., 1998) and one Vent2 half-site (Trindade et al., 1999). Vent1 is a direct target of Vent2 (Friedle and Knochel, 2002) which is a direct target of BMP signaling (Rastegar et al., 1999) and both are expressed in the non-neural ectoderm when sox3 is restricted to the neuroectoderm (Gawantka et al., 1995; Onichtchouk et al., 1996). To determine if these sites are important for restriction of sox3 expression, the Vent1 consensus site was mutated to generate p8 and the Vent2 consensus site was deleted to generate p9. The sites were both altered to generate p10 (Fig. 6C). While p8 and p9 transgenic embryos expressed GFP in the dorsal ectoderm in late gastrula embryos, p10 embryos expressed GFP throughout the ectoderm (Fig. 6C). These deletion studies demonstrate that a Vent1 and Vent2 consensus motif are required for the restriction of sox3 expression to the neuroectoderm.
Vent1 and Vent2 repress the expression of endogenous sox3 and sox3-GFP in gastrula embryos
Transgenic analysis demonstrated that Vent consensus binding sites are required for restriction of sox3 to the presumptive neural plate, thus supporting our interpretation of CHX experiments that sox3 expression is inhibited by a target of BMP. To determine if Vent1 or Vent2 alters the expression of sox3-GFP, we injected 1-cell embryos with sox3-GFP DNA and either vent1 (V1), vent2 (V2), or the dominant active VPvent1 (VPV1) or VPvent2 (VPV2) mRNA. As predicted, expression of Vent1 or Vent2 decreased expression of sox3-GFP, and VPV1 and VPV2 increased expression (Fig. 7A). To demonstrate the the two Vent consensus motifs are required for the Vent proteins to alter expression, we injected the vent mRNAs (0.7 ng vent1 and 3 ng vent2) with p10 DNA in which both Vent sites are mutated (Fig. 7A). Overexpression of Vent1 or Vent2 mRNA had no effect on the expression of p10. Therefore, loss of the two Vent consensus motifs in R1 prevents Vent1 or Vent2 from repressing sox3-GFP expression.
Fig. 7. Vent1 and Vent2 repress sox3 and sox3-GFP expression.

(A) Animal pole view of WISH for GFP in stage 12.5 embryos injected at the 1-cell stage with 50 pg sox3-GFP DNA or 50 pg of p10 DNA (V1 and V2 sites mutated), lacZ mRNA as a tracer (light blue) and vent (V1), vent2 (V2), VPvent1 (VPV1) or VPvent2 (VPV2) mRNA. (B) WISH of sox3 in stage 12.5 embryos injected with lacZ mRNA and vent mRNA as indicated. One of 32-cells was injected with V1 or V2 mRNA and 1 of 2-cells was injected with VPV1 or VPV2 mRNA. Embryos are a dorsal view. The numbers in the right hand corner are the number of embryos with the same phenotype as that shown over the total number of embryos. (C) RT-PCR using primers to sox3, geminin or ODC as a loading control of either uninjected ectodermal explants or those injected with noggin, V1, V2, V1+V2, VPV1 and/or VPV2 mRNA.
We next asked whether Vent1 and/or Vent2 are required for repression of endogenous sox3 expression by testing the effect of overexpression of Vent1, Vent2, VPV1 or VPV2. Overexpression of Vent1 and Vent2 resulted in inhibition of sox3 in 56% and 40% of the embryos, respectively (Fig. 7B). Some cells expressing Vent proteins (as indicated by β-galactosidase activity) also expressed sox3. These data suggest that while Vent1 and 2 may be required for complete restriction of sox3 expression to the neuroectoderm, neither can repress sox3 expression entirely in the neural plate. One possibility is that Vent function may require interaction with another protein. This is supported by experiments in which the dominant activator forms, VPV1 and VPV2, were overexpressed (Fig 7B). Upon binding the VPV proteins should effectively activate the expression of target genes without this protein interaction. Expression of either VPV protein resulted in an expansion of sox3 in non-neural ectoderm in over 90% of late gastrula embryos (Fig. 7B).
BMP inhibition induces sox3 expression in ectodermal explants (Fig. 1C) and requires protein synthesis to repress expression of sox3 (Fig. 3A). To determine if overexpression of Vent1 or Vent2 inhibits the induction of sox3 by Noggin, sox3 expression was analyzed in stage 12.5 ectodermal explants from embryos injected with noggin and vent1 or vent2 mRNA (Fig. 7C). Overexpression of Vent2 alone had no effect, while overexpression of Vent1 slightly decreased the induction of sox3 by Noggin and VPV1 and VPV2 induced sox3. These responses in explants mimic those in whole embryos with Vent1 decreasing sox3 expression more effectively than Vent2. We also tested the response of geminin to the Vent proteins in explants since a geminin reporter construct was shown to require Vent binding sites for restriction of expression to the neuroectoderm (Taylor et al., 2006). In this case, both Vent1 and Vent2 reduced geminin expression in response to Noggin but neither VPV1 nor VPV2 induced expression.
Discussion
Many studies have investigated the signals required to induce neural tissue in vertebrates. Yet, controversy remains concerning the signaling pathways required and the combinations of transcription factors necessary to activate expression of early neural genes. Are FGF and Wnt signaling pathways required for the regulation of early neural genes? How is inhibition of BMP translated into a transcriptional response? Is there a neurogenic code? We addressed these questions by studying the regulation of the early neural gene sox3 and comparing it to that of its paralog sox2. Here we show that neither gene requires Wnt or FGF signaling for induction by BMP inhibition, but both require FGF signaling for the maintenance of expression. In transgenic embryos we identified three cis-modules important for the regulation of sox3 expression. Through dissection of these modules, we uncover evidence indicating that an unknown forkhead binding protein is involved in the induction of sox3 and that two BMP targets, Vent1 and Vent2 restrict expression of sox3 to the neuroectoderm. Surprisingly, none of these elements are conserved within the sox2 locus.
FGF signaling is required for the maintenance of sox2 and sox3 expression
Our studies indicate that FGF signaling is needed for the maintenance of sox3 and sox2 expression (Fig. 2), however both were induced by Noggin in explants when FGF signaling through FGFR1 and 2 was inhibited. This differs from previous work demonstrating that sox2 is not expressed in whole embryos treated with the chemical FGF inhibitor SU5402 (Delaune et al., 2005). SU5402 can inhibit the tyrosine kinase activity of other FGF receptors (Grand et al., 2004; Sun et al., 1999) and therefore the use of different inhibitors may explain the discrepant results. Thus, together these data indicate that while FGFR1 is not required for induction of sox2 and sox3 expression, other FGF signaling pathways may be. An alternate possibility is that with excess amounts of Noggin, inhibition of FGF inhibition has no effect. In this case, FGF signaling is required to inhibit Smad activity and therefore BMP signaling (Pera et al., 2003) and this is rendered nonessential in the presence of large quantities of Noggin.
Inhibition of Wnt signaling with ΔTcf3 and ΔXfz8, did not induce sox2 or sox3 expression in animal caps or affect their expression in response to Noggin. However, recent studies indicate that the early neural gene geminin requires the Wnt pathway for expression in early gastrulae (Taylor et al., 2006). While there are two TCF sites upstream of X. laevis sox3, they are not required for expression of the sox3-GFP transgene (p17, Table S1). Thus, distinct molecular mechanisms appear to be used to induce sox3 and geminin expression in the neuroectoderm.
Sox3 and sox2 are induced by Noggin in animal caps but have different requirements for expression
Sox3 and sox2 have distinct spatiotemporal expression patterns in ectodermal explants and in the early embryo. In contrast to sox2, sox3 mRNA is initially detected throughout the ectoderm and in untreated explants of gastrula embryos. This difference in expression could be due to maternal sox3 mRNA and in which case, zygotic sox3 and sox2 expression could be identical. However, our transgenic analysis of sox3 demonstrates that sox3-GFP is initially expressed throughout the ectoderm and gradually restricted to the neuroectoderm. Thus, zygotic sox3 and sox2 are likely to have different patterns of expression in the early gastrula embryo. Given this, different mechanisms of regulation are expected.
Interestingly, CHX experiments provide further evidence that distinct regulatory factors are required for sox2 and sox3 expression in response to BMP antagonism. One interpretation is that a protein must be synthesized for sox2 to be expressed, while sox3 is simply derepressed in neuroectoderm in response to BMP inhibition and thus, does not require protein synthesis. Comparisons of the sox2 and sox3 regulatory regions also reveal innate differences between these genes; while Xenopus sox2 flanking sequences are highly conserved with other vertebrates and contain modules identified to be important for expression in the chick node, Xenopus sox3 regulatory regions are not highly conserved with Xenopus sox2 or even with sox3 across species other than X. tropicalis. Thus, in toto, our experiments indicate that two closely related genes have unique mechanisms of gene regulation at the onset of neural development.
Vent proteins are required to restrict expression to the neural ectoderm
Overexpression studies and deletion analyses in transgenic embryos indicate that sox3 expression is restricted to the neuroectoderm via the actions of the BMP target Vent2 and its target Vent1 (Friedle and Knochel, 2002; Peiffer et al., 2005; Rastegar et al., 1999). We present evidence that Vent1 and Vent2 interact with the R1 enhancer to restrict sox3-GFP expression to the neuroectoderm (Fig. 5C). In whole embryos, Vent1 is most effective at reducing endogenous sox3 expression in the neural plate and selectively reduces sox3 expression in response to Noggin, in animal cap assays. However, both VPVent1 and VPVent2 can induce sox3 expression in whole embryos and animal caps.
Sox3 expression is dynamic; early pan-ectodermal expression is followed by gradual restriction to dorsal ectoderm. Our findings, similar to those for the regulation of the early neural gene zic1 (Tropepe et al., 2006), suggest that generation of this complex pattern requires more than derepression from BMP signaling. Indeed, analysis of deletion constructs in transgenic embryos revealed an additional upstream module (A1) that is necessary but not sufficient for expression of the sox3 transgene. Mutation of a forkhead consensus binding motif within this module decreased expression in neurula embryos with the exception of the anterior CNS. Since the mutation of the forkhead binding site did not mimic the deletion of the A1 module, we suspect that additional elements within or upstream of A1 are required activity.
Conclusion
Our analyses of sox3 expression identified a module required for restriction of expression to the neuroectoderm in late gastrula and another required for expression in gastrula and neurula embryos. These studies combined with animal cap assays reveal that there are at least two other modules required to control expression in gastrula embryos: (i) one required to restrict expression from the marginal zone and (ii) one required to respond to FGF signals to maintain expression in the neuroectoderm. These studies when considered in light of previous studies of SOX2 regulation in chick and our comparison of sox2 and sox3 expression in frog, indicate that sox3 does not share the same mechanism of regulation with sox2 or potentially even across species. While conservation of regulatory modules is a common way to identify important regulatory domains, the absence of conserved transcription factor sites for genes with similar expression profiles in different species is not unusual (Dermitzakis and Clark, 2002). In fact, there are many other genes which have different regulatory codes but maintain the same expression profile (Ludwig et al., 2000). The question remains: is there a neural transcription code? It would be tempting to say “no” given the distinct regulation of sox2 and sox3. Studies of the regulation of additional early neural genes will help to elucidate this complex question of neural development.
Supplementary Material
Acknowledgments
We thank C. Niehrs and D. Kimelman for supplying plasmids and R. Harland, M. Donoghue and all members of R.H.’s and E.C’s lab for helpful discussions. This work was supported by the American Cancer Society IRG97-152-04 and The National Institute of Health NS048918 (to E.S.C.). Funding for T.G. was provided by GM42341 (to RM.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–70. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–40. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JC, Beddington RS, Harland RM. Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 1999;13:3149–59. doi: 10.1101/gad.13.23.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand V, Hudson C, Caillol D, Popovici C, Lemaire P. Neural tissue in ascidian embryos is induced by FGF9/16/20, acting via a combination of maternal GATA and Ets transcription factors. Cell. 2003;115:615–27. doi: 10.1016/s0092-8674(03)00928-0. [DOI] [PubMed] [Google Scholar]
- Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227:239–55. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- Brunelli S, Casey ES, Bell D, Harland RM, Lovell-Badge R. Sox3 expression during central nervous system development is regulated by modular elements and this regulation is evolutionary conserved. Genesis 2003 [Google Scholar]
- Dalton S, Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992;68:597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- Darras S, Nishida H. The BMP signaling pathway is required together with the FGF pathway for notochord induction in the ascidian embryo. Development. 2001;128:2629–38. doi: 10.1242/dev.128.14.2629. [DOI] [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. [DOI] [PubMed] [Google Scholar]
- Dermitzakis ET, Clark AG. Evolution of transcription factor binding sites in Mammalian gene regulatory regions: conservation and turnover. Mol Biol Evol. 2002;19:1114–21. doi: 10.1093/oxfordjournals.molbev.a004169. [DOI] [PubMed] [Google Scholar]
- Domingos PM, Itasaki N, Jones CM, Mercurio S, Sargent MG, Smith JC, Krumlauf R. The Wnt/beta-catenin pathway posteriorizes neural tissue in Xenopus by an indirect mechanism requiring FGF signalling. Dev Biol. 2001;239:148–60. doi: 10.1006/dbio.2001.0431. [DOI] [PubMed] [Google Scholar]
- Fainsod A, Deissler K, Yelin R, Marom K, Epstein M, Pillemer G, Steinbeisser H, Blum M. The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech Dev. 1997;63:39–50. doi: 10.1016/s0925-4773(97)00673-4. [DOI] [PubMed] [Google Scholar]
- Friedle H, Knochel W. Cooperative interaction of Xvent-2 and GATA-2 in the activation of the ventral homeobox gene Xvent-1B. J Biol Chem. 2002;277:23872–81. doi: 10.1074/jbc.M201831200. [DOI] [PubMed] [Google Scholar]
- Friedle H, Rastegar S, Paul H, Kaufmann E, Knochel W. Xvent-1 mediates BMP-4-induced suppression of the dorsal-lip-specific early response gene XFD-1′ in Xenopus embryos. Embo J. 1998;17:2298–307. doi: 10.1093/emboj/17.8.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furthauer M, Thisse C, Thisse B. A role for FGF-8 in the dorsoventral patterning of the zebrafish gastrula. Development. 1997;124:4253–64. doi: 10.1242/dev.124.21.4253. [DOI] [PubMed] [Google Scholar]
- Gawantka V, Delius H, Hirschfeld K, Blumenstock C, Niehrs C. Antagonizing the Spemann organizer: role of the homeobox gene Xvent-1. Embo J. 1995;14:6268–79. doi: 10.1002/j.1460-2075.1995.tb00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Skarmeta J, de La Calle-Mustienes E, Modolell J. The Wnt-activated Xiro1 gene encodes a repressor that is essential for neural development and downregulates Bmp4. Development. 2001;128:551–60. doi: 10.1242/dev.128.4.551. [DOI] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–65. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Grand EK, Chase AJ, Heath C, Rahemtulla A, Cross NC. Targeting FGFR3 in multiple myeloma: inhibition of t(4;14)-positive cells by SU5402 and PD173074. Leukemia. 2004;18:962–6. doi: 10.1038/sj.leu.2403347. [DOI] [PubMed] [Google Scholar]
- Hardcastle Z, Chalmers AD, Papalopulu N. FGF-8 stimulates neuronal differentiation through FGFR-4a and interferes with mesoderm induction in Xenopus embryos. Curr Biol. 2000;10:1511–4. doi: 10.1016/s0960-9822(00)00825-3. [DOI] [PubMed] [Google Scholar]
- Hardcastle Z, Papalopulu N. Distinct effects of XBF-1 in regulating the cell cycle inhibitor p27(XIC1) and imparting a neural fate. Development. 2000;127:1303–14. doi: 10.1242/dev.127.6.1303. [DOI] [PubMed] [Google Scholar]
- Hawley SH, Wunnenberg-Stapleton K, Hashimoto C, Laurent MN, Watabe T, Blumberg BW, Cho KW. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev. 1995;9:2923–35. doi: 10.1101/gad.9.23.2923. [DOI] [PubMed] [Google Scholar]
- Heeg-Truesdell E, Labonne C. Neural induction in Xenopus requires inhibition of Wnt-beta-catenin signaling. Dev Biol. 2006 doi: 10.1016/j.ydbio.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Hudson C, Darras S, Caillol D, Yasuo H, Lemaire P. A conserved role for the MEK signalling pathway in neural tissue specification and posteriorisation in the invertebrate chordate, the ascidian Ciona intestinalis. Development. 2003;130:147–59. doi: 10.1242/dev.00200. [DOI] [PubMed] [Google Scholar]
- Kaufmann E, Muller D, Knochel W. DNA recognition site analysis of Xenopus winged helix proteins. J Mol Biol. 1995;248:239–54. doi: 10.1016/s0022-2836(95)80047-6. [DOI] [PubMed] [Google Scholar]
- Kishi M, Mizuseki K, Sasai N, Yamazaki H, Shiota K, Nakanishi S, Sasai Y. Requirement of Sox2-mediated signaling for differentiation of early Xenopus neuroectoderm. Development. 2000;127:791–800. doi: 10.1242/dev.127.4.791. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Fuentealba L, Ikeda A, Reversade B, De Robertis EM. Default neural induction: neuralization of dissociated Xenopus cells is mediated by Ras/MAPK activation. Genes Dev. 2005;19:1022–7. doi: 10.1101/gad.1306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F, Ronce N, Hamel BC, Thomas P, Lespinasse J, Raynaud M, Paringaux C, Van Bokhoven H, Kalscheuer V, Fryns JP, Chelly J, Moraine C, Briault S. Transcription factor SOX3 is involved in X-linked mental retardation with growth hormone deficiency. Am J Hum Genet. 2002;71:1450–5. doi: 10.1086/344661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire P, Bertrand V, Hudson C. Early steps in the formation of neural tissue in ascidian embryos. Dev Biol. 2002;252:151–69. doi: 10.1006/dbio.2002.0861. [DOI] [PubMed] [Google Scholar]
- Linker C, Stern CD. Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development. 2004;131:5671–81. doi: 10.1242/dev.01445. [DOI] [PubMed] [Google Scholar]
- Loots GG, Ovcharenko I. rVISTA 2.0: evolutionary analysis of transcription factor binding sites. Nucleic Acids Res. 2004;32:W217–21. doi: 10.1093/nar/gkh383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig MZ, Bergman C, Patel NH, Kreitman M. Evidence for stabilizing selection in a eukaryotic enhancer element. Nature. 2000;403:564–7. doi: 10.1038/35000615. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–87. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–9. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Nitta KR, Takahashi S, Haramoto Y, Fukuda M, Onuma Y, Asashima M. Expression of Sox1 during Xenopus early embryogenesis. Biochem Biophys Res Commun. 2006;351:287–93. doi: 10.1016/j.bbrc.2006.10.040. [DOI] [PubMed] [Google Scholar]
- Onichtchouk D, Gawantka V, Dosch R, Delius H, Hirschfeld K, Blumenstock C, Niehrs C. The Xvent-2 homeobox gene is part of the BMP-4 signalling pathway controlling [correction of controling] dorsoventral patterning of Xenopus mesoderm. Development. 1996;122:3045–53. doi: 10.1242/dev.122.10.3045. [DOI] [PubMed] [Google Scholar]
- Ovcharenko I, Loots GG, Hardison RC, Miller W, Stubbs L. zPicture: dynamic alignment and visualization tool for analyzing conservation profiles. Genome Res. 2004;14:472–7. doi: 10.1101/gr.2129504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer DA, Von Bubnoff A, Shin Y, Kitayama A, Mochii M, Ueno N, Cho KW. A Xenopus DNA microarray approach to identify novel direct BMP target genes involved in early embryonic development. Dev Dyn. 2005;232:445–56. doi: 10.1002/dvdy.20230. [DOI] [PubMed] [Google Scholar]
- Pera EM, Ikeda A, Eivers E, De Robertis EM. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev. 2003;17:3023–8. doi: 10.1101/gad.1153603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, Ward-van Oostwaard D, Mummery C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004;117:1269–80. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development. 1998;125:1967–78. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–98. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VN, Reddy ES. A divergent ets-related protein, elk-1, recognizes similar c-ets-1 proto-oncogene target sequences and acts as a transcriptional activator. Oncogene. 1992;7:65–70. [PubMed] [Google Scholar]
- Rastegar S, Friedle H, Frommer G, Knochel W. Transcriptional regulation of Xvent homeobox genes. Mech Dev. 1999;81:139–49. doi: 10.1016/s0925-4773(98)00239-1. [DOI] [PubMed] [Google Scholar]
- Rex M, Orme A, Uwanogho D, Tointon K, Wigmore PM, Sharpe PT, Scotting PJ. Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Dev Dyn. 1997;209:323–32. doi: 10.1002/(SICI)1097-0177(199707)209:3<323::AID-AJA7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R. SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet. 2004;36:247–55. doi: 10.1038/ng1309. [DOI] [PubMed] [Google Scholar]
- Roel G, Hamilton FS, Gent Y, Bain AA, Destree O, Hoppler S. Lef-1 and Tcf-3 transcription factors mediate tissue-specific Wnt signaling during Xenopus development. Curr Biol. 2002;12:1941–5. doi: 10.1016/s0960-9822(02)01280-0. [DOI] [PubMed] [Google Scholar]
- Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol. 1996;6:1456–67. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–8. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]
- Streit A, Lee KJ, Woo I, Roberts C, Jessell TM, Stern CD. Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development. 1998;125:507–19. doi: 10.1242/dev.125.3.507. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mech Dev. 1999;82:51–66. doi: 10.1016/s0925-4773(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Sun L, Tran N, Liang C, Tang F, Rice A, Schreck R, Waltz K, Shawver LK, McMahon G, Tang C. Design, synthesis, and evaluations of substituted 3-[(3- or 4-carboxyethylpyrrol-2-yl)methylidenyl]indolin-2-ones as inhibitors of VEGF, FGF, and PDGF receptor tyrosine kinases. J Med Chem. 1999;42:5120–30. doi: 10.1021/jm9904295. [DOI] [PubMed] [Google Scholar]
- Takemoto T, Uchikawa M, Kamachi Y, Kondoh H. Convergence of Wnt and FGF signals in the genesis of posterior neural plate through activation of the Sox2 enhancer N-1. Development. 2006;133:297–306. doi: 10.1242/dev.02196. [DOI] [PubMed] [Google Scholar]
- Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, Pevny LH. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JJ, Wang T, Kroll KL. Tcf- and Vent-binding sites regulate neural-specific geminin expression in the gastrula embryo. Dev Biol. 2006;289:494–506. doi: 10.1016/j.ydbio.2005.10.047. [DOI] [PubMed] [Google Scholar]
- Treisman R, Marais R, Wynne J. Spatial flexibility in ternary complexes between SRF and its accessory proteins. Embo J. 1992;11:4631–40. doi: 10.1002/j.1460-2075.1992.tb05565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trindade M, Tada M, Smith JC. DNA-binding specificity and embryological function of Xom (Xvent-2) Dev Biol. 1999;216:442–56. doi: 10.1006/dbio.1999.9507. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30:65–78. doi: 10.1016/s0896-6273(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Uchikawa M, Ishida Y, Takemoto T, Kamachi Y, Kondoh H. Functional analysis of chicken Sox2 enhancers highlights an array of diverse regulatory elements that are conserved in mammals. Dev Cell. 2003;4:509–19. doi: 10.1016/s1534-5807(03)00088-1. [DOI] [PubMed] [Google Scholar]
- Uchikawa M, Kamachi Y, Kondoh H. Two distinct subgroups of Group B Sox genes for transcriptional activators and repressors: their expression during embryonic organogenesis of the chicken. Mech Dev. 1999;84:103–20. doi: 10.1016/s0925-4773(99)00083-0. [DOI] [PubMed] [Google Scholar]
- Uwanogho D, Rex M, Cartwright EJ, Pearl G, Healy C, Scotting PJ, Sharpe PT. Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech Dev. 1995;49:23–36. doi: 10.1016/0925-4773(94)00299-3. [DOI] [PubMed] [Google Scholar]
- Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–21. doi: 10.1016/j.ydbio.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Vogeli KM, Harland RM. Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway. Int J Dev Biol. 2001;45(1):225–7. [PubMed] [Google Scholar]
- Weinstein DC, Hemmati-Brivanlou A. Neural induction in Xenopus laevis: evidence for the default model. Curr Opin Neurobiol. 1997;7:7–12. doi: 10.1016/s0959-4388(97)80114-6. [DOI] [PubMed] [Google Scholar]
- Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–3. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Graziano E, Harland R, Jessell TM, Edlund T. An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr Biol. 2000;10:421–9. doi: 10.1016/s0960-9822(00)00431-0. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Rydstrom A, Trimborn T, Willert K, Nusse R, Jessell TM, Edlund T. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325–30. doi: 10.1038/35077115. [DOI] [PubMed] [Google Scholar]
- Wood HB, Episkopou V. Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre- gastrulation to early somite stages. Mech Dev. 1999;86:197–201. doi: 10.1016/s0925-4773(99)00116-1. [DOI] [PubMed] [Google Scholar]
- Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–6. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



