Abstract
A thorough understanding of histone acetyltransferase CBP/p300-mediated regulation of gene expression and cell growth is essential to identify mechanisms relevant to the development of histone deacetylase (HDAC) inhibitor-based preventive and therapeutic strategies. We found that knockdown of CITED2 (CBP/p300 interacting coactivator with glutamic acid/aspartic acid-rich tail 2) increased colon cancer cell invasiveness in vitro. Gene expression profiling revealed that CITED2 knockdown induced matrix metalloproteinase 13 (MMP-13) gene expression in colon cancer cells. Butyrate, a naturally occurring HDAC inhibitor, induced CITED2 expression and downregulated MMP-13 expression in RKO cells. Additionally, ectopic expression of CITED2 arrested RKO cell growth. Thus, CITED2 regulates colon cancer invasion and might be a target for HDAC inhibitor-based intervention of colon cancer.
Keywords: histone deacetylase inhibitor, butyrate, matrix metalloproteinase 13, β-catenin
Colon cancer is the second leading cause of cancer death in the United States. Colorectal cancer commonly metastasizes to the liver [1], and as with most cancers, it is the metastasis that is mainly responsible for high mortality rates. Thus, elucidation of the mechanisms responsible for initiation, progression and eventually metastasis of colon cancer metastases is required for the ultimate control of this disease. Evolution of the metastatic phenotype requires enhanced cell invasiveness to enable the tumor cell to seperate from the primary site and successfully establish a metastatic colony [2]. Unlike the molecular events described for the pathogenesis of primary colon tumors, the genes and pathways responsible for metastasis in these tumors have not been well characterized.
Aberrant gene expression due to epigenetic changes has been postulated to be a driving force underlying tumor progression, and HDAC and DNA methyltransferase inhibitors-based epigenetic therapy has emerged as a reliable approach for the intervention of cancer [3]. Functional CBP/p300 is critical for transcriptional regulation of gene expression and control of cell growth [4,5]. CBP/p300 loss-of-function is associated with a variety of malignant cancers, including colon cancer [6–8]. In the human colon cancer cell line HCT116, p300 deletion leads to aggressive “cancer” phenotypes, including increased migration and invasion in vitro [9].
CITED2 is a bifunctional protein that belongs to a family of transcriptional cofactors that is characterized by a conserved ED-rich domain at the C-terminus. A functional motif (LPXL) within this domain is necessary and sufficient for binding to the first cysteine-histidine–rich region of CBP/p300 [10]. Initially described as a corepressor of hypoxia-inducing factor 1α (HIF1α) by competing for CBP/p300 binding [11], CITED2 also functions as a coactivator of activator protein 2 (AP-2) [12], PPARα and PPARγ [13], and LIM-homeodomain protein Lhx2 [14] by recruiting CBP/p300. Loss of CITED2 in mice results in embryonic lethality—a consequence of multiple developmental defects [15,16]. Ectopic expression of melanocyte-specific gene related gene (MRG1), an alternatively spliced isoform of CITED2, results in oncogenic transformation in rat fibroblasts [17]. However, it is not clear if CITED2 functions as a tumor-promoter or suppressor. A recent study showed that knockdown of CITED2 in the breast cancer cell line MDA-MB-231 attenuates TGFβ1–mediated upregulation of MMP-9 and cell invasiveness in vitro [18]. This study raised the possibility that CITED2 affects tumorigenesis by modulating tumor invasion rather than proliferation. Using CITED2 specific small hairpin (sh) RNA to knockdown CITED2 expression in human colon cancer cells, we observed that CITED2 knockdown induced changes in cell morphology, concomitant with increased cancer cell invasiveness in vitro. Our results suggest a pivotal role for CITED2 in colon cancer cell growth regulation and may have important implications in targeting CITED2 for nutrition-based chemoprevention and chemotherapy for colon cancer.
Materials and methods
Reagents
Monoclonal antibody against p21waf1 and rabbit polyclonal antibody against HDAC1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); rabbit polyclonal antibody against acetyl-H2A was obtained from Cell Signaling (Danvers, MA); mouse monoclonal anti-CITED2 (JA22) was obtained from Novus Biologicals (Littleton, CO); and mouse monoclonal anti-β-catenin was obtained from BD Biosciences Pharmingen. A control scrambled siRNA was obtained from Ambion (Austin, TX).
Plasmids
The pMK17, which contains 598 bp of human CITED2 proximal promoter, was kindly provided by Dr. Shoumo Bhattacharya (University of Oxford). The plko1 lentiviral vector or plko1-shCITED2 which expresses shRNA targeting the human CITED2 cDNA coding region from +428 to +448 nt (BC004377) were obtained from the Open Biosystems (Huntsville, AL). The lentiviruses were produced at the University of Michigan Vector Core. The pOBT7-CITED2, which contains the full-length human CITED2 cDNA was purchased from the Origene (Rockville, MD). To generate HA-tagged CITED2 expression vector, the following primers were used to amplify full-length CITED2 cDNA using the pOBT7-CITED2 as the template. Forward: 5’-CCGACAAGCTTGCAGACCATATGATGGCAATGAACC-3’; backward: 5’-TTAAGCGTAATCTGGAACATCGTATGGGTAACAGCTCACTCTGCTGGGC-3’. The PCR fragments were digested with Hind III and inserted into the pCMV10 vector and verified by sequencing. The expression of HA-CITED2 was verified by immunoblots.
Cell culture
The human colon cancer cell line RKO was purchased from the ATCC (Manassas, VA) and cultured in minimum essential medium with 10% fetal bovine serum. To generate CITED2 knockdown stable cell line, RKO cells were transduced with lentiviral empty vector or CITED2 shRNA expressing vector and selected with puromycin at 0.1 mg/ml for 3–4 weeks. The pools of puromycin-resistent cells were used for further analyses. To generate stable cell line that expresses HA-tagged CITED2, cells were transfected with pCMV10 vector or pCMV10-HA-CITED2 vector and selected with G418 at 0.5 mg/ml for 3 weeks. The pools of G418-resistent cells were used for further analyses.
Reporter assay
Cells cultured in 48-well plates were transfected with human CITED2 reporter pMK17 using FUGENE 6 (Roche). The cells were treated with 2.5 mM sodium butyrate for 20 h prior to performing luciferase reporter assays that were normalized to protein [19]. Luciferase assay was performed on Perkin Elmer VICTOR3 1420 Multilabel Counter using the Luciferase Reporter Assay System (Promega).
Small interfering RNA
RNA interference experiments with small interfering RNA (siRNA) were carried out as described before [20]. The region of CITED2 cDNA targeted for siRNA was: +519 5'-AAGGTTTAACAACTCCCAGTT-3'. A scrambled siRNA (Ambion) was used as control. siRNAs were transfected into cells with Oligofectamine (Invitrogen).
RNA isolation and RT-PCR analysis
Total RNA was isolated from cells using RNeasy mini kit (Qiagen) following the manufacturer’s protocol. First-strand cDNA synthesis was performed using the SuperScript® III First-Strand Synthesis System (Invitrogen). The sequences of primers and amplification conditions are available upon request.
Matrigel invasion assay
Matrigel invasion assays were performed using BD Matrigel Invasion Chamber (6-well plates, 8 μm pore size, BD Biosciences). Cells were first cultured in serum free medium for 20–24 h, then collected and resuspended in medium with 0.1% BSA at a density of 2.5 × 105 cells/ml. Culture medium with 10% FBS was added to the lower chamber and 500 μl of the resuspended cells were added onto the top of the Matrigel. Forty hours later, the noninvaded cells and Matrigel on the topside of the transwell were scrapped off with cotton swab. Cells on the lower surface of the membrane were fixed with methanol, stained with haematoxylin and eosin (H&E) and viewed with Olympus BX60 microscope using SPOT software.
MMP-13 activity assay
Cells were seeded in 12-well plates at a density of 2 × 105/well. Twelve hours later, cells were washed with PBS and incubated in 0.5 mL serum-free medium for another 24 h. The conditional medium was collected, centrifuged at 10,000 rpm for 5 min; and 25 μl of the supernatant was used to detect MMP-13 activity using the SensoLyte Plus™ 520 MMP-13 Assay Kit (AnaSpec, San Jose, CA) following the manufacturer’s instruction. The fluorescence signal was measured by VICTOR3 Multilabel Counter (Perkin Elmer) with a filter set of excitation/emission=495 nm/535 nm.
Confocal microscopy
Cells were fixed in 3.7% formaldehyde solution for 10 minutes, treated with 0.1% Triton X-100 for 5 minutes, and incubated with Alexa fluor 488 phalloidin (Molecular Probes, Eugene, OR) for 30 minutes at room temperature. The cells were mounted and examined with a confocal microscope (Olympus FV-500) at the University of Michigan Microscopy & Image Analysis Lab.
Results and discussion
CITED2 knockdown induced morphological changes in colon cancer cells
To explore the functions of CITED2 in colonic cells, we used CITED2-specific shRNA to knockdown its expression in the colon cancer cell line RKO. As shown in Fig 1A and 1B, both CITED2 mRNA and protein levels were significantly reduced by CITED2 shRNA. The expression of CITED4 was not affected (Fig 1A), which validated the specificity of CITED2 shRNA. Cells with reduced CITED2 expression induced a flattened morphology compared to the control cells (Fig 1C). Notably, a flattened appearance is also a feature of CITED2 (−/−) mouse fibroblasts [21]. To determine whether the morphological changes were associated with alterations in the actin cytoskeleton, the cells were stained with phalloidin. Phalloidin staining revealed cytoskeleton reorganization in CITED2 knockdown RKO cells (Fig 1D).
Fig 1. CITED2 knockdown induced morphological changes in RKO cells.

(A) RKO cells were transduced with plko1 empty lentiviral vector or plko1-shCITED2, and selected with puromycin for 3 weeks. The pools of puromycin-resistent cells were used for total RNA isolation and RT-PCR analyses. (B) Whole cell extracts were prepared and mouse anti-CITED2 (Novus Biologicals) and anti-GAPDH (Chemicon) antibodies were used for immunoblotting (IB). (C) Phase contrast microscopy of live cells (magnification: 200×). (D) Cells were stained with Alexa fluor 488 phalloidin (Molecular Probes) and examined with confocal microscope (magnification: 400×).
CITED2 knockdown increased colon cancer cell invasiveness in vitro
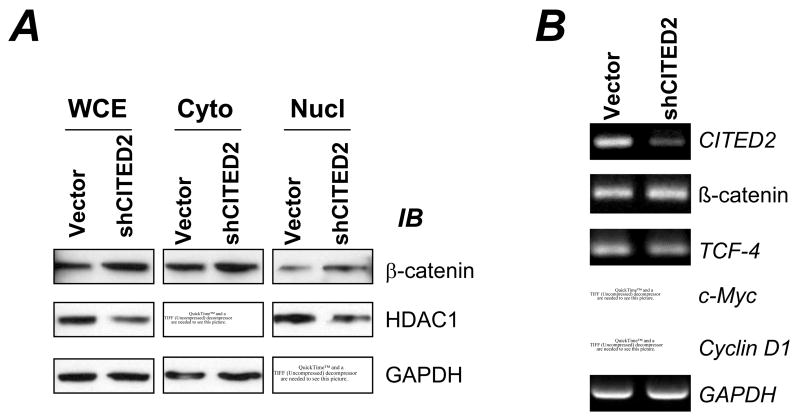
Actin reorganization is normally associated with changes in cancer cell migration and invasion [22]. Therefore, we assessed whether the morphological changes in CITED2 knockdown cells were accompanied by changes in cell migration and invasion in vitro. Wound healing assays showed that CITED2 knockdown had no affect on cell migration (data not shown). However, the Boyden chamber invasion assay demonstrated that the CITED2 knockdown significantly increased the invasiveness of RKO cells (Fig 2). Given the importance of Wnt signaling in colon cancer progression, we examined whether the phenotypic changes in CITED2 knockdown cells were accompanied by alterations in the Wnt/β-catenin pathway. As shown in Fig 3A, reduced levels of CITED2 had no significant affect on the subcellular localization of β-catenin in RKO cells, nor the expression of β-catenin and TCF (Fig 3B). The lack of induction of the β-catenin pathway correlates with the lack of induction of downstream targets, e.g., cyclin D1 and c-myc (Fig 3B). Therefore, reduced levels of CITED2 induced phenotypic changes that were independent of the β-catenin/TCF pathway.
Fig 2. CITED2 knockdown increased cell invasive capacity.
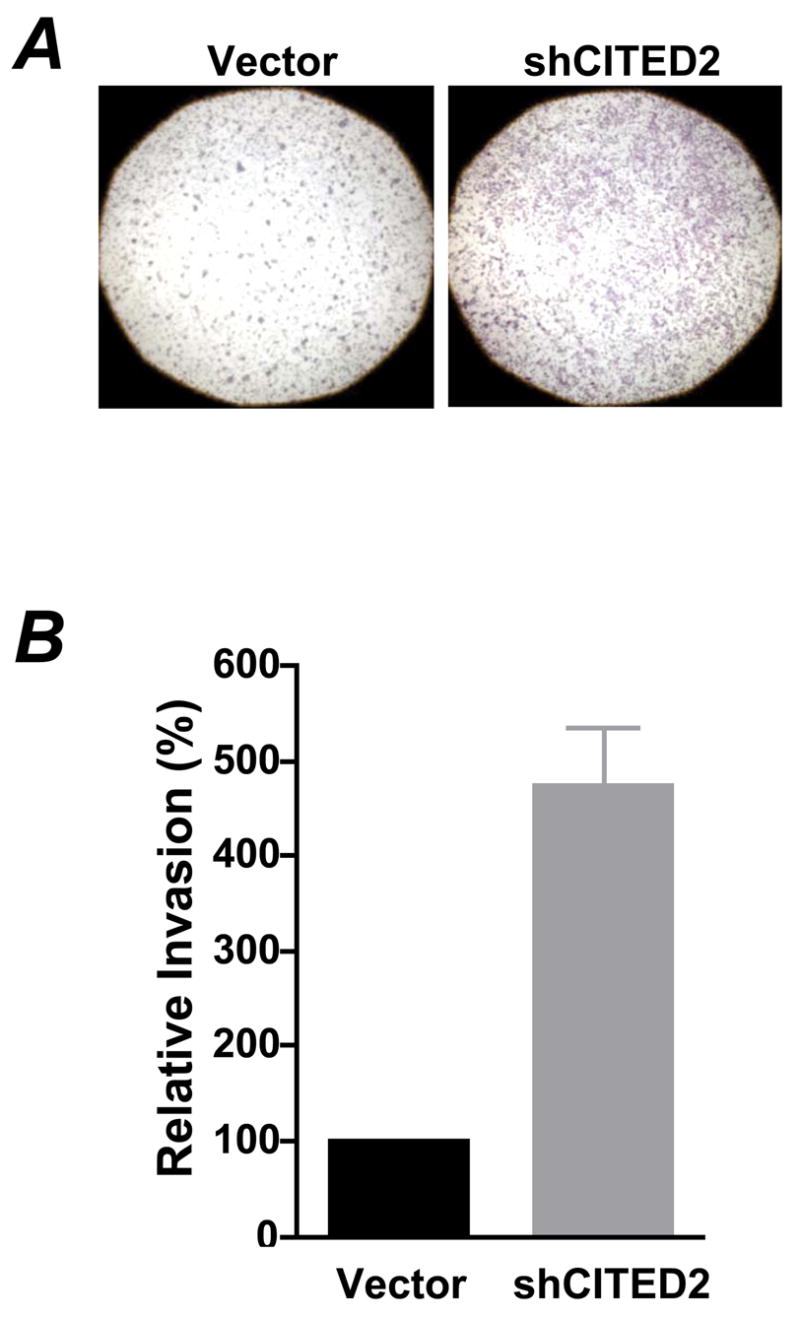
(A) RKO cells were first cultured in serum free medium for 20–24 h, then collected and resuspended in medium with 0.1% BSA. Culture medium with 10% FBS was added to the lower chamber of BD Matrigel Invasion Chamber and the resuspended cells were added onto the top of the Matrigel. Forty hours later, cells on the lower surface of the membrane were fixed with methanol, stained with H&E and viewed with microscope (magnification: 20×). Representative images from three independent experiments are shown. (C) Cells on the lower surface of the membrane were counted under a microscope. Data shown are means ± SEM for numbers of cells from three independent experiments, and ten fields were counted from each experiment.
Fig 3. CITED2 knockdown does not affect the β-catenin pathway.
(A) Whole cell extract (WCE), cytoplasmic (Cyto) and nuclear (Nucl) fractions of RKO cells were prepared for immunoblotting. Rabbit anti-HDAC1 (Santa Cruz Biotechnology) and mouse anti-β-catenin (BD Biosciences) were used for immunoblotting. HDAC1 was used as a marker for the nuclear fraction, and GAPDH was used as a marker for the cytoplasmic fraction. (B) Total RNA was isolated from cells for RT-PCR as described above.
CITED2 knockdown upregulated MMP-13 expression
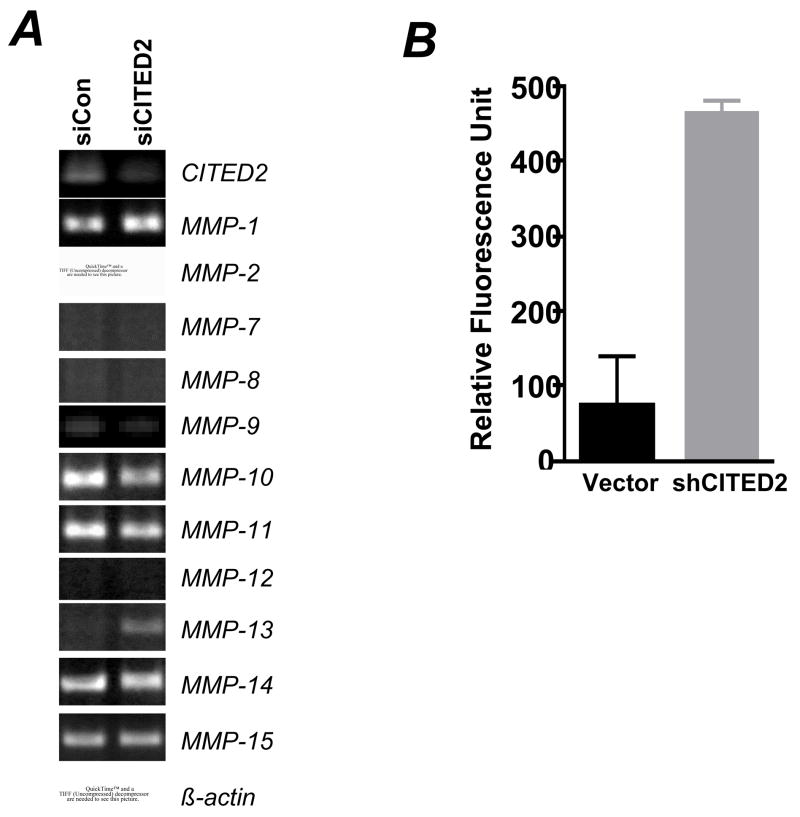
To identify key genes involved in the phenotypic changes in CITED2 knockdown of the RKO cells, we performed DNA microarray analysis using the Affymetrix GeneChip Human U133 Plus 2.0 Array. A total of 739 genes were differentially regulated by a factor of 2.5-fold when CITED2 levels were decreased: 235 genes were upregulated and 504 genes were downregulated. Among the differentially regulated genes, MMP-13 was significantly upregulated in CITED2 knockdown cells (4-fold), suggesting that this metalloproteinase was specifically inhibited by CITED2. To determine whether the upregulation of MMP-13 in CITED2 knockdown cells is an early response of CITED2 knockdown or the consequence of chronic cellular physiological changes, we performed transient siRNA transfection experiments. Total RNA was isolated 20 h after siRNA transfection, and RT-PCR was performed to survey the expression of MMP1–3 and 7–16. Of the MMPs detected in RKO cells, MMP-13 was significantly upregulated by CITED2 silencing (Fig 4A). Notably, it has been reported that CITED2 mediates flow shear regulated expression of MMP-1 and MMP-13 in human chondrocytes [18]. However, MMP-9 was essentially undetectable in RKO cells and the levels were not significantly induced in contrast to what has been reported for this target of CITED2 in human breast cancer cell line MDA-MB-231 [18]. Thus regulation of MMP-9 by CITED2 may be cell dependent. Enzyme-linked immunosorbent assay for MMP-13 confirmed that CITED2 knockdown significantly increased the cell levels of MMP-13 protein (data not shown). Accordingly, MMP-13 activity was also significantly increased in the medium of CITED2 knockdown cells (Fig 4B). Previous studies have established a critical role for MMPs in colon cancer invasion and metastasis [23]. In particular, elevated MMP-13 expression is associated with poor prognosis in colon cancer [24]. We therefore assessed whether neutralizing MMP13 activity with its antibody blocked RKO cell invasion in vitro. Incubation of cells with the anti-MMP-13 monoclonal antibody VIIA2 (Calbiochem) reduced the invasive capacity of cells expressing reduced levels of CITED2 by ~25% (data not shown), suggesting that other changes in CITED2 knockdown cells (e.g., cytoskeletal reorganization) also contribute to the altered invasiveness of these cells.
Fig 4. CITED2 knockdown increased MMP-13 expression and enzyme activity in RKO cells.
(A) RKO cells were transfected with a control siRNA (Ambion) or CITED2-specific siRNA. Twenty hours after transfection, total RNA was isolated for RT-PCR. (B) Cells were seeded in 12-well plates. Twelve hours later, cells were washed and incubated in serum-free medium for another 24 h. The medium was collected, centrifuged; and the supernatant was used to assay MMP-13 activity.
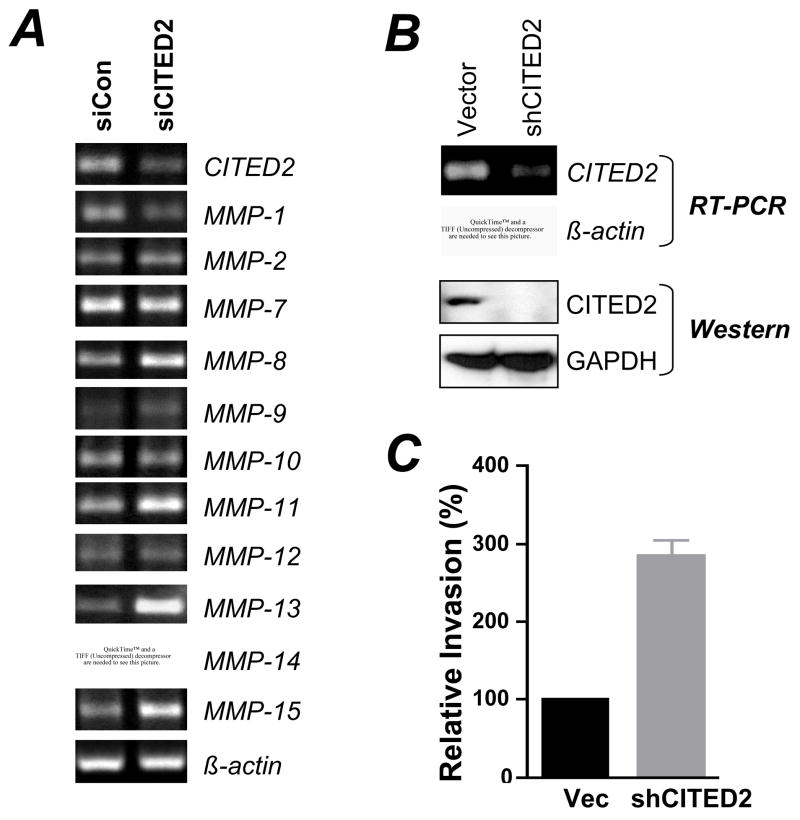
To investigate whether CITED2 commonly regulates MMP-13 expression in colon cancer cell lines, we silenced CITED2 expression in SW480 colon cancer cells with siRNA. As shown in Fig 5A, CITED2-specific siRNA reduced CITED2 expression by ~70% (Fig 5A). Of the 11 MMPs detected in this cell line, MMP-13 was most significantly up-regulated (Fig 5A) as observed with RKO cells (Fig 4A). The expression of MMP-8, −9, −11, and −15 was also slightly upregulated with the knockdown of CITED2. Contrary to the slight upregulation of MMP-1 in CITED2-knockdown RKO cells, MMP-1 was downregulated in CITED2-knockdown SW480 cells (Fig 5A), suggesting the different genetic and epigenetic background of colon cancer cells might also contribute to CITED2 functions. To further determine whether CITED2 regulates SW480 invasiveness, we generated CITED2-knockdown stable cell line (Fig 5B) and performed Matrigel invasion assay. Boyden chamber invasion assay demonstrated that CITED2-knockdown significantly increased the invasiveness of SW480 cells (Fig 5C). Together, our data strongly support the notion that CITED2 regulates MMP-13 expression and invasiveness in colon cancer cell lines.
Fig 5. CITED2 knockdown increased MMP-13 expression and cell invasiveness in SW480 cells.
(A) SW480 cells were transfected with a control siRNA (Ambion) or CITED2-specific siRNA. Twenty hours after transfection, total RNA was isolated for RT-PCR. (B) SW480 cells were transduced with plko1 empty lentiviral vector or plko1-shCITED2, and selected with puromycin for 3 weeks. The pools of puromycin-resistent cells were used for RT-PCR (upper panels) and Western blot (lower panels) analyses. (C) Matrigel invasion assays of cells in (B) were performed as described in Fig 2B. Twenty-four hours after invasion, cells on the lower surface of the membrane were stained with H&E and counted. Data shown are means ± SEM of cells from three independent experiments, and ten fields were counted from each experiment.
Butyrate upregulated CITED2 expression and downregulated MMP-13 expression in colon cancer cells
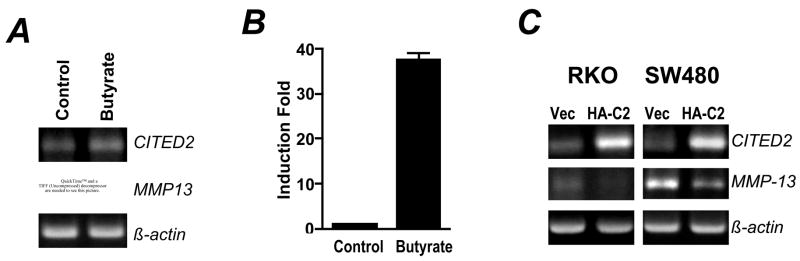
Numerous studies have shown that butyrate, a major dietary fermentation product and potent HDAC inhibitor, inhibits colon cancer invasion [25–27]. Research also suggests that MMPs expression can be epigenetically dysregulated as a result of histone hypoacetylation during tumor progression [28]. It is known that butyrate inhibits the expression of MMP-9 in colon cancer cells [27,29], and MMP-1 and MMP-13 in chondrocytes [30]. Thus, we tested whether butyrate regulated MMP-13 and/or CITED2 expression in RKO cells. As shown in Fig 6A, butyrate upregulated CITED2 expression and downregulated MMP-13 expression in RKO cells. Reporter assays demonstrated strong activation of the CITED2 promoter by butyrate (Fig 6B). To explore whether there is a direct link between CITED2 and MMP-13 expression in colon cancer cells, we overexpressed HA-tagged CITED2 in RKO and SW480 cells. RT-PCR confirmed the overexpression of CITED2 in both cell lines. Ectopic expression of CITED2 was accompanied by downregulation of MMP-13 expression (Fig 6C). This observation was consistent with a previous report that overexpression of CITED2 represses MMP-13 expression in human chondrocytes [18]. The expression of MMP-1, −9 and other MMPs was not significantly affected by CITED2 overexpression (data not shown). Thus, butyrate stimulates CITED2 expression, and CITED2 expression is inversely correlated with MMP-13 expression in colon cancer cells.
Fig 6. Butyrate upregulated CITED2 expression and downregulated MMP-13 expression in RKO cells.
(A) RKO cells were treated with 5 mM sodium butyrate for 16 h. Total RNA was isolated for RT-PCR. (B) RKO cells transfected with pMK17 reporter, then treated with 2.5 mM sodium butyrate for 20 h prior to luciferase reporter assays that were normalized to protein. Results are presented as means ± SEM of three experiments. (C) RKO and SW480 cells were transfected with pCMV10 vector (Vec) or pCMV10/HA-CITED2 (HA-C2). Two days later, cells were collected and total RNA was isolated for RT-PCR analyses.
CITED2 arrested cell growth when overexpressed in colon cancer cells
Having established that CITED2 expression is upregulated during stimulation to repress cell growth (Fig 4), we investigated its biological activity by overexpressing it in RKO cells. As transfection efficiency in RKO cells is low by conventional transient transfection, the cells were transfected with an empty vector (pCMV10) or HA-tagged CITED2 expression vector (pCMV10/HA-CITED2) followed by G418 selection to enrich the transfected cells. Using this approach, we observed a 2–3-fold increase of CITED2 mRNA in the enriched HA-CITED2–expressing RKO cells (data not shown). Because an antiproliferative effect of CITED2 was apparent during the selection of the “stable” populations, we analyzed cell cycle transition by flow cytometry. We found that ectopic expression of CITED2 arrested RKO cell growth (Fig 7A). Accordingly, overexpression of CITED2 increased cyclin-dependent kinase inhibitor p21waf1 expression and significantly enhanced butyrate-induced p21waf1 expression (Fig 7B).
Fig 7. Ectopic expression of CITED2 arrested RKO cells.

(A) RKO cells were transfected with pCMV10 vector or pCMV10/HA-CITED2 and selected with G418 at 0.5 mg/ml for 3 weeks. Cells were then seeded in 12-well plates at a confluency of ~25%. Forty hours later, cells were collected for flow cytometry. Data shown are mean±SEM of three repeats. * p<0.05. (B) Cells were incubated with 2.5 mM sodium butyrate for up to 8 h. Western blot was performed.
Among the CITEDs, CITED2 and CITED4 share some similarities. Firstly, both CITED2 and CITED4 are transcriptional co-repressors of HIF1α by competing with HIF1α for CBP/p300 binding [31]. HIF1α responsive genes are involved in many biological processes including angiogenesis, invasion/metastasis, cytoskeletal structure, and cell proliferation/survival [32]. Secondly, both CITED2 and CITED4 also function as coactivators of transcription factor AP-2 by bridging the interaction between AP-2 and CBP/p300 [12,33]. AP-2 is important to epithelial cells and has been implicated in activities such as mediation of growth arrest through activation of p21waf1 [34], maintenance of homotypic cell-cell adhesion through activation of E-cadherin [35] and promotion of apoptosis [36]. Fox et al. [31] documented that breast cancer development is characterized by either nuclear loss or cytoplasmic translocation of CITED4, with subsequent loss of HIF1α transcriptional antagonist activity. The striking similarities between CITED2 and CITED4 suggest a role for CITED2 in human tumorigenesis. A recent study showed that knockdown of CITED2 in breast cancer cell line MDA-MB-231 diminishes stimulation of MMP-9 and cell invasiveness by TGFβ1 in vitro [18]. However, our study suggests that CITED2 might act as a repressor of colon cancer progression. In fact, we found that CITED2 suppressed colon cancer cell growth when overexpressed (data not shown). Thus, it may prove fruitful to study the function of CITED2 in other types of cancers and determine whether the state of CITED2 (e.g., expression and subcellular localization) changes during tumor progression. Collectively, our study provides a strong rationale for further exploring the roles of CITED2 in colon cancer progression and the possibilities of targeting CITED2 for chemoprevention and chemotherapy for colon cancer.
Acknowledgments
Supported by NIH grant R01-DK55732 (JLM), Michigan Gastrointestinal Peptide Research Center Pilot Feasibility Grant P30-DK34933 (LB) and the John and Suzanne Munn Endowed Research Fund of the University of Michigan Cancer Center P30-CA46592 (LB).
Abbreviations
- CITED2
CBP/p300 interacting coactivator with glutamic acid/aspartic acid-rich tail 2
- MMP
matrix metalloproteinase
- HDAC
histone deacetylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Markowitz SD, Dawson DM, Willis J, Willson JK. Focus on colon cancer. Cancer Cell. 2002;1:233–6. doi: 10.1016/s1535-6108(02)00053-3. [DOI] [PubMed] [Google Scholar]
- 2.Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–603. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- 3.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–92. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iyer NG, Ozdag H, Caldas C. p300/CBP and cancer. Oncogene. 2004;23:4225–31. doi: 10.1038/sj.onc.1207118. [DOI] [PubMed] [Google Scholar]
- 5.Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–77. [PubMed] [Google Scholar]
- 6.Muraoka M, Konishi M, Kikuchi-Yanoshita R, Tanaka K, Shitara N, Chong JM, Iwama T, Miyaki M. p300 gene alterations in colorectal and gastric carcinomas. Oncogene. 1996;12:1565–9. [PubMed] [Google Scholar]
- 7.Giles RH, Peters DJ, Breuning MH. Conjunction dysfunction: CBP/p300 in human disease. Trends Genet. 1998;14:178–83. doi: 10.1016/s0168-9525(98)01438-3. [DOI] [PubMed] [Google Scholar]
- 8.Gayther SA, et al. Mutations truncating the EP300 acetylase in human cancers. Nat Genet. 2000;24:300–3. doi: 10.1038/73536. [DOI] [PubMed] [Google Scholar]
- 9.Krubasik D, et al. Absence of p300 induces cellular phenotypic changes characteristic of epithelial to mesenchyme transition. Br J Cancer. 2006;94:1326–32. doi: 10.1038/sj.bjc.6603101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharya S, Michels CL, Leung MK, Arany ZP, Kung AL, Livingston DM. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev. 1999;13:64–75. doi: 10.1101/gad.13.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharya S, Michels CL, Leung MK, Arany ZP, Kung AL, Livingston DM. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1 [In Process Citation] Genes Dev. 1999;13:64–75. doi: 10.1101/gad.13.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braganca J, Eloranta JJ, Bamforth SD, Ibbitt JC, Hurst HC, Bhattacharya S. Physical and functional interactions among AP-2 transcription factors, p300/CREB-binding protein, and CITED2. J Biol Chem. 2003;278:16021–9. doi: 10.1074/jbc.M208144200. [DOI] [PubMed] [Google Scholar]
- 13.Tien ES, Davis JW, Vanden Heuvel JP. Identification of the CREB-binding protein/p300-interacting protein CITED2 as a peroxisome proliferator-activated receptor alpha coregulator. J Biol Chem. 2004;279:24053–63. doi: 10.1074/jbc.M401489200. [DOI] [PubMed] [Google Scholar]
- 14.Glenn DJ, Maurer RA. MRG1 binds to the LIM domain of Lhx2 and may function as a coactivator to stimulate glycoprotein hormone alpha-subunit gene expression. J Biol Chem. 1999;274:36159–67. doi: 10.1074/jbc.274.51.36159. [DOI] [PubMed] [Google Scholar]
- 15.Bamforth SD, et al. Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat Genet. 2001;29:469–74. doi: 10.1038/ng768. [DOI] [PubMed] [Google Scholar]
- 16.Barbera JP, Rodriguez TA, Greene ND, Weninger WJ, Simeone A, Copp AJ, Beddington RS, Dunwoodie S. Folic acid prevents exencephaly in Cited2 deficient mice. Hum Mol Genet. 2002;11:283–93. doi: 10.1093/hmg/11.3.283. [DOI] [PubMed] [Google Scholar]
- 17.Sun HB, Zhu YX, Yin T, Sledge G, Yang YC. MRG1, the product of a melanocyte-specific gene related gene, is a cytokine-inducible transcription factor with transformation activity. Proc Natl Acad Sci U S A. 1998;95:13555–60. doi: 10.1073/pnas.95.23.13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou YT, Wang H, Chen Y, Danielpour D, Yang YC. Cited2 modulates TGF-beta-mediated upregulation of MMP9. Oncogene. 2006;25:5547–60. doi: 10.1038/sj.onc.1209552. [DOI] [PubMed] [Google Scholar]
- 19.Bai L, Merchant JL. Transcription factor ZBP-89 cooperates with histone acetyltransferase p300 during butyrate activation of p21waf1 transcription in human cells. J Biol Chem. 2000;275:30725–33. doi: 10.1074/jbc.M004249200. [DOI] [PubMed] [Google Scholar]
- 20.Bai L, Kao JY, Law DJ, Merchant JL. Recruitment of ataxia-telangiectasia mutated to the p21(waf1) promoter by ZBP-89 plays a role in mucosal protection. Gastroenterology. 2006;131:841–52. doi: 10.1053/j.gastro.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Kranc KR, Bamforth SD, Braganca J, Norbury C, van Lohuizen M, Bhattacharya S. Transcriptional coactivator Cited2 induces Bmi1 and Mel18 and controls fibroblast proliferation via Ink4a/ARF. Mol Cell Biol. 2003;23:7658–66. doi: 10.1128/MCB.23.21.7658-7666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamazaki D, Kurisu S, Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005;96:379–86. doi: 10.1111/j.1349-7006.2005.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mc Donnell S, Chaudhry V, Mansilla-Soto J, Zeng ZS, Shu WP, Guillem JG. Metastatic and non-metastatic colorectal cancer (CRC) cells induce host metalloproteinase production in vivo. Clin Exp Metastasis. 1999;17:341–9. doi: 10.1023/a:1006651019335. [DOI] [PubMed] [Google Scholar]
- 24.Leeman MF, McKay JA, Murray GI. Matrix metalloproteinase 13 activity is associated with poor prognosis in colorectal cancer. J Clin Pathol. 2002;55:758–62. doi: 10.1136/jcp.55.10.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emenaker NJ, Calaf GM, Cox D, Basson MD, Qureshi N. Short-chain fatty acids inhibit invasive human colon cancer by modulating uPA, TIMP-1, TIMP-2, mutant p53, Bcl-2, Bax, p21 and PCNA protein expression in an in vitro cell culture model. J Nutr. 2001;131:3041S–6S. doi: 10.1093/jn/131.11.3041S. [DOI] [PubMed] [Google Scholar]
- 26.Emenaker NJ, Basson MD. Short chain fatty acids inhibit human (SW1116) colon cancer cell invasion by reducing urokinase plasminogen activator activity and stimulating TIMP-1 and TIMP-2 activities, rather than via MMP modulation. J Surg Res. 1998;76:41–6. doi: 10.1006/jsre.1998.5279. [DOI] [PubMed] [Google Scholar]
- 27.Lee JC, et al. Butyrate regulates the expression of c-Src and focal adhesion kinase and inhibits cell invasion of human colon cancer cells. Mol Carcinog. 2005;43:207–14. doi: 10.1002/mc.20117. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Salvador J, Armas-Pineda C, Perezpena-Diazconti M, Chico-Ponce de Leon F, Sosa-Sainz G, Lezama P, Recillas-Targa F, Arenas-Huertero F. Effect of sodium butyrate on pro-matrix metalloproteinase-9 and -2 differential secretion in pediatric tumors and cell lines. J Exp Clin Cancer Res. 2005;24:463–73. [PubMed] [Google Scholar]
- 29.Aparicio A, Gardner A, Tu Y, Savage A, Berenson J, Lichtenstein A. In vitro cytoreductive effects on multiple myeloma cells induced by bisphosphonates. Leukemia. 1998;12:220–9. doi: 10.1038/sj.leu.2400892. [DOI] [PubMed] [Google Scholar]
- 30.Young DA, Lakey RL, Pennington CJ, Jones D, Kevorkian L, Edwards DR, Cawston TE, Clark IM. Histone deacetylase inhibitors modulate metalloproteinase gene expression in chondrocytes and block cartilage resorption. Arthritis Res Ther. 2005;7:R503–12. doi: 10.1186/ar1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox SB, Braganca J, Turley H, Campo L, Han C, Gatter KC, Bhattacharya S, Harris AL. CITED4 inhibits hypoxia-activated transcription in cancer cells, and its cytoplasmic location in breast cancer is associated with elevated expression of tumor cell hypoxia-inducible factor 1alpha. Cancer Res. 2004;64:6075–81. doi: 10.1158/0008-5472.CAN-04-0708. [DOI] [PubMed] [Google Scholar]
- 32.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 33.Braganca J, Swingler T, Marques FI, Jones T, Eloranta JJ, Hurst HC, Shioda T, Bhattacharya S. Human CREB-binding protein/p300-interacting transactivator with ED-rich tail (CITED) 4, a new member of the CITED family, functions as a co-activator for transcription factor AP-2. J Biol Chem. 2002;277:8559–65. doi: 10.1074/jbc.M110850200. [DOI] [PubMed] [Google Scholar]
- 34.Zeng YX, Somasundaram K, el-Deiry WS. AP2 inhibits cancer cell growth and activates p21WAF1/CIP1 expression. Nat Genet. 1997;15:78–82. doi: 10.1038/ng0197-78. [DOI] [PubMed] [Google Scholar]
- 35.Batsche E, Muchardt C, Behrens J, Hurst HC, Cremisi C. RB and c-Myc activate expression of the E-cadherin gene in epithelial cells through interaction with transcription factor AP-2. Mol Cell Biol. 1998;18:3647–58. doi: 10.1128/mcb.18.7.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilger-Eversheim K, Moser M, Schorle H, Buettner R. Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis and cell-cycle control. Gene. 2000;260:1–12. doi: 10.1016/s0378-1119(00)00454-6. [DOI] [PubMed] [Google Scholar]