Abstract
The purpose of this study was to examine effects of d-amphetamine on choice controlled by reinforcement delay. Eight pigeons responded under a concurrent-chains procedure in which one terminal-link schedule was always fixed-interval 8 s, and the other terminal-link schedule changed from session to session between fixed-interval 4 s and fixed-interval 16 s according to a 31-step pseudorandom binary sequence. After sufficient exposure to these contingencies (at least once through the pseudorandom binary sequence), the pigeons acquired a preference for the shorter reinforcement delay within each session. Estimates of the sensitivity to reinforcement immediacy were similar to those obtained in previous studies. For all pigeons, at least one dose of d-amphetamine attenuated preference and, hence, decreased estimates of sensitivity to reinforcement immediacy; in most cases, this effect occurred without a change in overall response rates. In many cases, the reduced sensitivity to reinforcement delay produced by d-amphetamine resulted primarily from a decrease in the asymptotic level of preference achieved within the session; in some cases, d-amphetamine produced complete indifference. These findings suggest that a reduction in the sensitivity to reinforcement delay may be an important behavioral mechanism of the effects of psychomotor stimulants.
Keywords: preference, reinforcement delay, acquisition, behavioral mechanisms, d-amphetamine, key peck, pigeons
Effects of drugs on behavior maintained by delayed reinforcement have received recent attention. Much of this work has focused on effects of drugs under “self-control” preparations in which subjects choose between a larger, more delayed reinforcer and a smaller, more immediate one. Drugs classified as psychomotor stimulants (e.g., amphetamines, methylphenidate) typically increase the likelihood of choosing the larger, more delayed reinforcer (Pietras, Cherek, Lane, Tcheremissine, & Steinberg, 2003; Pitts & Febbo, 2004; Pitts & McKinney, 2005; Richards, Sabol, de Wit, 1999; Wade, de Wit, & Richards, 2000; but see Charrier & Thiebot, 1996; Evenden & Ryan, 1996). There are a number of potential behavioral mechanisms of this effect (see Pitts & Febbo, 2004; Richards et al., 1999). One hypothesis is that these drugs attenuate the discounting effects of reinforcement delay. According to this view, stimulants increase choices of a larger more delayed reinforcer by altering the delay–discount function such that, relative to nondrug conditions, a given increase in delay produces a smaller discounting effect on the effectiveness (value) of a reinforcer.
Support for the above-mentioned hypothesis was provided by Pitts and Febbo (2004). Pigeons were trained under a concurrent-chains schedule (see Autor, 1969; Herrnstein, 1964) in which two keys were simultaneously available as initial links and access to the terminal links was available according to a variable-interval (VI) schedule. The terminal link associated with one of the keys provided a smaller reinforcer according to a fixed-time (FT) 2-s schedule (i.e., the delay to the smaller reinforcer was 2 s). The terminal link associated with the other key provided a larger reinforcer according to an FT schedule whose value increased across the session (i.e., the delay to the larger reinforcer increased across the session). With this procedure, a delay-of-reinforcement function was obtained within each session. The data were analyzed using a generalized-matching model similar to that proposed by Logue, Rodriguez, Pena-Correal, and Mauro (1984), with separate parameters for sensitivity to reinforcement delay and amount. Pitts and Febbo reported that methamphetamine reduced estimates of sensitivity to the effects of reinforcement delay in all their pigeons. It should be noted that, for 3 of the 4 pigeons, this effect occasionally was accompanied by what may have been a reduction in the sensitivity to the effects of reinforcement amount. Nevertheless, Pitts and Febbo suggested that their data provided support for the notion that a reduced sensitivity to effects of reinforcement delay was a potential behavioral mechanism of the stimulant-induced increases in preference for a larger, more delayed reinforcer that frequently has been reported in the literature.
If a reduction in the sensitivity to reinforcement delay is an important behavioral mechanism of the effects of stimulant drugs on self-control choices, then it is reasonable to predict that these drugs will attenuate effects of reinforcement delay under other conditions. Interestingly, there have been relatively few investigations of drug effects on behavior maintained by reinforcement delay under circumstances other than those involving self-control choices. Walker and Branch (1996) found that intermediate doses of cocaine either slightly increased or did not affect, and larger doses decreased, response rates maintained by both briefly and completely signaled delayed reinforcement. LeSage, Byrne, and Poling (1996) investigated effects of d-amphetamine on acquisition of lever pressing in rats with delayed reinforcement. They found that the lowest dose (1.0 mg/kg) tested enhanced acquisition in some of their subjects; higher doses suppressed responding in all subjects. Sagvolden, Slatta, and Arntzen (1988) reinforced nose-poking a target hole within a 4 × 5 matrix of holes under a fixed-interval (FI) schedule. Under baseline conditions, an accelerated pattern of nose-poking developed in which both the rate and the proportion of pokes to the target and adjacent locations increased across the FI. Lower doses of methylphenidate increased the proportion of pokes to the target and adjacent locations during the early portion of the interval. Although their study did not involve explicitly programmed reinforcement delays, and there are a number of potential accounts of their findings, Sagvolden et al. suggested that this effect may have resulted from a methylphenidate-induced change in the delay-of-reinforcement gradient (i.e., an attenuation of the effects of reinforcement delay).
Interpretation of drug effects on behavior maintained by delayed reinforcement under single schedules is complicated by other, more general, effects (e.g., direct effects on motor functioning). Choice arrangements may offer a more promising approach. Grace, Bragason, and McLean (2003) developed a procedure capable of producing rapid acquisition of preference under control of reinforcement delay. Pigeons responded under a concurrent-chains procedure with VI initial-link schedules and FI terminal-link schedules; reinforcement amount (3-s access to grain) was the same for both terminal links. The FI terminal-link schedules (i.e., the delays to reinforcement associated with the initial-links) changed across sessions unpredictably. For example, in Grace et al.'s Experiment I, one terminal-link schedule always was FI 8 s, whereas the other terminal-link schedule changed between FI 4 s and FI 16 s across sessions according to a 31-step pseudorandom binary sequence (PRBS; see Hunter & Davison, 1985; Schofield & Davison, 1997). Grace et al. found that after sufficient training under this procedure (after one or two PRBS cycles), response allocation during the initial links adjusted rapidly within each session, and preference for the terminal link with the shorter delay to reinforcement reached asymptote by approximately midway through the session. Multiple-regression analyses indicated that response allocation during the initial link within a given session was controlled by the delay values in effect during that session (i.e., estimates of sensitivity ranged from 0.5 to 2.0 across subjects), and not by those in effect during previous sessions (i.e., estimates of sensitivity in a given session to the delay values in effect for each of the nine immediately preceding sessions were near zero).
Several features of the procedure used by Grace et al. (2003) commend it as a preparation for studying effects of drugs on behavior controlled by reinforcement delay. First, as a choice procedure, it allows changes in response allocation to occur without dramatic changes in overall response output. Thus, there is a potential for separating drug-induced changes in control by delayed reinforcement from other drug effects. Second, because it is not a self-control procedure, assessment of drug effects on sensitivity to reinforcement delay is not contaminated by different reinforcement amounts associated with each option. Third, the PRBS design appears to provide a rapid and valid technique for determination of sensitivity to changes in reinforcement variables (also see Grace & McLean, 2006; Hunter & Davison, 1985; Maguire, Hughes, & Pitts, 2007; Schofield & Davison, 1997). Fourth, this procedure is capable of generating stable within-session acquisition in relatively few sessions and, thus, it appears to provide an effective and convenient baseline against which to assess effects of drugs. Drug effects on acquisition have received considerable attention in behavioral pharmacology (e.g., Cohn & Paule, 1995; Thompson & Moerschbaecher, 1979). Relatively few studies, however, have focused on the effects of drugs on acquisition with delayed reinforcement (e.g., LeSage et al., 1996).
The purpose of the present study was to examine effects of d-amphetamine under a rapid-acquisition procedure similar to the one reported by Grace et al. (2003). If a reduction in sensitivity to reinforcement delay is an important behavioral mechanism of the effects of psychomotor stimulants, then d-amphetamine would be expected to alter within-session acquisition, and/or attenuate asymptotic levels, of preference controlled by delayed reinforcement.
Method
Subjects
Eight pigeons of mixed breed, numbered 105–108 and 225–228, were maintained at 85% (± 15 g) of their free-feeding body weights by providing postsession feeding as needed. The pigeons were housed individually in a colony room with a 12/12 hr light/dark cycle (lights on at 6:00 a.m.). Water and health grit were available continuously in the home cages. Pigeons 105–108 were experimentally naïve at the beginning of the study. Pigeons 225–228 all had previous experience key pecking under concurrent-chains schedules of food presentation.
Apparatus
Eight custom-built operant-conditioning chambers were used. The experimental space within each chamber was 32.0 cm deep by 34.0 cm wide by 34.0 cm high. One wall of each chamber was constructed of aluminum and contained three response keys arranged in a row, 21.0 cm from the floor and 10.0 cm apart (center to center). Each key was 2.5 cm in diameter, could be transilluminated by a white, red, or green light, and required a force of 0.15 N to operate its corresponding switch. A 5.0- by 5.5-cm aperture, into which a food magazine containing wheat could be raised, was located 15.0 cm directly below the center key. While the magazine was raised, the aperture was illuminated with a 28-VDC lamp and all other lights in the chamber were off. General chamber illumination was provided by a 28-VDC houselight located 7 cm directly above the center key. Each chamber was enclosed within a sound-attenuating cubicle equipped with an exhaust fan that provided ventilation and masking noise. Experimental events were programmed and data were recorded by a Windows®-controlled microcomputer using Med Associates® (Georgia, VT) software and interfacing equipment located in an adjoining room.
Behavioral Procedure
Preliminary Training
Following adaptation to the chamber and magazine training, Pigeons 105–108 were trained to peck the keys via an autoshaping procedure. After key-peck training, these pigeons were exposed to a concurrent variable-interval (VI) 1-s, VI 1-s schedule, using the two white side keys, for one session. The values of the VI schedules were increased to 10 s over the course of three to four sessions. Once responding occurred reliably on both keys, a concurrent-chains procedure was implemented. These, and all subsequent sessions, ended after 72 initial-/terminal-link cycles or 70 min, whichever occurred first. During the initial link, the white side keys were illuminated and a VI 10-s schedule operated. Once the interval elapsed, the next response to the preselected key provided entry to its associated terminal link. Entry was assigned randomly to the left or right terminal link with the restriction that every six cycles contained three entries to each terminal link. The VI 10-s schedule began timing upon the first peck to either key (i.e., pausing after completion of the previous terminal link was excluded from initial-link time). Separate lists of 12 intervals (constructed from the exponential distribution described by Fleshler & Hoffman, 1962) were used for cycles in which the left or right key was selected. Each list was sampled without replacement so that each interval was used three times for both the left and right keys within each session. A 1-s changeover delay (COD) was in effect such that a peck on a given key could not gain entry into a terminal link until 1 s had elapsed since a changeover to that key.
Upon entry into a terminal link, the color of the associated side key changed to red or green and the other key darkened (terminal-link color/position assignments for each pigeon are listed below). Responding in the terminal links was reinforced with 3-s access to the food magazine, arranged according to FI schedules. After one session in which both terminal links were FI 1-s schedules, one terminal link schedule was changed to FI 4 s and the other terminal link schedule was changed to FI 8 s (i.e., the pigeons chose between FI 4-s or FI 8-s terminal links). After five consecutive sessions in which more responses occurred on the initial-link key associated with the FI 4-s schedule, that terminal-link schedule then was changed to an FI 16 s, while the other terminal link remained FI 8 s (i.e., the pigeons chose between FI 16-s or FI 8-s terminal links). After five consecutive sessions in which more responses occurred on the initial-link key associated with the FI 8-s schedule, the rapid-acquisition procedure was initiated.
Rapid Acquisition
Once preliminary training was completed, pigeons 105–108 were exposed to a rapid-acquisition concurrent-chains procedure similar to the one described by Grace et al. (2003). Throughout the remainder of the study, one terminal-link schedule was always FI 8-s (the standard delay), whereas the other terminal-link schedule (the variable delay) changed between FI 4-s or FI 16-s across sessions according to the same 31-step PRBS used by Grace et al. (see Hunter & Davison, 1985). That is, the side key associated with the shorter delay to reinforcement varied unpredictably across sessions, but remained constant within a given session.
The color/position combination associated with the standard and variable delays was counterbalanced for Pigeons 105–108 and held constant throughout the experiment. For Pigeons 105 and 107, a red left key was associated with the variable delay and a green right key was associated with standard delay; for Pigeons 106 and 108, a red right key was associated with the variable delay and a green left key was associated with the standard delay. All other features of the concurrent-chains procedure were as described above.
Pigeons 225–228 already were responding on a rapid-acquisition procedure at the conclusion of a previous experiment and had completed several 31-session PRBS series. The procedure used with these pigeons in the present experiment was the same as the one used with Pigeons 105–108, with two exceptions. First, the colors/positions associated with the terminal links were the same for all pigeons; the fixed delay (FI 8 s) was associated with the red left key and the variable delay (FI 4 s or FI 16 s) was associated with the green right key. Second, because the previous study with these pigeons involved an examination of the effects of reinforcement amount on sensitivity to reinforcement delay, two types of concurrent-chains cycles occurred within each session. For one cycle type, the reinforcement amount associated with both terminal links was 1.5-s access to grain (the smaller reinforcer); for the other cycle type, the reinforcement amount associated with both terminal links was 4.5-s access to grain (the larger reinforcer). Cycle type was signaled by different houselight conditions: For one type, the houselight blinked off for 0.25-s intervals (i.e., 0.25 s on, 0.25 s off, and so on) during the first 3 s of the cycle, after which it remained on until grain presentation; and for the other type, the houselight remained on throughout the cycle (except during grain presentation). The blinking houselight signaled cycles ending in the larger reinforcer for Pigeons 225 and 226 and signaled cycles ending in the smaller reinforcer for Pigeons 227 and 228. Sessions consisted of 72 cycles (36 of each type), composed of 12 blocks of six cycles each. All cycles were the same type (larger or smaller reinforcer) within a block. The cycle type for the first block was determined randomly and the cycle types strictly alternated thereafter. Sessions ended after 70 min if all 72 cycles had not been completed.
With few exceptions, sessions were conducted 7 days per week at approximately the same time of day. Drug testing was initiated when a) at least one 31-session PRBS series had been completed (Pigeons 105–108), and b) when the ratio of responses on the two keys consistently tracked the ratio of immediacies for a minimum of 10 consecutive sessions (all pigeons), as determined by visual inspection of daily graphs of immediacy ratios and response ratios.
Pharmacological Procedure
d-Amphetamine sulphate (Sigma®, generously donated by Professor Larry Kokkinidis, University of Canterbury) was dissolved in 0.9% sodium chloride (saline) and injected 15 min prior to selected experimental sessions. Injections were given into the breast muscle (i.m.), usually in a volume of 1.0 ml/kg. Injections were administered once or twice per week, provided that the data from the session conducted the day before (the “control session”) were within the range of the previous 10 noninjection sessions. If this was not the case, the injection for that day was cancelled and the session was conducted as scheduled. Injection sites alternated between the left and right breast muscle. Effects of the saline vehicle (“saline sessions”) were determined at least twice prior to the initiation of drug administration and periodically throughout the dosing regimen. All sessions preceded by injections were separated by a minimum of 2 days. The following doses (expressed as the salt) of d-amphetamine were tested in each bird: 0.3, 1.0, 1.7, 3.0 mg/kg; Pigeon 108 received a single administration of 5.6 mg/kg. Doses were administered in an irregular order, and the effects of each dose and saline usually were determined at least twice at each variable delay (i.e., four determinations total). The number of determinations occasionally was reduced when a higher dose (3.0 or 5.6 mg/kg) substantially suppressed responding and/or when the lowest dose (0.3 mg/kg) did not affect responding.
Data Analysis
The primary dependent measure was the ratio of responses on the option associated with the standard delay (FI 8 s) to responses on the option associated with the variable delay (FI 16 s or FI 4 s) during the initial links. For each session, the log (base 10) of the response ratio was expressed as a function of the log-obtained immediacy ratio (immediacy was calculated as the reciprocal of the delay). A quantitative evaluation of the degree to which response ratios were controlled by the delay values in effect for the current and for the previous sessions was conducted using a generalized matching equation similar to the one reported by Davison and McCarthy (1988):
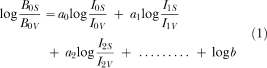 |
1 |
where B represents initial-link responses and I represents reinforcement immediacy (the reciprocal of delay) for each alternative (S = standard delay and V = variable delay). The parameters a0…a9 represent sensitivity to reinforcement immediacy at each lag and b represents bias; a positive value of b indicates a bias for the standard alternative and a negative value indicates a bias the variable alternative. Sensitivity coefficients from Lag 0 (the current session) through Lag 9 (the ninth preceding session) were obtained via multiple-regression analyses. In the regressions, all the predictor variables (i.e., lag reinforcer immediacy ratios) were entered in a single step.
Effects of d-amphetamine on preference were assessed by plotting dose-effect functions for log response ratios (standard/variable) separately for each pair of terminal-link schedules (FI 8 s/FI 16 s and FI 8 s/FI 4 s). For each terminal-link pair (session type), the effect produced by each dose of d-amphetamine was quantified by expressing the difference between the log-ratio at that dose (drug log ratio) and the log ratio under saline conditions (saline log ratio) as a proportion of the log ratio under saline conditions [i.e., (drug log ratio–saline log ratio)/saline log ratio]. For each dose, the size of the drug-induced change in log ratios was compared across pairs of terminal-link schedules via a repeated-measures t-test.
Dose-effect functions also were obtained for sensitivity and bias. Sensitivity and bias were obtained for individual pigeons by plotting average log response ratios as a function of log-obtained immediacy ratios separately for control sessions, saline sessions, and sessions preceded by each dose; the slope and y-intercept of the resulting regression line for each condition was used to estimate sensitivity and bias, respectively. Drug effects on performance within sessions were characterized by plotting both log response ratios and estimates of sensitivity in the initial links across session 12ths (i.e., blocks of six cycles) under drug and nondrug conditions. Finally, effects of d-amphetamine on overall response rates in the initial link and on response rates in each of the terminal links were obtained. Overall initial-link response rates were calculated by dividing the sum of responses on both keys by the total time spent in the initial link. Separate one-way, repeated-measures analyses of variance were conducted for sensitivity, bias, and overall response rate, using the data for saline and each of the doses (for sensitivity and bias, only the data for 0.3, 1.0, and 1.7 mg/kg were used because estimates could not be obtained for Pigeons 106, 107, and 228 at 3.0 mg/kg). Post-hoc analyses were conducted using a Tukey's HSD test. All statistical tests were conducted with α = .05.
For Pigeons 225–228, all measures of performance were virtually identical across the two reinforcement amounts. Thus, the data for the two reinforcement amounts were combined for all of the analyses presented below.
Results
Performance Under The Rapid-acquisition Procedure
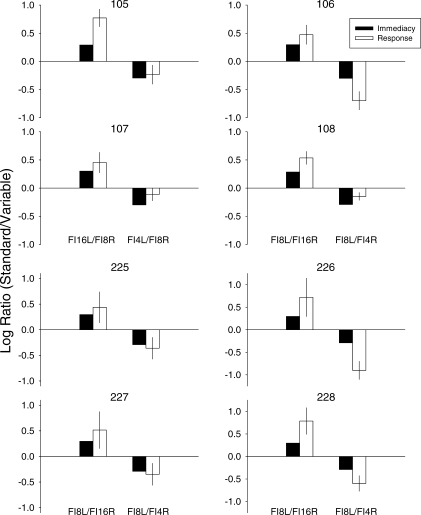
Once stable performance was achieved, initial-link response allocation for all pigeons consistently tracked the terminal-link delays in effect. Figure 1 shows log ratios (standard/variable) averaged across control sessions (those immediately preceding injections) for individual pigeons. Note that, for sessions in which the variable schedule was FI 16 s, a preference for the FI 8-s schedule results in a positive value, and for sessions in which the variable schedule was FI 4 s, a preference for the FI 4-s schedule results in a negative value. The corresponding log-obtained immediacy ratios also are shown. For all pigeons, response allocation in a given session was well controlled by the terminal-link values arranged in that session. Indeed, for several pigeons (106 and 225–228), the response ratios were more extreme than the immediacy ratios (i.e., overmatching). Several pigeons showed a larger preference for the shorter delay when the terminal-link schedules were FI 8 s and FI 16 s than when the terminal-link schedules were FI 8 s and FI 4 s (i.e., these pigeons showed a bias for the standard alternative, illustrated in Table 1 by the positive values for control sessions). This effect was most pronounced for Pigeons 105, 107, and 108. In contrast, Pigeons 106 and 226 showed a larger preference for the shorter delay when terminal-link schedules were FI 8 s and FI 4 s than when the terminal-link schedules were FI 8 s and FI 16 s (i.e., these pigeons showed a bias for the variable alternative, illustrated in Table 1 by the negative values for control sessions).
Fig 1.
Log obtained initial-link immediacy (filled bars) and response ratios (unfilled bars) in individual pigeons; the ratios shown are expressed as data for the option associated with the standard-delay (FI 8 s) to data for the option associated with the variable-delay (FI 4 s or FI 16 s). Values are means from all control sessions. For Pigeons 105 and 107, the standard-delay schedule (FI 8 s) occurred on the right key and the variable-delay schedule (FI 4 s or FI 16 s) occurred on the left key; for the remaining pigeons, this was reversed. Error bars show ±1 SD.
Table 1.
Estimates of bias for individual pigeons.
| Pigeon | Control | Saline | 0.3 mg/kg | 1.0 mg/kg | 1.7 mg/kg | 3.0 mg/kg |
| 105 | 0.27 | 0.13 | 0.37 | 0.23 | 0.27 | −0.28 |
| 106 | −0.10 | 0.03 | −0.13 | −0.43 | −0.14 | — |
| 107 | 0.16 | 0.28 | 0.14 | 0.17 | −0.05 | — |
| 108 | 0.19 | 0.16 | 0.18 | 0.09 | 0.13 | 0.06 |
| 225 | 0.01 | −0.12 | 0.09 | −0.003 | −0.25 | −0.38 |
| 226 | −0.14 | −0.11 | −0.14 | 0.35 | 0.05 | 0.16 |
| 227 | 0.09 | 0.15 | 0.30 | 0.02 | −0.17 | −0.63 |
| 228 | 0.06 | −0.10 | −0.57 | 0.29 | 0.37 | — |
Note. Positive values indicate a bias for the standard alternative. Dashes indicate instances in which bias could not be estimated (either because responding was completely suppressed or because the particular dose was not given at least once under each delay condition).
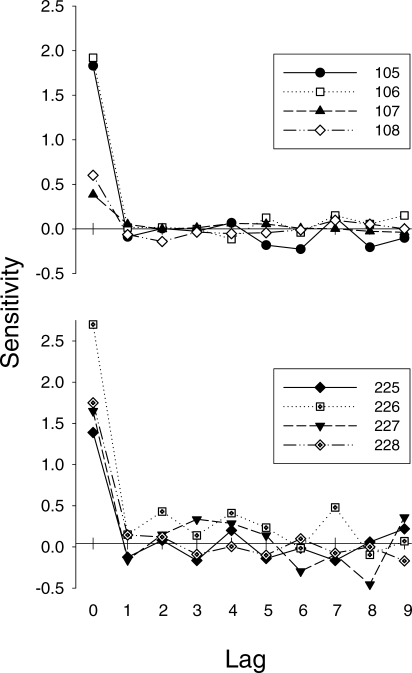
Figure 2 shows the results of the multiple-regression analysis. Displayed are sensitivity coefficients for all lags over the course of the 30 sessions preceding initiation of the injection regimen. The top panel shows data for Pigeons 105–108 and the bottom panel shows data for Pigeons 225–228. Lag 0 sensitivities ranged from approximately 0.5 to 2.0 for Pigeons 105–108 and 1.4 to 2.7 for Pigeons 225–228. Sensitivities at other lags generally were near 0 (except in a few cases for Pigeons 226 and 227). In each case, the Lag 0 coefficient was statistically significant. Although there were a few exceptions (e.g., Lags 2, 4, and 7 for Pigeon 226), coefficients at other lags typically were not significant. Overall, Equation 1 described these data reasonably well, accounting for an average of 88% (range: 72–97%) of the variance across pigeons.
Fig 2.
Estimates of sensitivity to reinforcement immediacy for Lag 0 through Lag 9 for Pigeons 105–108 (upper panel) and 225–228 (lower panels) obtained by multiple-regression analyses of the 30 sessions immediately preceding initiation of the drug regimen. Data for individual pigeons are shown by different symbols.
Effects of D-amphetamine
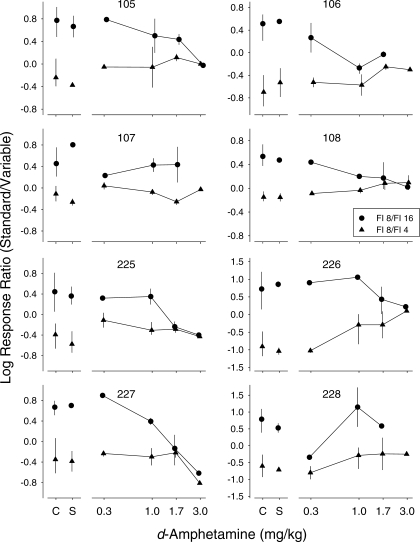
Figure 3 shows individual dose-effect functions for log response ratios. In each graph, circles show data from sessions in which the variable-delay schedule was FI 16 s and triangles show data from sessions in which the variable-delay schedule was FI 4 s. d-Amphetamine typically produced a dose-related reduction in preference. That is, response ratios usually were less extreme following d-amphetamine administration than under nondrug conditions such that the functions tended to converge. This effect was evident to some degree with at least one dose in all of the pigeons. For most of the pigeons, this effect was an increasing function of dose; the 1.7 and 3.0 mg/kg doses often completely, or nearly completely, eliminated preference. This convergence can be described as a reduction in sensitivity to reinforcement delay, or immediacy (see Figures 5 and 6). The functions for Pigeons 107 and 228 were atypical in that the largest effect occurred at 0.3 mg/kg.
Fig 3.
Log initial-link response ratios (standard/variable) as a function of d-amphetamine dose for each pigeon, shown separately for sessions in which the variable terminal-link schedule was 16 s (circles) and 4 s (triangles). For Pigeons 105 and 107, the standard-delay schedule (FI 8 s) occurred on the right key and the variable-delay schedule (FI 4 s or FI 16 s) occurred on the left key; for the remaining pigeons, this was reversed. Points above C show data from control sessions and points above S show data from sessions preceded by saline administration. Data points show means and error bars show ranges. Note that the y-axis ranges for Pigeons 226 and 228 are greater than those for the other pigeons. The 3.0 mg/kg dose completely eliminated responding in Pigeons 106 and 228 when the terminal links were FI 8 s/FI 16 s. The 3.0 mg/kg dose inadvertently was not tested in Pigeon 107 under the FI 8 s/FI 16 s condition.
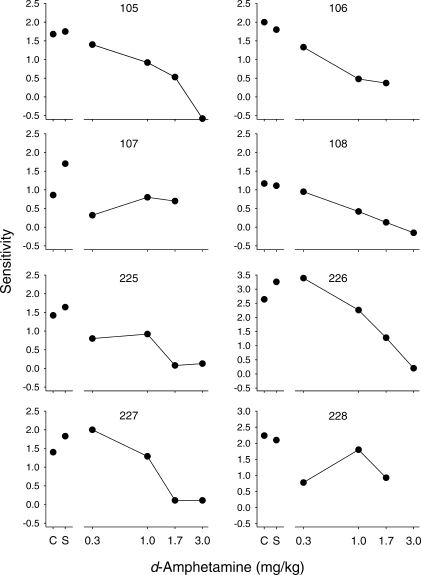
Fig 5.
Dose-effect functions for d-amphetamine on sensitivity to reinforcement immediacy for each pigeon. See text for description of the method used to estimate sensitivity. Note that the y-axis range for Pigeons 226 and 228 is greater than that for the other pigeons.
Fig 6.
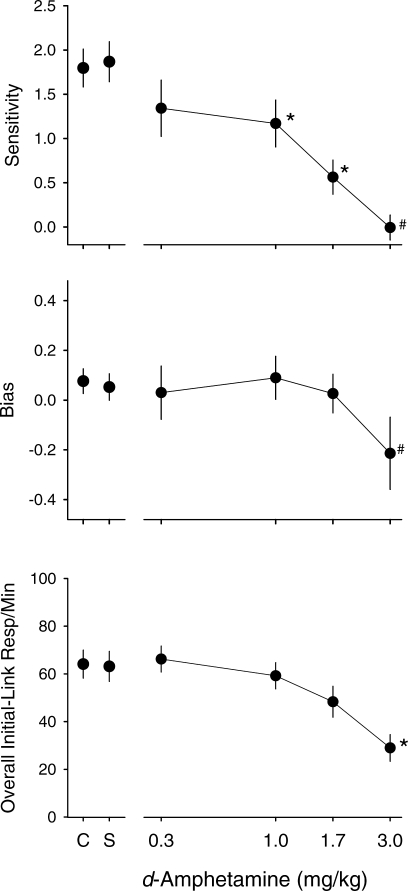
Group dose-effect functions for sensitivity (upper panel), bias (middle panel), and overall initial-link response rates (lower panel). Data points are means from all pigeons (except at 3.0 mg/kg for sensitivity and bias, where the data points are means of 6 pigeons); error bars show SE. For each pigeon, overall initial-link response rate was obtained by averaging the rates across the different terminal-link pairs. Asterisks show values significantly different from those under saline; for sensitivity and bias, data for 3.0 mg/kg were not included in the statistical analysis (indicated by #).
Table 2 shows the change produced by each dose, expressed as a proportion of the log ratio obtained under saline sessions, in individual pigeons across the two values of the variable-delay schedule (see Data Analysis for a description of the calculations used to obtain these values). Positive values indicate an increase in preference for the more immediate reinforcer and negative values indicate a decrease in preference for the more immediate reinforcer; values close to −1.0 indicate a near complete elimination of preference and negative values more extreme than −1.0 indicate a switch in preference. A couple of features of these data are worth noting. First, the vast majority of the values are negative, which confirms the drug-induced reduction in preference illustrated in Figure 3. Second, in some cases, the change in the log ratio produced by a dose differed depending on whether the variable delay was FI 16 s or FI 4 s. The particular schedule associated with the larger effect, however, was not consistent across instances. For example, for Pigeon 105 at all doses (except 3.0 mg/kg), the reduction in preference was proportionally larger when the variable-delay schedule was FI 4 s than when it was FI 16 s, whereas for Pigeon 106 at the same doses, the opposite was the case. For Pigeon 225, the proportional reduction in preference was larger when the variable-delay schedule was FI 4 s with 0.3 and 1.0 mg/kg, but was larger when the variable-delay schedule was FI 16 s with 1.7 and 3.0 mg/kg. Analyses of the group data failed to yield significant differences across schedules at any of the doses (see Table 2).
Table 2.
The change in log ratio (standard/variable) produced by each dose, expressed as a proportion of the log ratio during saline sessions, for sessions in which the variable-delay schedule was FI 16 s and sessions in which the variable-delay schedule was FI 4 s.
| Pigeon | 0.3 mg/kg |
1.0 mg/kg |
1.7 mg/kg |
3.0 mg/kg |
||||
| FI 16 s | FI 4 s | FI 16 s | FI 4 s | FI 16 s | FI 4 s | FI 16 s | FI 4 s | |
| 105 | 0.19 | −0.86 | −0.25 | −0.85 | −0.34 | −1.31 | −1.04 | −1.00 |
| 106 | −0.52 | −0.01 | −1.49 | 0.08 | −1.06 | −0.53 | — | −0.43 |
| 107 | −0.71 | −1.16 | −0.47 | −0.71 | −0.46 | −0.02 | — | −0.90 |
| 108 | −0.07 | −0.42 | −0.58 | −0.77 | −0.64 | −1.55 | −0.96 | −1.62 |
| 225 | −0.11 | −0.81 | −0.03 | −0.47 | −1.66 | −0.51 | −2.11 | −0.25 |
| 226 | 0.05 | −0.01 | 0.23 | −0.72 | −0.50 | −0.72 | −0.74 | −1.10 |
| 227 | 0.28 | −0.39 | −0.44 | −0.23 | −1.19 | −0.43 | −1.88 | 1.11 |
| 228 | −1.65 | 0.12 | 1.17 | −0.60 | 0.11 | −0.66 | — | −0.65 |
| Mean | −0.32 | −0.44 | −0.23 | −0.53 | −0.72 | −0.72 | −1.35 | −0.61 |
| SD | 0.64 | 0.47 | 0.76 | 0.32 | 0.56 | 0.49 | 0.61 | 0.81 |
| t-test | t(7) = .39, p = .71 | t(7) = .89, p = .40 | t(7) = .00, p = 1.0 | |||||
Note. See Data Analysis for an explanation of the method used to calculate these values. Results of t-tests comparing values across the two schedules are presented in the bottom row (no test was conducted for the data at 3.0 mg/kg because of missing values for 3 of the pigeons).
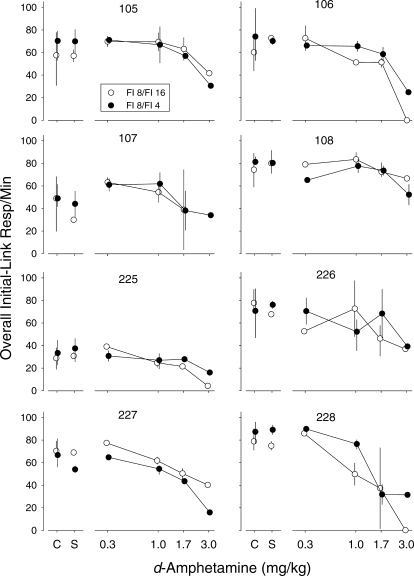
In several instances, d-amphetamine affected the distribution of responses across the two options in the initial link without substantially affecting overall response rate. Figure 4 shows dose-effect functions for overall initial-link response rates. For most of the pigeons, except 107 and 228, doses up to and including 1.7 mg/kg produced relatively little effect on overall response rates. For Pigeons 107 and 228, effects of 1.7 mg/kg were variable, particularly when the terminal links were FI 8 and 16 s; sometimes this dose did not affect overall response rate and sometimes it substantially reduced it. In some of the pigeons (e.g., 106, 225, 227, and 228), 3.0 mg/kg decreased initial-link response rates associated with one of the terminal-link pairs more than the other, although the particular pair varied across pigeons. For Pigeon 108, 5.6 mg/kg completely eliminated responding (data not shown).
Fig 4.
Dose-effect functions for response rates during the initial links. Data were obtained by adding responses on the left and right keys and dividing by the time spent in the initial link. Open symbols show rates from sessions in which the terminal links were FI 8 s and FI 16 s and filled symbols show rates from sessions in which the terminal links were FI 8 s and FI 4 s. Other characteristics are as described in Figure 3.
The drug-induced reductions in preference for the shorter delay shown in Figure 3 typically occurred without consistent changes in terminal-link response rates or without changes in the obtained delays (i.e., the durations of the terminal links). Obtained delays usually decreased only when response rates in the terminal links were substantially reduced by the higher doses (terminal link response rates and obtained delays are presented in the Appendix).
Figure 5 shows d-amphetamine dose-effect functions for estimates of sensitivity to reinforcement immediacy. For most of the pigeons, sensitivity decreased as a function of d-amphetamine dose. For Pigeons 107 and 228, at least one dose decreased sensitivity, but the effects were not monotonically related to dose. Figure 6 shows dose-effect curves for estimates of sensitivity (upper panel) and bias (middle panel) averaged across all pigeons; individual-subject data for bias are shown in Table 1 (a positive value indicates a bias for the standard alternative and a negative value indicates a bias for the variable alternative). Again, these data illustrate the decrease in sensitivity to immediacy as a function of dose. Sensitivity decreased from an average of 1.67 under control conditions to an average of 1.12 and 0.52 at the 1.0 and 1.7 mg/kg doses, respectively. One-way ANOVAs yielded a significant effect of dose on sensitivity [F (3,21) = 15.27, p < .01], but not on bias [F (3,21) = 0.13, p = .93)]; note that the data for the 3.0 mg/kg dose was not included in these analyses because this dose substantially reduced response rates and, thus, estimates could not be obtained for all of the pigeons. Post hoc analyses for sensitivity indicated that the data for both 1.0 and 1.7 mg/kg were significantly different from those for saline (ps < .01). For comparison, a group average dose-effect function for overall initial-link response rate is presented in the bottom panel (note that because there was no consistent difference in overall initial-link response rates across the different pairs of terminal links, these rates were averaged for each pigeon to yield a single number). The ANOVA revealed a significant effect of d-amphetamine on rate [F (4,28) = 17.33, p < .01]; only the data at 3.0 mg/kg were significantly different from saline (p < .01).
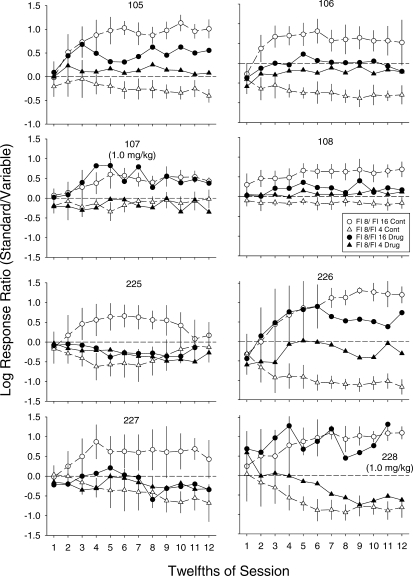
Figures 3–6 illustrate effects of d-amphetamine on aggregate session measures. It is possible, however, that d-amphetamine differentially affected choice at different points within the session. Figure 7 shows effects of 1.0 mg/kg (107 and 228) or 1.7 mg/kg (all other pigeons) on log response ratios across session 12ths. For each pigeon, the dose selected was the highest one administered that did not substantially affect overall response rate. In these graphs, log response ratios (standard/variable) during control sessions are shown by the open symbols and those following administration of the selected dose are shown by the filled symbols. Data for sessions in which the variable delay was FI 16 s are shown by circles, and data for sessions in which the variable delay was FI 4 s are shown by triangles. These plots show the attenuation of preference following administration of the selected dose of d-amphetamine for the majority of the pigeons. Under control conditions, responding was relatively indifferent at the beginning of the session, followed by a transition across the session such that an asymptotic preference was reached by about midway through. As with the data shown in Figures 1 and 3, these functions for control conditions illustrate the stronger preference for the shorter delay when the terminal links were FI 8 and FI 16 s than when they were FI 8 and FI 4 s for some of the pigeons (e.g., 105, 107, 108, and 227). For all pigeons except 107 the selected dose shifted preference toward indifference. For some pigeons (e.g., 105, 106, and 108), the selected dose attenuated preference without substantially affecting bias (i.e., the midline between the two functions did not appear to change). For some pigeons (e.g., 225 and 227), the attenuation of preference was accompanied by a shift in bias (i.e., an apparent change in the midline between the functions).
Fig 7.
Log initial-link response ratios (standard/variable) across successive 12ths of the session for individual pigeons, shown separately for sessions in which the variable terminal-link schedule was 16 s (circles) and 4 s (triangles). Data from control sessions are shown by open symbols, and data from sessions following administration of 1.7 mg/kg (or 1.0 mg/kg for Pigeons 107 and 228) d-amphetamine are shown by filled symbols. Data points are means; error bars around the points for control show ± 1 SD. Missing points for Pigeons 225 and 228 at 12 on the x axis are from conditions in which these birds did not finish one or more of the sessions at this dose.
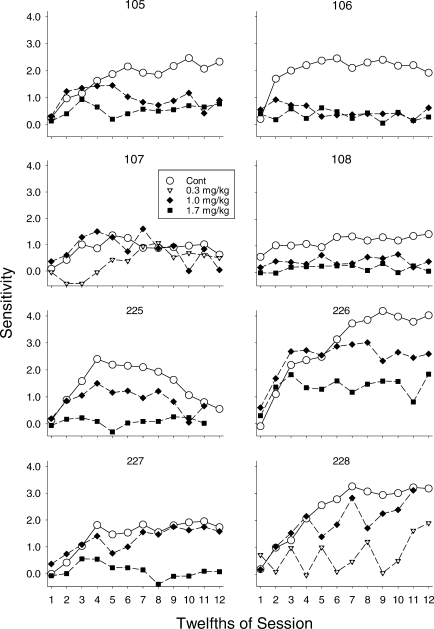
Figure 8 shows estimates of sensitivity for each pigeon across session 12ths under control conditions (open symbols) and following administration of two d-amphetamine doses (0.3 and 1.0 mg/kg for Pigeons 107 and 228; 1.0 and 1.7 mg/kg for the rest of the pigeons). For all pigeons, the functions during control conditions increased early in the session, then reached a maximum. The functions during control conditions typically reached their asymptote within the first seven blocks, and, except for Pigeon 225, remained at asymptote for the remainder of session; the function for Pigeon 225 decreased during the second half of the session. For all of the pigeons, at least one dose of d-amphetamine altered the function. A common effect was a decrease in the asymptotic level of sensitivity reached within the session, but no change in the rate of acquisition to that asymptote. The function for Pigeon 107 at 0.3 mg/kg was somewhat unique in that acquisition of preference was delayed, after which the function increased to an asymptote that was slightly lower than that obtained under control. Finally, in several cases, particularly at 1.7 mg/kg, evidence of control by reinforcement delay was almost completely eliminated (i.e., the function essentially was flat).
Fig 8.
Estimates of sensitivity across successive 12ths of the session for individual pigeons. The open circles show data from control sessions and the filled diamonds and squares show data from sessions preceded by 1.0 and 1.7 mg/kg d-amphetamine, respectively; for Pigeons 107 and 228, 1.7 mg/kg substantially decreased response rates, so for these pigeons effects of 0.3 mg/kg are shown by the inverted triangles with a white dot in the center.
Discussion
Performance Under The Rapid-acquisition Procedure
All pigeons showed rapid acquisition of preference controlled by reinforcement delay. Typically, responding was relatively indifferent early in the session, followed by a transition across the session such that an asymptotic preference level was reached by about midway through. These data closely resemble those reported by Grace et al. (2003) and Grace and McLean (2006). Indeed, estimates of sensitivity to delay (lag 0) were quite similar across these three studies. The present results, then, provide additional support for the use of this type of procedure as a rapid and efficient method for estimating sensitivity to effects of reinforcement variables (also see Hunter & Davison, 1985; Maguire et al., 2007; Schofield & Davison, 1997).
For some of the pigeons in the present study, preference for the more immediate reinforcer was more extreme when the terminal links were FI 8 s versus FI 16 s than when they were FI 8 s versus FI 4 s; Grace et al. (2003) reported similar effects in several of their subjects. This appears to be an example of the “terminal-link effect,” in which preference under a constant ratio of terminal-link delays increases as a function of the absolute duration of the terminal links. The terminal-link effect is accommodated by several models of choice under concurrent-chains schedules (e.g., Fantino, 1977; Grace, 1994; Mazur, 2001).
Effects of D-amphetamine
d-Amphetamine decreased preference for the more immediate reinforcer. That is, d-amphetamine shifted response allocation toward indifference and, thus, decreased estimates of the sensitivity to reinforcement delay. In all pigeons, at least one dose reduced sensitivity to delay without substantially changing overall response rates and without changing obtained delays. Thus, it is unlikely that d-amphetamine's effects on response allocation in the initial-link were an indirect result of drug-induced changes in either of these two variables. Furthermore, d-amphetamine reduced sensitivity to delay without systematically changing bias. Although in some instances, the magnitude of the attenuation in preference depended upon whether the variable-delay schedule was FI 4 s or FI 16 s, the particular schedule associated with the larger effect was not consistent across pigeons or doses.
One effect of d-amphetamine in the present experiment was a reduction in asymptotic levels of preference reached within the session. In many instances, acquisition early in the session following d-amphetamine administration was similar to that obtained under control. In a few instances, acquisition appeared to be slightly faster, and in one instance it was attenuated, following d-amphetamine administration. These changes, however, were almost always accompanied by a decrease in the asymptotic preference levels reached within the session. At 1.7 mg/kg, the acquisition function often was completely, or nearly completely, flat; that is, control by reinforcement delay essentially was eliminated.
The d-amphetamine-induced reduction in asymptotic level of preference achieved within the session suggests that responding was no longer as sensitive to reinforcement delay as under control conditions. The present data, then, are consistent with the notion that a reduction in the sensitivity to reinforcement delay is an important behavioral mechanism of the effects of psychomotor stimulants. Several lines of evidence indicate that this effect occurs with considerable generality. As noted in the introduction, these drugs usually increase choices of a larger, more delayed reinforcer under self-control procedures (Pietras et al., 2003; Pitts & Febbo, 2004; Pitts & McKinney, 2005; Richards et al., 1999; Wade et al., 2000), and quantitative analyses of choice are consistent with this view (Pitts & Febbo, 2004). Interestingly, quantitative analyses also indicate that lesions of the orbital prefrontal cortex can increase estimates of sensitivity to reinforcement delay (e.g., Mobini et al., 2002; Kheramin et al., 2003). This finding supports the above interpretation in that lesions of this brain region reduce levels of dopamine, a neurochemical effect opposite that of administering d-amphetamine. Finally, the finding that cocaine can increase response rates maintained by delayed reinforcement under single schedules (Walker & Branch, 1996) also is consistent with the notion that stimulants attenuate effects of reinforcement delay.
The present data are interesting in light of those previously reported by LeSage et al. (1996). Although there was considerable variability across subjects, LeSage et al. reported that a low dose of d-amphetamine (1.0 mg/kg) slightly enhanced rates of acquisition of lever pressing with delayed reinforcement in a subset of their subjects. It should be noted that this dose also increased rates of pressing an inactive lever, suggesting that the drug effect was not selective to acquisition. Nevertheless, the effects in both the present study and the LeSage et al. study are consistent with the notion that d-amphetamine reduces the sensitivity of behavior to the effects of reinforcement delay. Such a mechanism would be expected to shift preference toward indifference when choice is controlled by delay, but would be expected to attenuate the detrimental effects of delay on the acquisition of a single response.
Although a drug-induced attenuation of sensitivity to delay is viable account of the present data, as well as those from a number of previous studies, other variables and/or behavioral mechanisms certainly may have been involved. In the present study, the dose-dependent decrease in sensitivity to delay produced by d-amphetamine was quite reliable. Nevertheless, for Pigeons 107 and 228, although at least one dose of d-amphetamine decreased sensitivity to delay, these effects were not monotonically related to dose. Indeed, for these 2 pigeons, the largest drug effect on sensitivity occurred at the lowest dose, and higher doses often decreased response rates substantially. Response rates for these 2 pigeons were most susceptible to the response-rate decreasing effects of d-amphetamine, which may have interacted with effects on sensitivity to delay. In addition, initial sensitivity to delay also may influence d-amphetamine's effects; sensitivity to delay for Pigeon 107 under control conditions tended to be lower than for the other pigeons.
It also is possible that the present results relate to a drug-induced alteration of temporal stimulus control. It is not unreasonable to postulate that acquisition of preference within any given session in the present study involved acquisition of stimulus control by the delay durations in effect for that session (see Grace et al., 2003 and Grace & McLean, 2006 for a discrimination-based account of performance under this procedure). Thus, any manipulation that affected stimulus control by duration might be expected to produce a corresponding change in preference. Following administration of certain doses of psychomotor stimulants, subjects often respond earlier than usual under procedures typically used to assess temporal discrimination (e.g., Eckerman, Segbefia, Manning, & Breese, 1987; Maricq, Roberts, & Church, 1981; Meck, 1983). One interpretation of this effect is that dopamine activation (e.g., via stimulant administration) produces an overestimation of the passage of time; subjects respond as if more time has elapsed than actually has (e.g., Meck, 1996). Moreover, Maricq et al. found that certain doses of methamphetamine produced a constant proportional change in time perception across all tested durations. A straightforward application of this account to the present results, however, predicts no change in the distribution of responses in the initial links following administration of d-amphetamine (i.e., perception of the durations of the terminal links should have changed by the same proportion). Interestingly, however, a number of investigators have reported that psychomotor stimulants can flatten temporal psychophysical functions (e.g., Chiang et al., 2000; McLure, Saulsgiver, & Wynne, 2005; Odum, Lieving, & Schaal, 2002; Santi, Weise, & Kuiper, 1995; Stubbs & Thomas, 1974). Whether or not psychomotor stimulants shift temporal psychophysical functions to the left, or simply flatten them, may depend upon the specific procedure used. For example, Chiang et al. (2000) found that d-amphetamine both flattened the function and shifted it to the left under a free-operant timing task in which responding during an ongoing temporal interval was controlled by the passage of time. In contrast, d-amphetamine only flattened the function under an interval-bisection procedure in which responding was under stimulus control of a previously experienced stimulus duration. The attenuation of preference controlled by reinforcement delay in the present study is consistent with a flattening of the psychophysical timing function. Such an effect makes sense if choice in the present study was under stimulus control of the delay durations previously experienced within a given session. Indeed, the present procedure may share features in common with the interval-bisection task used by Chiang et al. (i.e., each can be viewed as a “retrospective”-timing task; see Killeen & Fetterman, 1988).
Interpretations of the present results in terms of behavioral mechanisms such as a reduction in the sensitivity to delay and/or an alteration of stimulus control are complicated by several factors. For instance, only two delay ratios were examined in the present study; a more complete characterization of the effects of d-amphetamine on sensitivity to delay will require investigation across a wide range of delay ratios. Furthermore, it has been difficult to distinguish effects of psychomotor stimulants on stimulus control from other effects on response rates (e.g., Katz, 1982, 1983, 1988). Under a variety of conditions, intermediate doses of psychomotor stimulants tend to produce “rate-dependent” effects, which often are characterized by an increase in relatively low response rates and a decrease in (or no effect upon) relatively high response rates (see Dews & Wenger, 1977; Sanger & Blackman, 1976). Some recent investigations have indicated that effects of these drugs under a number of temporal-discrimination procedures can be described quite well by rate-dependent analyses (e.g., Odum et al., 2002; Saulsgiver, McLure, & Wynne, 2006). Indeed, it could be argued that the effects of d-amphetamine in the current study were simply rate-dependent (or, given that the rates on the different keys during the initial links tended to converge after drug administration, perhaps the effect is better described as “rate-constant,” see Gonzalez & Byrd, 1977). Thus, it is possible that the attenuation of preference produced by the intermediate doses of d-amphetamine in the current study was an artifact of an increase in the relatively low response rates on the nonpreferred option and a decrease in the relatively high response rates on the preferred option. Although a rate-dependent account of the current data cannot be ruled out, we find an account based upon behavioral mechanisms (e.g., a reduction in sensitivity to delay) more convincing. As noted in the introduction, data from a number of studies indicate that psychomotor stimulants increase choices of larger, more delayed reinforcers. In several of these studies, a drug-induced shift in the distribution of responses across options was obtained from a baseline of indifference (i.e., when response probabilities for the different options were equal) (e.g., Pitts & Febbo, 2004; Richards et al., 1999; Wade et al., 2000). Such effects are difficult to interpret as rate-dependent, but are consistent with an account in terms of a reduction in the sensitivity to reinforcement delay. Furthermore, although rate dependency provides an effective empirical description of drug effects on operant behavior under some conditions (e.g., Dews & Wenger, 1977), its status as an explanatory concept has been questioned (see Branch, 1984; Odum et al., 2002). Indeed, it is quite possible that what could be described as rate-dependent effects in the present study actually were an artifact of a specific behavioral mechanism of drug action (e.g., a reduction in sensitivity to delay), rather than the other way around.
In summary, d-amphetamine attenuated rapid acquisition of preference in concurrent chains. Although there are a number of potential interpretations, these data are consistent with the notion that a reduction in the sensitivity to reinforcement delay is an important behavioral effect of psychomotor stimulants. The present findings add to a growing body of literature indicating that choice procedures, and their associated quantitative analyses, provide relatively effective methods for identifying behavioral mechanisms of drug action.
Acknowledgments
This paper is dedicated to the memory of Professor Larry Kokkinidis of the University of Canterbury—he is sorely missed. This work would not have been possible without his generous donation of d-amphetamine. This work also was made possible by a Research Reassignment, a Charles L. Cahill Grant, and a Summer Research Initiative awarded to R. C. Pitts by the University of North Carolina Wilmington. The authors thank Mark Berg, Darren Christensen, and Karla Mattson for their help in running the experiment.
Appendix
Mean response rates (Responses/minute) in the terminal links and obtained delays (s) from the initial link to the terminal link when the terminal links were either FI 4 s and FI 8 s or FI 8 s and FI 16 s from each condition. Ranges are in parentheses. Dashes indicate instances in which there were no obtained-delay data because responding was completely suppressed.
| Subject Condition | Terminal-link rates |
Mean obtained delays |
||||||
| 4 | 8 | 16 | 8 | 4 | 8 | 16 | 8 | |
| 105 | ||||||||
| Control | 154.55 (128.39–175.97) | 73.39 (62.04–97.46) | 60.96 (43.27–73.41) | 74.03 (20.01–110.50) | 4.14 (4.11–4.18) | 8.17 (8.13–8.26) | 16.25 (16.17–16.49) | 8.32 (8.14–9.11) |
| Saline | 155.65 (141.43–169.86) | 72.57 (63.54–81.60) | 65.95 (59.51–72.40) | 73.78 (62.70–84.86) | 4.16 (4.16–4.17) | 8.20 (8.19–8.21) | 16.20 (16.16–16.23) | 8.20 (8.17–8.24) |
| 0.3 mg/kg | 148.07 (145.17–150.97) | 65.56 (65.38–65.73) | 74.66 (74.41–74.92) | 67.12 (59.57–74.78) | 4.13 (4.11–4.14) | 8.25 (8.19–8.31) | 16.20 (16.15–16.24) | 8.21 (8.18–8.24) |
| 1.0 mg/kg | 136.72 (112.04–161.39) | 65.68 (33.33–98.03) | 56.84 (54.64–59.03) | 51.39 (39.53–63.25) | 4.15 (4.11–4.18) | 8.28 (8.11–8.45) | 16.27 (16.15–16.38) | 8.24 (8.18–8.30) |
| 1.7 mg/kg | 115.55 (103.17–127.93) | 40.50 (40.04–40.96) | 61.51 (46.14–76.89) | 40.20 (25.18–55.22) | 4.17 (4.13–4.20) | 8.21 (8.20–8.22) | 16.50 (16.30–16.70) | 8.26 (8.18–8.34) |
| 3.0 mg/kg | 8.37 | 32.08 | 15.31 | 2.79 | 4.19 | 8.59 | 154.26 | 56.97 |
| 106 | ||||||||
| Control | 172.42 (159.77–184.96) | 68.04 (55.42–91.84) | 78.51 (67.97–93.13) | 68.46 (51.72–89.81) | 4.11 (4.09–4.14) | 8.17 (8.11–8.23) | 16.178 (16.14–16.29) | 8.16 (8.11–8.25) |
| Saline | 159.62 (158.19–161.04) | 75.56 (74.23–76.89) | 66.28 | 66.55 | 4.14 (4.13–4.15) | 8.29 (8.15–8.42) | 16.22 | 8.34 |
| 0.3 mg/kg | 172.14 (167.88–176.4) | 60.59 (51.76–69.43) | 77.81 (59.00–96.61) | 73.22 (70.23–76.20) | 4.11 (4.11) | 8.28 (8.21–8.34) | 16.2 (16.13–16.27) | 8.16 (8.14–8.18) |
| 1.0 mg/kg | 166.26 (153.59–178.92) | 62.09 (40.75–83.44) | 68.33 (31.00–140.59) | 64.22 (36.59–110.5) | 4.08 (4.08) | 8.14 (8.09–8.18) | 16.33 (16.17–16.42) | 8.23 (8.16–8.29) |
| 1.7 mg/kg | 156.86 (122.14–191.58) | 43.06 (20.11–66.01) | 75.68 (41.00–128.28) | 47.30 (35.71–62.04) | 4.08 (4.08) | 8.38 (8.13–8.62) | 16.35 (16.15–16.48) | 8.37 (8.14–8.57) |
| 3.0 mg/kg | 131.15 | 28.31 | 0 | 0 | 4.13 | 9.36 | — | — |
| 107 | ||||||||
| Control | 316.91 (288.40–364.94) | 108.46 (94.06–138.92) | 85.47 (62.25–109.98) | 120.59 (99.29–152.28) | 4.07 (4.06–4.09) | 8.08 (8.05–8.11) | 16.16 (16.10–16.26) | 8.08 (8.05–8.14) |
| Saline | 306.64 (258.76–354.52) | 96.85 (82.32–111.39) | 80.74 | 168.30 | 4.09 (4.09) | 8.14 (8.08–8.20) | 16.18 | 8.07 |
| 0.3 mg/kg | 356.56 | 96.30 | 89.67 (79.69–110.66) | 96.76 (79.53–120.91) | 4.09 | 8.10 | 16.52 (16.13–17.21) | 8.09 (8.05–8.11) |
| 1.0 mg/kg | 301.08 (298.03–304.14) | 110.3 (96.42–124.17) | 89.89 (72.75–107.03) | 117.22 (112.69–121.76) | 4.09 (4.06–4.11) | 8.09 (8.08–8.09) | 16.27 (16.22–16.32) | 8.09 (8.09) |
| 1.7 mg/kg | 225.82 (206.50–245.15) | 79.66 (78.76–80.57) | 73.58 (61.82–85.34) | 132.49 (94.68–170.29) | 4.13 (4.12–4.14) | 8.16 (8.13–8.19) | 16.36 (16.21–16.50) | 8.22 (8.15–8.28) |
| 3.0 mg/kg | 256.63 | 59.71 | 4.15 | 8.43 | ||||
| 108 | ||||||||
| Control | 90.26 (74.98–105.92) | 62.72 (53.31–70.72) | 52.02 (40.00–69.05) | 68.21 (49.14–93.69) | 4.35 (4.27–4.49) | 8.50 (8.29–8.74) | 16.35 (16.22–16.54) | 8.47 (8.29–8.74) |
| Saline | 90.97 (86.53–95.4) | 58.31 (44.42–72.20) | 51.45 (49.01–55.32) | 73.68 (67.00–64–83.03) | 4.39 (4.35–4.43) | 8.4 (8.31–8.48) | 16.3 (16.24–16.39) | 8.44 (8.35–8.60) |
| 0.3 mg/kg | 65.15 | 39.14 | 57.97 | 68.50 | 4.40 | 8.56 | 16.33 | 8.71 |
| 1.0 mg/kg | 83.07 (69.28–96.86) | 46.93 (39.50–54.35) | 61.1 (55.79–66.42) | 51.72 (50.92–52.52) | 4.29 (4.25–4.33) | 8.48 (8.31–8.65) | 16.37 (16.21–16.52) | 8.40 (8.38–8.41) |
| 1.7 mg/kg | 72.46 (69.80–75.12) | 35.84 (34.00–37.68) | 57.30 (50.50–64.18) | 52.37 (34.84–81.54) | 4.26 (4.25–4.26) | 8.55 (8.43–8.67) | 16.32 (16.24–16.36) | 8.41 (8.38–8.43) |
| 3.0 mg/kg | 46.51 (12.40–80.61) | 15.59 (12.16–19.01) | 65.54 | 41.57 | 5.90 (4.30–7.52) | 10.65 (9.38–11.92) | 16.25 | 8.42 |
| 225 | ||||||||
| Control | 62.71 (27.05–120.74) | 35.57 (17.74–66.10) | 39.18 (33.50–41.24) | 50.24 (41.68–56.68) | 4.8 (4.21–5.73) | 8.88 (8.30–9.58) | 16.25 (16.17–16.49) | 8.32 (8.14–9.11) |
| Saline | 67.85 (35.76–104.73) | 38.45 (11.26–52.39) | 33.46 (28.93–37.89) | 61.95 (46.28–75.62) | 4.90 (4.44–5.36) | 9.05 (8.24–10.07) | 16.62 (16.23–16.89) | 8.46 (8.19–8.75) |
| 0.3 mg/kg | 60.96 (47.29–74.63) | 35.62 (32.85–38.39) | 45.23 | 51.09 | 4.90 (4.69–5.11) | 9.00 (8.98–9.03) | 16.43 | 8.51 |
| 1.0 mg/kg | 90.09 (47.02–112.94) | 34.51 (29.19–43.34) | 26.52 (23.31–29.73) | 39.91 (30.10–49.73) | 4.27* (4.25–4.36) | 8.72* (8.62–9.08) | 18.25* (17.63–18.86) | 8.40* (8.30–8.49) |
| 1.7 mg/kg | 62.80 (26.96–98.65) | 20.53 (12.72–28.34) | 21.58 (14.13–28.67) | 19.58 (6.74–30.25) | 4.29* (4.19–4.39) | 9.96* (9.82–10.09) | 18.06* (17.09–19.20) | 9.47* (8.81–10.62) |
| 3.0 mg/kg | 55.31 | 18.11 | 44.70 | 9.34 | 4.52* | 9.42* | 18.69* | 9.64* |
| 226 | ||||||||
| Control | 117.62 (83.30–133.41) | 101.75 (82.57–122.70) | 84.54 (77.44–98.02) | 111.46 (101.51–115.89) | 4.40* (4.16–4.69) | 8.31* (8.11–8.72) | 16.24 (16.15–16.31) | 8.15 (8.09–8.29) |
| Saline | 124.72 (121.11–128.33) | 110.54 (106.08–115.01) | 82.29 (78.72–85.86) | 106.06 (98.46–113.66) | 4.26 (4.13–4.39) | 8.15 (8.13–8.17) | 16.26* (16.07–16.45) | 8.13* (8.13–8.14) |
| 0.3 mg/kg | 111.88 (95.39–128.38) | 109.71 (107.04–112.38) | 82.58 | 98.91 | 4.58 (4.31–4.84) | 8.27 (8.19–8.35) | 16.23* | 8.13* |
| 1.0 mg/kg | 122.08 (119.15–127.73) | 110.99 (99.10–117.60) | 79.34 (72.92–85.75) | 101.91 (100.83–102.99) | 4.38 (4.16–4.70) | 8.18 (8.13–8.22) | 16.28 (16.22–16.34) | 8.24 (8.12–8.37) |
| 1.7 mg/kg | 99.29 (69.59–128.98) | 63.99 (23.01–104.96) | 89.90 (67.84–110.11) | 109.95 (106.08–114.10) | 4.48 (4.29–4.67) | 8.85 (8.13–9.56) | 16.52* (16.15–17.19) | 8.19* (8.14–8.23) |
| 3.0 mg/kg | 127.90 | 90.04 | 99.15 | 114.72 | 4.17 | 8.20 | 16.46 | 8.21 |
| 227 | ||||||||
| Control | 122.59 (97.88–156.60) | 87.49 (64.30–108.24) | 79.93 (64.35–92.12) | 103.00 (90.59–111.59) | 4.23 (4.14–4.31) | 8.56 (8.13–10.42) | 16.27 (16.21–16.37) | 8.13 (8.08–8.18) |
| Saline | 99.25 (96.24–102.26) | 103.22 (99.35–107.08) | 75.68 (71.84–79.52) | 107.16 (99.14–115.18) | 4.27 (4.26–4.27) | 8.16 (8.14–8.17) | 16.32 (16.24–16.39) | 8.18 (8.17–8.19) |
| 0.3 mg/kg | 99.54 (95.35–103.74) | 73.33 (69.02–77.64) | 72.47 | 109.47 | 4.37 (4.37) | 8.15 (8.09–8.20) | 16.26 | 8.13 |
| 1.0 mg/kg | 127.39 (118.54–119.50) | 70.73 (62.12–71.63) | 54.30 (53.69–54.91) | 65.20 (60.77–69.63) | 4.17 (4.14–4.19) | 8.19 (8.18–8.21) | 16.38 (16.36–16.39) | 8.32 (8.31–8.33) |
| 1.7 mg/kg | 113.14 (106.10–120.19) | 41.00 (19.33–62.68) | 58.18 (29.30–84.84) | 59.48 (43.50–78.13) | 4.19 (4.16–4.21) | 11.30 (8.19–14.40) | 16.82 (16.36–17.52) | 8.33 (8.17–8.43) |
| 3.0 mg/kg | 7.10 | 0.12 | 5.50 | 4.98 | 5.29* | 149.72* | 22.15* | 11.36* |
| 228 | ||||||||
| Control | 116.93 (83.14–132.94) | 143.08 (99.51–178.15) | 66.96 (54.26–79.70) | 149.30 (123.62–181.05) | 4.21 (4.12–4.33) | 8.13 (8.10–8.22) | 16.33 (16.27–16.38) | 8.12 (8.10–8.17) |
| Saline | 133.02 (131.98–134.06) | 181.77 (181.03–182.51) | 79.90 (77.84–81.95) | 163.65 (147.10–180.20) | 4.16 (4.14–4.18) | 8.11 (8.10–8.12) | 16.35 (16.31–16.38) | 8.11 (8.09–8.13) |
| 0.3 mg/kg | 113.78 (98.20–129.37) | 136.25 (108.92–163.58) | 91.21 | 166.46 | 4.23 (4.20–4.26) | 8.11 (8.10–8.11) | 16.30 | 8.09 |
| 1.0 mg/kg | 127.20 (118.07–132.21) | 124.04 (102.50–166.67) | 61.13 (53.07–69.18) | 131.15 (120.91–141.38) | 4.15 (4.15–4.16) | 8.12 (8.11–8.13) | 16.34* (16.28–16.40) | 8.12* (8.12) |
| 1.7 mg/kg | 94.56 (90.80–98.33) | 15.98 (15.62–16.34) | 29.98 (0.00–89.92) | 50.75 (0.00–152.26) | 4.22* (4.15–4.29) | 16.94* (10.83–23.10) | 16.31 (16.31) | 8.10 (8.10) |
| 3.0 mg/kg | 9.78 | 2.61 | 0.00 | 0.00 | 4.49* | 35.01* | — | — |
* Due to a programming error, in a few of the sessions in which response rates were substantially suppressed (and obtained delays were substantially lengthened), it could not be determined conclusively to which alternative 1–2 of the obtained delays belonged. Therefore, under these conditions, we made our best guess regarding the assignment of these delays to a given alternative.
References
- Autor S.M. The strength of conditioned reinforcers as a function of frequency and probability of reinforcement. In: Hendry D.P, editor. Conditioned reinforcement. Homewood, IL: Dorsey Press; 1969. pp. 127–162. [Google Scholar]
- Branch M.N. Rate dependency, behavioral mechanisms and behavioral pharmacology. Journal of the Experimental Analysis of Behavior. 1984;42:511–522. doi: 10.1901/jeab.1984.42-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier D, Thiebot M.H. Effects of psychotropic drugs on rats responding in an operant paradigm involving choice between delayed reinforcers. Pharmacology Biochemistry and Behavior. 1996;54:149–157. doi: 10.1016/0091-3057(95)02114-0. [DOI] [PubMed] [Google Scholar]
- Chiang T.-J, Al-Ruwaitea A.S.A, Mobini S, Ho M.-Y, Bradshaw C.M, Szabadi E. The effect of d-amphetamine on performance on two operant timing schedules. Psychopharmacology. 2000;150:170–184. doi: 10.1007/s002130000422. [DOI] [PubMed] [Google Scholar]
- Cohn J, Paule M.G. Repeated acquisition of response sequences: The analysis of behavior in transition. Neuroscience and Biobehavioral Reviews. 1995;19:397–406. doi: 10.1016/0149-7634(94)00067-b. [DOI] [PubMed] [Google Scholar]
- Davison M, McCarthy D. The matching law: A research review. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Dews P.B, Wenger G.R. Rate-dependency of the behavioral effects of amphetamine. In: Thompson T, Dews P.B, editors. Advances in behavioral pharmacology. Vol. 1. New York: Academic Press; 1977. pp. 167–227. [Google Scholar]
- Eckerman D.A, Segbefia D, Manning S, Breese G.S. Effects of methylphenidate and d-amphetamine on timing in the rat. Pharmacology, Biochemistry & Behavior. 1987;27:513–515. doi: 10.1016/0091-3057(87)90356-x. [DOI] [PubMed] [Google Scholar]
- Evenden J.L, Ryan C.N. The pharmacology of impulsive behavior in rats: The effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Fantino E. Conditioned reinforcement: Choice and information. In: Honig W.K, Staddon J.E.R, editors. Handbook of operant behavior. 1977. pp. 313–339. [Google Scholar]
- Fleshler M, Hoffman H.S. A progression for generating variable-interval schedules. Journal of the Experimental Analysis of Behavior. 1962;5:529–530. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F.A, Byrd L.D. Mathematics underlying the rate-dependency hypothesis. Science. 1977;195:546–550. doi: 10.1126/science.402028. [DOI] [PubMed] [Google Scholar]
- Grace R.C. A contextual model of concurrent-chains choice. Journal of the Experimental Analysis of Behavior. 1994;61:113–129. doi: 10.1901/jeab.1994.61-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace R.C, Bragason O, McLean A.P. Rapid acquisition of preference in concurrent chains. Journal of the Experimental Analysis of Behavior. 2003;80:235–251. doi: 10.1901/jeab.2003.80-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace R.C, McLean A.P. Rapid acquisition in concurrent chains: Evidence for a decision model. Journal of the Experimental Analysis of Behavior. 2006;85:181–202. doi: 10.1901/jeab.2006.72-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein R.J. Secondary reinforcement and rate of primary reinforcement. Journal of the Experimental Analysis of Behavior. 1964;7:27–36. doi: 10.1901/jeab.1964.7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter I, Davison M. Determination of a behavioral transfer function: White-noise analysis of session-to-session response ratio dynamics on concurrent VI VI schedules. Journal of the Experimental Analysis of Behavior. 1985;43:43–59. doi: 10.1901/jeab.1985.43-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J.L. Effects of drugs on stimulus control of behavior. I. Independent assessment of effects of response rates and stimulus control. Journal of Pharmacology and Experimental Therapeutics. 1982;223:617–623. [PubMed] [Google Scholar]
- Katz J.L. Effects of drugs on stimulus control of behavior. II. Degree of stimulus control as a determinant of effect. Journal of Pharmacology and Experimental Therapeutics. 1983;226:756–763. [PubMed] [Google Scholar]
- Katz J.L. Effects of drugs on stimulus control of behavior. III. Analysis of effects of pentobarbital and d-amphetamine. Journal of Pharmacology and Experimental Therapeutics. 1988;246:76–83. [PubMed] [Google Scholar]
- Kheramin S, Body S, Ho M.-Y, Velazquez-Martinez D.N, Bradshaw C.M, Szabadi E, Deakin J.F.W, Anderson I.M. Role of the orbital prefrontal cortex in choice between delayed and uncertain reinforcers: A quantitative analysis. Behavioural Processes. 2003;64:239–250. doi: 10.1016/s0376-6357(03)00142-6. [DOI] [PubMed] [Google Scholar]
- Killeen P, Fetterman J.G. A behavioral theory of timing. Psychological Review. 1988;95:274–295. doi: 10.1037/0033-295x.95.2.274. [DOI] [PubMed] [Google Scholar]
- LeSage M.G, Byrne T, Poling A. Effects of d-amphetamine on response acquisition with immediate and delayed reinforcement. Journal of the Experimental Analysis of Behavior. 1996;66:349–367. doi: 10.1901/jeab.1996.66-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue A.W, Rodriguez M.L, Pena-Correal T.E, Mauro B.C. Choice in a self-control paradigm: Quantification of experience-based differences. Journal of the Experimental Analysis of Behavior. 1984;41:53–67. doi: 10.1901/jeab.1984.41-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire D.R, Hughes C.E, Pitts R.C. Rapid acquisition of preference in concurrent schedules: Effects of reinforcement amount. Behavioural Processes. 2007;75:213–219. doi: 10.1016/j.beproc.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Maricq A.V, Roberts S, Church R.M. Methamphetamine and time estimation. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:18–30. doi: 10.1037//0097-7403.7.1.18. [DOI] [PubMed] [Google Scholar]
- Mazur J.E. Hyperbolic value addition and general models of animal choice. Psychological Review. 2001;108:96–112. doi: 10.1037/0033-295x.108.1.96. [DOI] [PubMed] [Google Scholar]
- McClure E.A, Saulsgiver K.A, Wynne C.D.L. Effects of d-amphetamine on temporal discrimination in pigeons. Behavioural Pharmacology. 2005;16:193–208. doi: 10.1097/01.fbp.0000171773.69292.bd. [DOI] [PubMed] [Google Scholar]
- Meck W.H. Selective adjustment of the speed of internal clock and memory processes. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:171–201. [PubMed] [Google Scholar]
- Meck W.H. Neuropharmacology of timing and time perception. Cognitive Brain Research. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho M.-Y, Bradshaw C.M, Szabadi E, Deakin J.F.W, Anderson I.M. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- Odum A.L, Lieving L.M, Schaal D.W. Effects of d-amphetamine in a temporal discrimination procedure: Selective changes in timing or rate dependency? Journal of the Experimental Analysis of Behavior. 2002;78:195–214. doi: 10.1901/jeab.2002.78-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras C.J, Cherek D.R, Lane S.D, Tcheremissine O.V, Steinberg J.L. Effects of methylphenidate on impulsive choice in adult humans. Psychopharmacology. 2003;170:390–398. doi: 10.1007/s00213-003-1547-2. [DOI] [PubMed] [Google Scholar]
- Pitts R.C, Febbo S.M. Quantitative analyses of methamphetamine's effects on self-control choices: Implications for elucidating behavioral mechanisms of drug action. Behavioural Processes. 2004;66:213–233. doi: 10.1016/j.beproc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Pitts R.C, McKinney A.P. Effects of methylphenidate and morphine on delay-discount functions obtained within sessions. Journal of the Experimental Analysis of Behavior. 2005;83:297–314. doi: 10.1901/jeab.2005.47-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J.B, Sabol K.E, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior. Psychopharmacology. 1999;146:432–439. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Slatta K, Arntzen E. Low doses of methylphenidate (Ritalin) may alter the delay-of-reinforcement gradient. Psychopharmacology. 1988;95:303–312. doi: 10.1007/BF00181938. [DOI] [PubMed] [Google Scholar]
- Sanger D.J, Blackman D.E. Rate-dependent effects of drugs: Review of the literature. Pharmacology, Biochemistry, & Behavior. 1976;4:73–83. doi: 10.1016/0091-3057(76)90178-7. [DOI] [PubMed] [Google Scholar]
- Santi A, Weise L, Kuiper D. Amphetamine and memory for event duration in rats and pigeons: Disruption of attention to temporal samples rather than changes in the speed of the internal clock. Psychobiology. 1995;23:224–232. [Google Scholar]
- Saulsgiver K.A, McClure E.A, Wynne C.D.L. Effects of d-amphetamine on the behavior of pigeons exposed to the peak procedure. Behavioural Processes. 2006;71:268–285. doi: 10.1016/j.beproc.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Schofield G, Davison M. Nonstable concurrent choice in pigeons. Journal of the Experimental Analysis of Behavior. 1997;68:219–232. doi: 10.1901/jeab.1997.68-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs D.A, Thomas J.R. Discrimination of stimulus duration and d-amphetamine in pigeons: A psychophysical analysis. Psychopharmacologia. 1974;36:313–322. doi: 10.1007/BF00422563. [DOI] [PubMed] [Google Scholar]
- Thompson D.M, Moerschbaecher J.M. Drug effects on repeated acquisition. In: Thompson T, Dews P.B, editors. Advances in behavioral pharmacology, Vol. 2. New York: Academic Press; 1979. pp. 229–259. [Google Scholar]
- Wade T.R, de Wit H, Richards J.B. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Walker D.J, Branch M.N. Effects of cocaine on briefly signaled versus completely signaled delays to reinforcement. Journal of the Experimental Analysis of Behavior. 1996;65:375–388. doi: 10.1901/jeab.1996.65-375. [DOI] [PMC free article] [PubMed] [Google Scholar]