Abstract
In contrast to growth factor-stimulated tyrosine phosphorylation of p120, its relatively constitutive serine/threonine phosphorylation is not well understood. Here we examined the role of serine/threonine phosphorylation of p120 in cadherin function. Expression of cadherins in cadherin-null cells converted them to an epithelial phenotype, induced p120 phosphorylation and localized it to sites of cell contact. Detergent solubility and immunofluorescence confirmed that phosphorylated p120 was at the plasma membrane. E-cadherin constructs incapable of traveling to the plasma membrane did not induce serine/threonine phosphorylation of p120, nor did cadherins constructs incapable of binding p120. However, an E-cadherin cytoplasmic domain construct artificially targeted to the plasma membrane did induce serine/threonine phosphorylation of p120, suggesting phosphorylation occurs independently of signals from cadherin dimerization and trafficking through the ER/Golgi. Solubility assays following calcium switch showed that p120 isoform 3A was more effective at stabilizing E-cadherin at the plasma membrane relative to isoform 4A. Since the major phosphorylation domain of p120 is included in isoform 3A but not 4A, we tested p120 mutated in the known phosphorylation sites in this domain, and found that it was even less effective at stabilizing E-cadherin. These data suggest that serine/threonine phosphorylation of p120 influences the dynamics of E-cadherin in junctions.
Keywords: p120 catenin, E-cadherin, membrane dynamics
Introduction
E-cadherin belongs to the classical subfamily of cadherins that mediates calcium-dependent cell-cell adhesion and plays important roles in the dynamic rearrangement of cells during morphogenesis [1]. E-cadherin is frequently lost in human tumor cells and the invasion suppressor property of E-cadherin has been well documented [2, 3]. Downregulation of the adhesive activity of E-cadherin in tumor cells that retain its expression can be achieved by a number of mechanisms including post-translational modification of components of the cadherin/catenin complex [4]. Destabilization and subsequent loss of E-cadherin protein correlates with a decrease in the expression of p120 catenin (p120) in the early stage of tumor progression [5], suggesting that p120 may be a modulator of cadherin function during tumorigenesis.
p120 is an armadillo repeat protein that binds to the membrane-proximal region of the cytoplasmic tail of E-cadherin [6]. p120 has multiple biological functions including regulation of cell adhesion by controlling cadherin turnover at the cell surface [7]. Cells expressing E-cadherin with a defective p120 binding site (764EED/AAA) failed to recruit p120 into the cadherin-catenin complex, and have reduced cell-cell adhesion, highlighting the importance of p120 in cadherin function [8].
p120 was first identified as a tyrosine kinase substrate that is heavily phosphorylated in response to growth factor stimulation and was later shown to be associated with the E-cadherin complex [9]. Mariner et al. showed that treatment of A431 cells with EGF resulted in enhanced phosphorylation of p120 on Y228, and this phosphorylated p120 was associated with E-cadherin, but Y228 phosphorylation itself was not essential for cell adhesion [10]. On the other hand, Ozawa and Ohkubo found that tyrosine phosphorylation of p120 in v-Src transfected E-cadherin-expressing L cells led to reduced cell-cell adhesion without influencing the affinity of p120 for E-cadherin [11]. In addition to its role in regulating E-cadherin function, p120 also functions as a Rho guanine nucleotide dissociation inhibitor, or Rho GDI [12, 13], and Castano et al. recently showed that this function of p120 is regulated by Fyn/Src-dependent tyrosine phosphorylation [14].
Interestingly, phosphoamino acid analysis showed that constitutive phosphorylation of p120 occurs extensively on serine, less on threonine, and not at all on tyrosine in L cells expressing E-cadherin [11]. Treatment of cells with the nonspecific kinase inhibitor, staurosporine, resulted in dephosphorylation of p120 on serine/threonine residues, which activated nonfunctional E-cadherin in Colo 205 cells and in L-cells [15] [6]. A report by Xia et al. showing that dephosphorylation of p120 via staurosporine or activation of PKC implicated S268 of p120 as a possible regulator of cadherin function [16]. However, the functional mechanism underlying the role of serine/threonine phosphorylation of p120 in cadherin-mediated adhesion is still not understood.
In the present study we show that forced expression of cadherins in the cadherin-deficient MiaPaCa-2 cell line converts these otherwise dispersed cells into epithelial-like cells, relocalizes p120 to the plasma membrane, and induces p120 phosphorylation on serine and threonine. Furthermore, using p120-deficient S2-013 cells, we examined the function of the p120 regulatory domain in cadherin activity.
Materials and Methods
Reagents, antibodies, and cultured cells
Reagents were from Sigma-Aldrich (St Louis, MO), or Fisher Chemicals (Fairlawn, NJ) unless otherwise indicated. Anti-E-cadherin mouse monoclonal antibody (mAb) that recognizes the cytoplasmic domain of E-cadherin, anti-p120 mouse mAb (pp120), anti-p120 phosphospecific mouse mAb (T310), and anti-p120 phosphospecific mouse mAb (T916) were from BD (Franklin Lakes, NJ). Anti-p120 phosphospecific mouse mAb (S268) and anti-p120 phosphospecific mouse mAb (S288) have been described [17]. Affinity-purified rabbit antibody (A300-977A; CTNND1) that recognizes a region between resides 625 and 681 of human p120-catenin (NP_001078927) was purchased from Bethyl Laboratories (Montgomery, TX). Mouse mAb (HECD1) against the extracellular region of human E-cadherin [18] was a kind gift from Dr. Masatoshi Takeichi (RIKEN Center for Developmental Biology, Kobe, Japan). Anti-tubulin mouse mAb (E7) was from the Developmental Studies Hybridoma Bank, Iowa City, IA. Anti-P-cadherin, N-cadherin, β-catenin and α-catenin have been described [19]. Human MiaPaCa-2 cells (CRL-1420) were obtained from American Type Culture Collection (ATCC, Manasass, VA), and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Hyclone Laboratories, Logan, UT) and 2.5% horse serum (Invitrogen, Carlsbad, CA). S2-013 cells were kindly provided by Dr. Tony Hollingsworth (UNMC, Omaha, NE), and maintained in DMEM supplemented with 10% FBS.
Detergent extraction, SDS-PAGE, immunoblots, and immunoprecipitations
Routinely, monolayers of subconfluent cultured cells were washed twice with ice-cold phosphate buffered saline (PBS) and extracted on ice with TNE buffer (10 mM Tris-HCl, pH 8.0, 0.5% Nonidet P-40, 1 mM EDTA) containing 2 mM orthovanadate, 20 μM calyculin A, 2 mM NaF, and protease inhibitor cocktail. Extracts were centrifuged at 14,000 g for 15 minutes at 4°C, and the supernatant was collected. Protein concentration was determined using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). TNE extracts were used for immunoprecipitation as described [20]. In experiments comparing soluble vs. insoluble proteins, we used the detergent solubility assay described by Gimond et al. [21], where the detergent insoluble pellet was resuspended in SDS sample buffer (2% SDS, 10% glycerol, 62.5 mM Tris-HCl, pH 6.8) followed by sonication. Cell extracts were resolved by SDS-PAGE and immunoblotted as described [19]. Immunoblots were quantified by densitometry using Adobe Photoshop© histogram.
Phosphatase treatment of cell extracts
Anti-mouse IgG affinity gel (300 μL, Cappel MP Biomedicals, Solon, OH) was incubated with 5 μg mouse anti-pp120 antibody (BD Biosciences Pharmingen, San Jose, CA) for 1 hour at 4°C. Subconfluent cell cultures were extracted in 10 mM Tris-HCl, pH 7.5, 0.1% Empigen BB (Calbiochem, La Jolla, CA), 5 mM EDTA, 2 mM EGTA, 30 mM sodium fluoride, 40 mM β-glycerophosphate, 10 mM sodium pyrophosphate, 2 mM sodium orthovanadate, and protease inhibitor cocktail (Calbiochem). The insoluble material was removed by centrifugation at 14,000 g for 15 minutes at 4°C, and the protein concentration of the resulting supernatant was determined using a Bio-Rad protein assay kit. 600 μg protein was used for immunoprecipitations. The immune complexes were washed four times with TBST (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% Tween 20, 2 mM sodium orthovanadate) and with TBS (TBST without 0.05% Tween 20) as the final wash. The washed beads were divided into two fractions that were incubated with Lambda phosphatase reaction buffer (New England BioLabs, Ipswich, MA) and MnCl2 either with or without Lambda phosphatase (1800 U/μl) for 30 minutes at 37°C, and then resolved by SDS-PAGE.
Immunofluorescence microscopy
Cells were fixed with HistoChoice tissue fixative (Amresco, Solon, OH) and processed as previously described [22]. For immunostaining with anti-p120 phosphospecific mouse mAb (T310 or T916), cells were fixed with ice-cold 10% trichloroacetic acid for 15 minutes, and blocked in PBS supplemented with 10% goat serum as described [23]. Cells were examined on a Zeiss Axiovert 200M microscope (Gottingen, Germany) equipped with an ORCA-ER digital camera (Hamamatsu, Houston, TX). Images were collected and processed using Slidebook software (Intelligent Imaging Innovations, Inc, Santa Monica, CA).
Constructs, transfections, and infections
cDNAs for human N-cadherin, human P-cadherin, mouse R-cadherin and human cadherin-11 were kind gifts from Dr. Avri Ben Ze'ev [24], Dr. Setsuo Hirohashi [25], Dr. Masatoshi Takeichi [26] and Dr. Akira Kudo [27], respectively. Mutant E-cadherin (Ep120AAA) that cannot bind to p120 has been described [8]. A mutation in N-cadherin (Np120AAA) that prevents binding to p120 was generated by recombinant PCR. The amino-acid alterations, E780A, E781A and D782A, correspond to those reported to be effective in uncoupling E-cadherin from p120 [8]. The details of the construction are available upon request. Full-length human E-cadherin fused at its C-terminus to GFP was constructed by first inserting an E-cadherin cDNA into pEGFP-N2 (Clontech, Mountain View, CA) and then inserting the tagged construct into a derivative of the LZRS retroviral vector. Details of the construction are available upon request.
A cDNA clone encoding the cytoplasmic domain of human E-cadherin (EcadCD) was generated through PCR amplification as described [28]. EcadM was generated by adding the myristylation sequence (MGSSKSKPKDPSQR) derived from v-Src as described [28]. EcadMOM was generated by adding a mitochondrial outer membrane targeting sequence [29] to the N-terminus of EcadCD. A chimeric construct comprised of the extracellular domain of N-cadherin and the cytoplasmic domain of E-cadherin has been described [22]. Constructs derived from in vitro DNA synthesis and/or PCR were sequenced and shown to contain only the intended changes. Constructs were inserted into pLZRS and transfected into Phoenix 293 cells using a TransIT-LT1 Reagent (Mirus, Madison, WT). Conditioned medium containing recombinant retrovirus was supplemented with 4 μg/ml polybrene and added to target cells as described [20, 30].
Transfected Phoenix 293 cells were selected with 4 μg/ml puromycin, and infected target cells were selected with 4 μg/ml G418. Retroviral constructs containing mouse p120 isoform 3A, mouse p120 isoform 3A with six residues of S/T (S252, S268, S288, T310, S312, 7916) mutated to alanine, and mouse p120 isoform 4A have been described [31, 32].
Pulse Chase
The culture medium of subconfluent monolayer of cells was replaced with methionine/cysteine-deficient DMEM (Sigma) containing 1% dialyzed FBS (Hyclone) and 0.04 M L-glutamine (GibcoBRL/Invitrogen) overnight. Cells were pulsed with fresh conditioned medium containing 250 μCi Trans 35S label (ICN MP Biomedicals, Irvine, CA) for 1 hour. Radioactive medium was removed, cells were washed with PBS and then chased by addition of non-radioactive medium for 0, 6, 12, and 24 hours respectively. Cells were lysed in TNE buffer at each time point, extracts were immunoprecipitated, resolved by SDS-PAGE, transferred to nitrocellulose, and exposed to film at -80°C for 3 days. Labeled proteins were quantified by densitometry using Adobe Photoshop© histogram.
Calcium Switch
Subconfluent monolayers of cells were serum and calcium starved overnight using calcium-free DMEM (Gibco). Culture medium was replaced with DMEM supplemented with 10% FBS, which contains 1.8 mM calcium.
Fluorescence Recovery After Photobleaching (FRAP)
Live-cell imaging was performed using the Marianas system from Intelligent Imaging Innovations equipped with the ERAP laser system (Photonic Instruments, St. Charles, IL). The intensity profiles were analyzed for the maximum intensity recovery (%) for areas after photobleaching. The measured values were fitted to a single exponential function up to 3 min after photobleaching to obtain the half-time of intensity recovery (t1/2).
Results
Cadherins mediate cell-cell contact, p120 membrane recruitment, and phosphorylation of p120 in MiaPaCa-2 cells
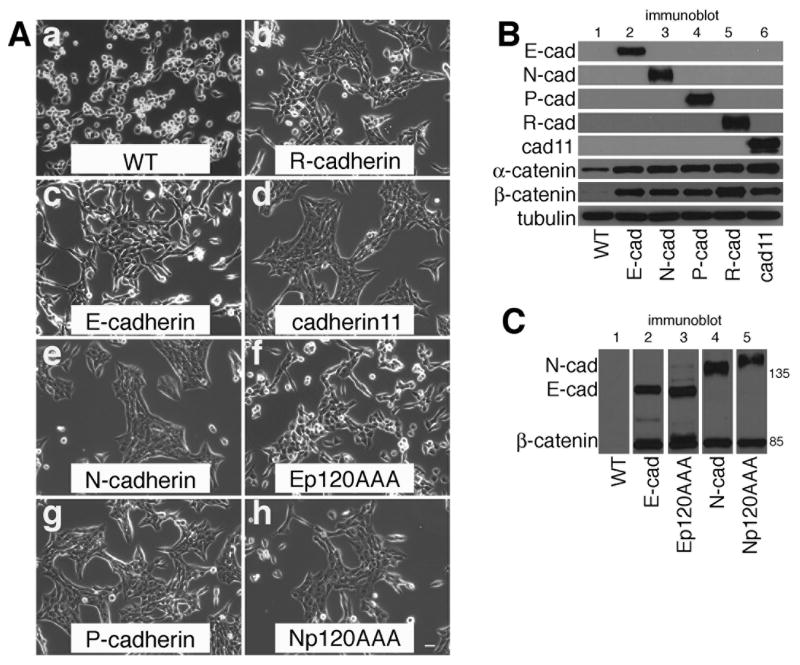
MiaPaCa-2 are poorly differentiated pancreatic tumor cells that display a rounded, disperse phenotype (Fig. 1A, panel a) and do not express any cadherins [33]. Expression of cadherins promoted an epithelial phenotype with significant cell-cell contact (Fig. 1A). There were no major differences in cellular morphology among cells expressing different cadherin family members including E-cadherin, N-cadherin, P-cadherin, R-cadherin and cadherin-11 (Fig. 1A). Fig. 1B shows the expression level of each cadherin in the cells shown in Fig. 1A, and shows that each cadherin is capable of stabilizing α-catenin and β-catenin as expected. Thoreson et al. developed E-cadherin mutants with a triple alanine substitution (764EED/AAA) in the p120 binding site that prevents the binding of p120 to the cadherin [8]. When E-cadherin with the AAA mutation (Ep120AAA) or N-cadherin with an analogous mutation (Np120AAA) were expressed in MiaPaCa cells they also conferred an epithelial phenotype on the cells (Fig. 1A, panels f and h, respectively). In addition, p120 uncoupled cadherins were as effective as wild type cadherins at stabilizing β-catenin (Fig. 1C).
Fig. 1. Forced cadherin expression promotes cell-cell adhesion in MiaPaCa-2 cells.
A: Phase micrographs of parental MiaPaCa-2 cells (a, WT), MiaPaCa-2 cells expressing E-cadherin (c), N-cadherin (e), P-cadherin (g), R-cadherin (b), cadherin-11 (d), E-cadherin with a p120 uncoupling mutation (f, Ep120AAA), or N-cadherin with a p120 uncoupling mutation (h, Np120AAA). Photographs were taken using a 10 X objective. Bar = 20 μm
B: TNE extracts of parental MiaPaCa-2 cells (lane 1, WT) MiaPaCa-2 cells expressing E-cadherin (lane 2), N-cadherin (lane 3), P-cadherin (lane 4), R-cadherin (lane 5) or cadherin-11 (lane 6) were immunoblotted for each cadherin, α-catenin, β-catenin and tubulin.
C: TNE extracts of parental MiaPaCa-2 cells (lane 1, WT), MiaPaCa-2 cells expressing E-cadherin (lane 2), E-cadherin with a p120 uncoupling mutation (lane 3), N-cadherin (lane 4), or N-cadherin with a p120 uncoupling mutation (lane 5) were immunoblotted for N-cadherin, E-cadherin and β-catenin.
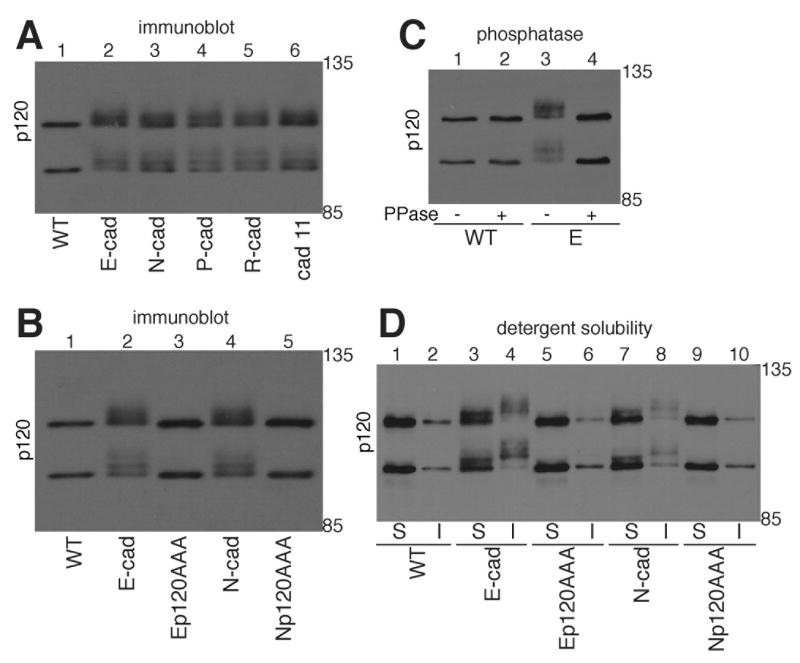
When we immunoblotted extracts of parental and cadherin-expressing MiaPaCa-2 cells for p120, we saw that cells expressing a cadherin had slower migrating p120 and that the p120 bands were diffuse (Fig. 2A, lanes 2-6) when compared to the parental cadherin-null MiaPaCa cells (Fig. 2A, lane 1). Immunoblotting for p120 typically produces two main bands. The slower migrating band corresponds to the longer isoforms (isoforms 1 and 2) and the faster migrating band corresponds to the shorter isoform 3. Interestingly, the banding pattern did not differ among cells expressing different cadherins. Cells expressing E- or N-cadherin with the AAA mutation had a p120 migration pattern identical to parental MiaPaCa cells (Fig. 2B), suggesting that association with the cadherin was necessary to alter p120 mobility. To determine if the diffuse appearance of p120 on SDS-PAGE was due to differences in phosphorylation, we treated the cell extract with lambda phosphatase and showed that p120 from E-cadherin-expressing MiaPaCa cells was converted to the sharp bands seen in cadherin-null cells (Fig. 2C, lane 4). To determine the subcellular localization of phosphorylated p120, we separated cell extracts into detergent soluble and detergent insoluble fractions and showed that p120 in the insoluble fraction migrated more slowly than p120 in the soluble fraction in cells expressing wild type E-cadherin (Fig. 2D lanes 3 and 4) or wild type N-cadherin (Fig. 2D lanes 7 and 8), suggesting that the more highly phosphorylated p120 proteins were associated with more mature adherens junctions. There is evidence for a small amount of phosphorylated p120 in the soluble fraction, which is expected since plasma membrane associated cadherin that is not yet incorporated into stable junctions is in this fraction. In cells expressing the AAA mutant forms of E-cadherin or N-cadherin, the majority of p120 was in the soluble fraction and that in the insoluble fraction migrated as a sharp band on SDS-PAGE. The data presented in Fig. 2 suggest that in MiaPaCa-2 cells, phosphorylation of p120 is mediated by its interaction with cadherin and likely occurs at the plasma membrane.
Fig. 2. Forced cadherin expression induces phosphorylation of p120.
A: TNE extracts of parental MiaPaCa-2 cells (lane 1, WT), MiaPaCa-2 cells expressing E-cadherin (lane 2), N-cadherin (lane 3), P-cadherin (lane 4), R-cadherin (lane 5) or cadherin-11 (lane 6) were immunoblotted for p120.
B: TNE extracts of parental MiaPaCa-2 cells (lane 1, WT), MiaPaCa-2 cells expressing E-cadherin (lane 2), E-cadherin with a p120 uncoupling mutation (lane 3), N-cadherin (lane 4) or N-cadherin with a p120 uncoupling mutation (lane 5) were immunoblotted for p120.
C: Extracts of MiaPaCa-2 cells and MiaPaCa cells expressing E-cadherin were immunoprecipitated with anti-p120 and treated with or without λ phosphatase. The immunoprecipitates were resolved by SDS-PAGE and immunoblotted for p120. Lane 1) untreated parental MiaPaCa-2 cells. Lane 2) phosphatase-treated parental MiaPaCa-2 cells. Lane 3) untreated MiaPaCa-2 cells expressing E-cadherin. Lane 4) phosphatase treated MiaPaCa-2 cells expressing E-cadherin.
D: Soluble (S) and insoluble (I) fractions of extracts from parental MiaPaCa-2 cells (lanes 1 and 2), MiaPaCa-2 cells expressing E-cadherin (lanes 3 and 4), E-cadherin with a p120 uncoupling mutation (lanes 5 and 6), N-cadherin (lanes 7 and 8) or N-cadherin with a p120 uncoupling mutation (lane 9 and 10) were immunoblotted for p120.
p120 serine/threonine phosphorylation sites include T310 and T916 residues in cadherin infected MiaPaCa-2 cells
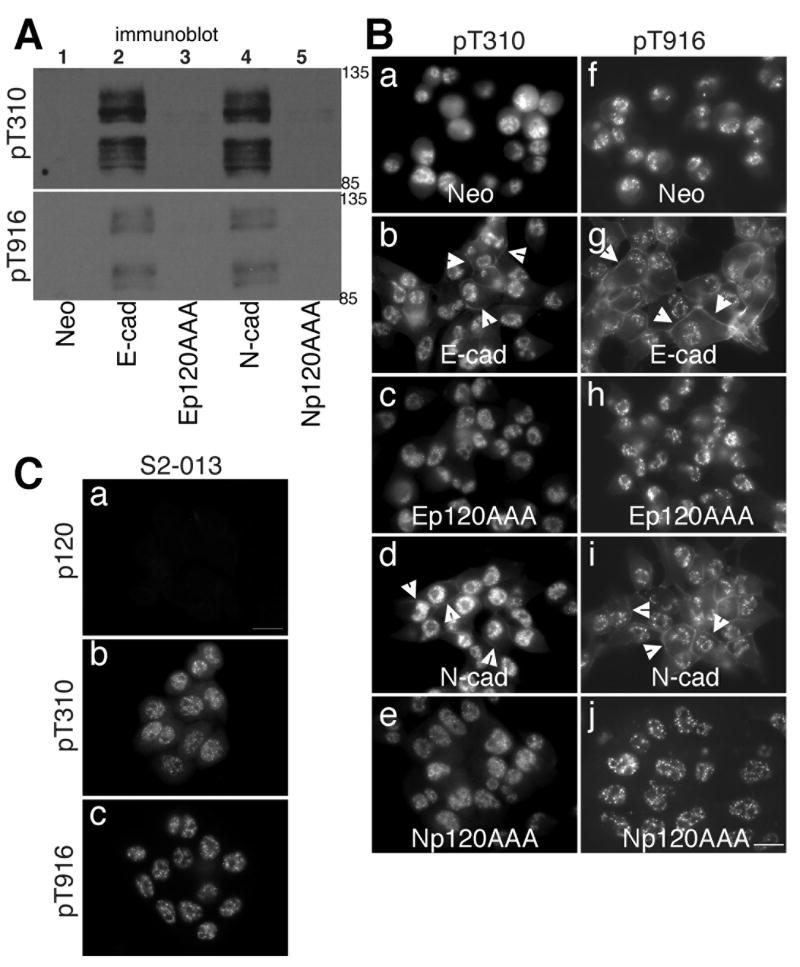
Several tyrosine and serine/threonine residues of p120 have been reported to be phosphorylated, including T310 and T916 [34]. T310 resides within the major phosphorylation domain, which is termed the regulatory domain and is a region of the amino-terminal domain of p120, whereas T916 is located near the carboxyl-terminus. Antibodies are available that specifically recognize p120 phosphorylated on T310 or T916 [16]. We used these antibodies to determine if T310 and/or T916 were phosphorylated under conditions that promote the changes in p120 mobility on SDS-PAGE that are shown in Fig. 2. Fig. 3A is an immunoblot showing that p120 was phosphorylated on both T310 and T916 in cells expressing wild type E-cadherin (lane 2) or wild type N-cadherin (lane 4). In cells expressing E-cadherin or N-cadherin that is unable to associate with p120, neither T310 nor T916 was phosphorylated (Fig. 3A, lanes 3 and 5 respectively). Fig. 3B compares the localization of p120 phosphorylated on T310 or T916 in MiaPaCa-2 cells expressing wild type E-cadherin, p120 uncoupled E-cadherin, wild type N-cadherin or p120 uncoupled N-cadherin. Each of these p120 phospho-specific antibodies shows significant non-p120 nuclear staining that is evident in Fig. 3C, panels b and c, which are immunofluorescence staining of p120 deficient S2-013 cells. This nuclear staining has been previously reported as non-specific [17], and despite our efforts to identify conditions that prevented nuclear staining, we could not diminish it. However, upon careful inspection of panels b, g, d and i in Fig. 3B, it is clear that cells expressing wild type E- or N-cadherin show plasma membrane staining with the phospho-specific antibodies (arrowheads), that is not seen in mock transfected cells (Fig. 3B, panels a and f) or in cells expressing p120 uncoupled AAA mutant cadherins (Fig. 3B, panels c, e, h, and j).
Fig. 3. Phosphorylation of p120 on T310 and T916 in cadherin-expressing MiaPaCa-2 cells.
A: TNE extracts of mock transduced MiaPaCa-2 cells (lane 1, Neo), MiaPaCa-2 cells expressing E-cadherin (lane 2), E-cadherin with a p120 uncoupling mutation (lane 3), N-cadherin (lane 4) or N-cadherin with a p120 uncoupling mutation (lane 5) were immunoblotted for phosphorylated T310 residue (top) or phosphorylated T916 residue (bottom) of p120.
B: Mock transduced MiaPaCa-2 cells (a and f), MiaPaCa-2 cells expressing E-cadherin (b and g), E-cadherin with a p120 uncoupling mutation (c and h), N-cadherin (d and i) or N-cadherin with a p120 uncoupling mutation (e and j) were fixed with 10% trichloroacetic acid and stained with antibodies specific for p120 phosphorylated at T310 residue (panels a-e) or T916 residue (panels f-j). Photographs were taken using a 60 X oil objective. Bar = 20 μm.
C: p120-deficient S2-013 cells were fixed with 10% trichloroacetic acid and stained with an antibody that recognizes all p120 isoforms (panel a), or antibodies that recognize only p120 that is phosphorylated at T310 (panels b) or T916 (panel c). Photographs were taken using a 60 X oil objective. Bar = 20 μm.
In contrast to the results with phospho T310 and phospho T916, we found no obvious difference in tyrosine phosphorylation in parental and cadherin-expressing MiaPaCa-2 cells. Supplemental Fig, S1 shows that p120 Y288 is phosphorylated in all cell lines. We also tested anti-phospho Y96, Y280 and Y291 and found no obvious difference (data not shown). Thus, we focused our investigation on phosphorylation of p120 on T310 and T916.
Phosphorylation of p120 requires plasma-membrane association but not signals from cadherin-cadherin engagement
To determine if p120 phosphorylation was induced by signals derived from cadherin-cadherin engagement at the plasma membrane, we made use of the following E-cadherin mutants (illustrated in Fig. 4A): 1) full length wild type E-cadherin; 2) the cytoplasmic domain of E-cadherin missing the transmembrane and extracellular domains that is thus expressed as a soluble cytoplasmic protein (E-cadCD); 3) the cytoplasmic domain of E-cadherin to which we have added a myristoylation signal (MGSSKSKPKDPSQR) derived from v-Src, thus targeting it to the plasma membrane (EcadM); 4) a chimera of N-cadherin and E-cadherin that is expressed by cells but does not fold properly and thus is trapped in the endoplasmic reticulum (ER) (N/E 5.5); and 5) the cytoplasmic domain of E-cadherin with a mitochondrial outer membrane-targeting sequence added to the amino-terminus (EcadMOM). Each construct was expressed in MiaPaCa cells and expression verified by immunoblotting extracts of the cells with an antibody that recognizes the cytoplasmic domain of E-cadherin (Fig. 4B). As a control, we expressed the neomycin resistance gene alone in MiaPaCa cells (lane 1).
Fig. 4. Modified E-cadherin mutants targeted to different cellular membranes.
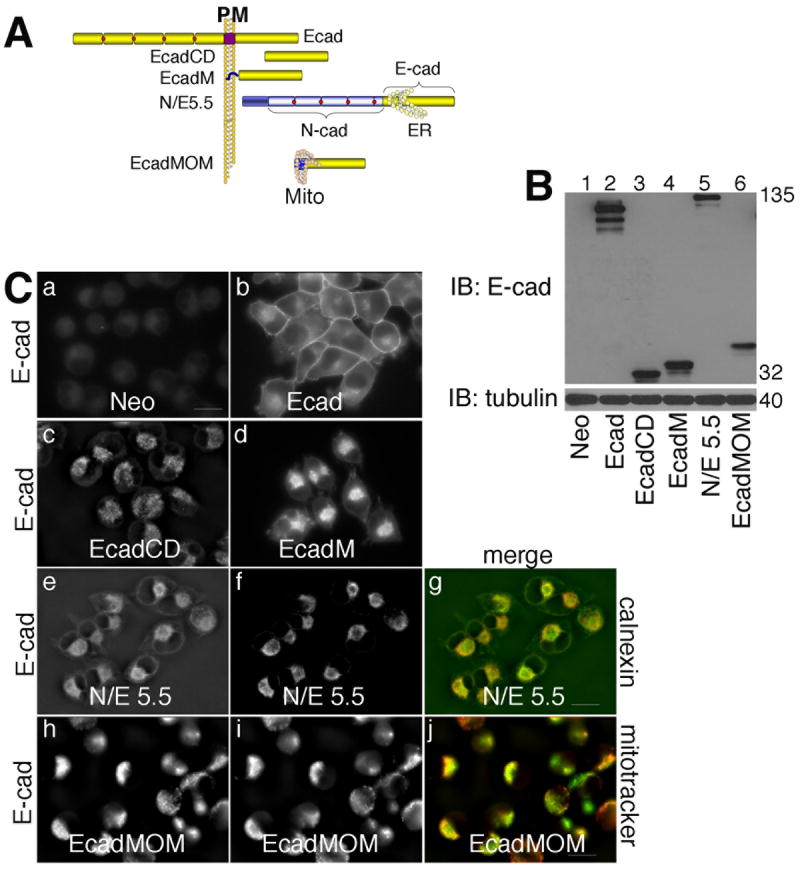
A: Schematic diagram of modified E-cadherin constructs: full length E-cadherin (E); the cytoplasmic tail of E-cadherin (amino acids 732-882, E-cadCD); the cytoplasmic tail of E-cadherin with the plasma membrane targeting sequence from v-Src added to the amino-terminus (E-cadM); a chimeric construct between N-cadherin and E-cadherin that remains trapped in the ER membrane (N/E5.5); and the cytoplasmic tail of E-cadherin with a mitochondrial outer membrane targeting sequence added to the amino-terminus (E-cadMOM).
B: TNE extracts of mock infected MiaPaCa-2 cells (lane 1), or cells expressing the constructs described in panel A were resolved by SDS-PAGE and immunoblotted for E-cadherin (top) and tubulin (bottom).
C: Mock infected MiaPaCa-2 cells (a), or cells expressing E-cadherin (b), EcadCD (c), EcadM (d), N/E 5.5 cadherin (e), or EcadMOM (h) were stained for E-cadherin. Cells expressing the N/E 5.5 construct were stained with the ER marker, calnexin (f). Cells expressing EcadMOM were stained with mitochondrial marker, Mitotracker (i). Merged pictures were taken of cells expressing N/E 5.5 (g) and EcadMOM (j) to illustrate co-localization with calnexin and Mitotracker, respectively. Photographs were taken using a 60 X oil objective. Scale bar = 20 μm.
Immunofluorescence showed that MiaPaCa cells expressing the Neo control had no staining for E-cadherin (Fig. 4C, panel a); wild type E-cadherin was localized to the plasma membrane (Fig. 4C, panel b); E-cadCD was diffusely localized in the cytosol (Fig. 4C, panel c); a fraction of EcadM was at the membrane, while some of it was cytosolic (Fig. 4C, panel d); the N/E chimera was trapped in the ER (Fig. 4C, panel e; and E-cadMOM was localized to mitochondria (Fig. 4C, panel h). To verify that the N/E chimera was in the ER, we showed that it co-localized with calnexin (Fig. 4C, panels e-g), and to verify that E-cadMOM was targeted to the mitochondria, we showed that it colocalized with Mitotracker (Fig. 4C, panels h-j).
We next tested whether these mutant E-cadherin molecules were associated with p120 in MiaPaCa cells. MiaPaCa cells expressing the neo control showed diffuse cytosolic localization of p120 (Fig. 5A, panel b) while cells expressing full length E-cadherin showed some cytosolic p120 together with a significant amount of plasma membrane associated p120 (Fig. 5A, panel e). p120 remained cytosolic with no plasma membrane localization in cells expressing the cytosolic EcadCD (Fig. 5A, panel h), however, when the cytoplasmic domain was targeted to the plasma membrane (EcadM), there was significant localization of p120 at the plasma membrane (Fig. 5A, panel k). When E-cadherin was trapped in the ER (N/E 5.5), p120 showed ER localization (Fig. 5A, panel m-o), and when E-cadherin was targeted to the mitochondria (EcadMOM), p120 was localized to mitochondria (Fig. 5A, panel p-r). In each case, p120 co-localized with the E-cadherin cytoplasmic domain, independent of where the cadherin was targeted, as shown in the merged pictures (Fig. 5A, panels c, f, i, l, o and r). To verify the co-localized proteins were actually associated with one another, we immunoprecipitated extracts of each cell line shown in Fig. 5A with antibodies against E-cadherin and immunoblotted the resolved proteins with antibodies against p120 (Fig. 5B). In each case, p120 was co-immunoprecipitated with the cadherin. The low level of p120 seen in the E-cadM immunoprecipitation is likely due to the small amount of E-cadM itself in this immunoprecipitation reaction, and the relatively small amount of the E-cadM that is localized at the plasma membrane.
Fig. 5. Modified E-cadherin constructs colocalize and physically interact with p120 in MiaPaCa-2 cells.
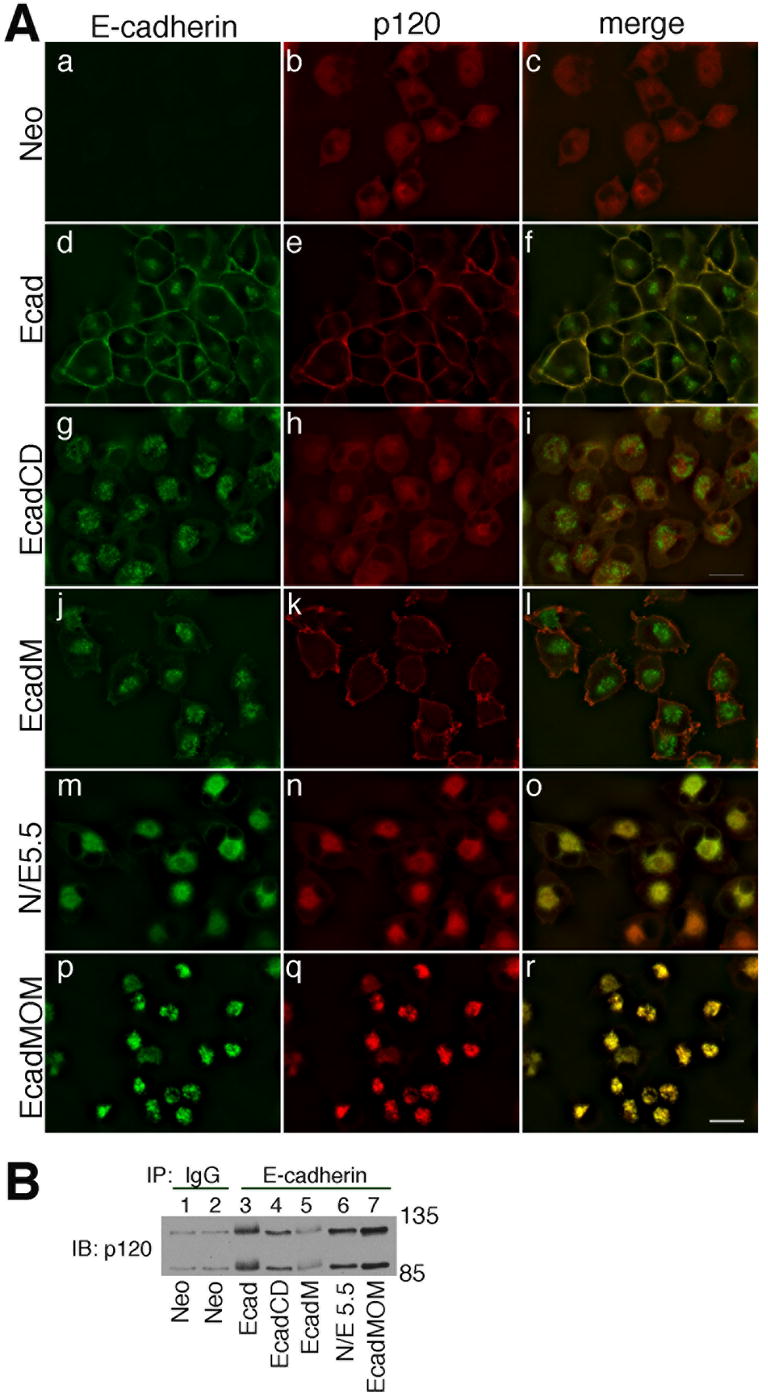
A: MiaPaCa-2 cells expressing the neomycin resistance gene (a-c), E-cadherin (d-f), EcadCD (g-i), EcadM (j-l), N/E 5.5 (m-o) or EcadMOM (p-r) were stained for E-cadherin and p120. Photographs were taken using a 60 X oil objective. Bar = 20 μm.
B: TNE extracts of cells shown in panel A were immunoprecipitated with anti-E-cadherin, resolved by SDS-PAGE and immunoblotted for p120 (lanes 2-7). The mock cells were immunoprecipitated with normal mouse IgG and immunoblotted for p120 as an antibody control (lane1).
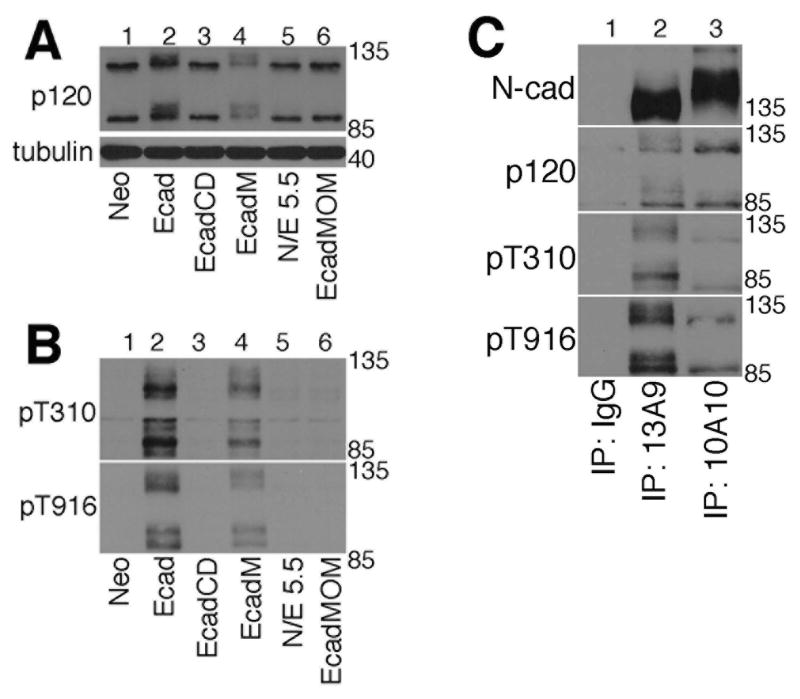
When we immunoblotted extracts of cells expressing the various E-cadherin constructs, the slower migrating, diffuse p120 bands were seen only in cells expressing full length wild type E-cadherin (Fig. 6A, lane 2) and E-cadherin that was targeted to the plasma membrane via a myristoylation signal (EcadM; Fig. 6A, lane 4). To verify that p120 was indeed phosphorylated when associated with full length E-cadherin and E-cadM, we immunoblotted extracts of each cell line with the antibodies that specifically recognize p120 that is phosphorylated on pT310 or pT916. Fig. 6B shows that p120 was phosphorylated on both T310 and T916 in cells expressing membrane targeted cadherin and was not phosphorylated on either threonine in cells expressing cadherin targeted to other cellular membranes. Since the E-cadM construct lacks the extracellular domain, these data suggest that phosphorylation of p120 requires plasma membrane association but does not require signals from cadherin-cadherin engagement. These data do not exclude the possibility that p120 targeted to the plasma membrane by other means would also be phosphorylated, as previously suggested [32].
Fig. 6. Phosphorylation of p120 requires plasma-membrane association but not signals from cadherin-cadherin engagement.
A: TNE extracts of MiaPaCa-2 cells expressing the neomycin resistance gene (lane 1), E-cadherin (lane 2), EcadCD (lane 3), EcadM (lane 4), N/E 5.5 (lane 5), or EcadMOM (lane 6) were resolved by SDS-PAGE and immunoblotted for p120 (top) and tubulin (bottom).
B: TNE extracts of MiaPaCa-2 cells expressing the neomycin resistance gene (lane 1), E-cadherin (lane 2), EcadCD (lane 3), EcadM (lane 4), N/E 5.5 (lane 5), or EcadMOM (lane 6) were resolved by SDS-PAGE and immunoblotted for p120 phosphorylated at T310 (top) and p120 phosphorylated at T916 residue (bottom).
C: TNE extracts of MiaPaCa-2 cells expressing N-cadherin were immunoprecipitated with an antibody that recognizes the pro-region of immature N-cadherin (10A10, lane 3) to pull down only the immature N-cadherin, or with an antibody that recognizes the cytoplasmic domain of N-cadherin (13A9, lane 2) to pull down all forms of N-cadherin. Immunoprecipitates were resolved by SDS-PAGE and immunoblotted for N-cadherin (using 13A9), p120, and p120 phosphorylated at T310 and T916. Normal mouse IgG was used as a negative control for immunoprecipitations (lane 1).
Since p120 phosphorylation appeared to occur at the plasma membrane and was dependent on its association with cadherin, we asked if p120 phosphorylation was limited to mature forms of the cadherin. Cadherins are synthesized with a pro-region that is removed by furin proteases upon processing in the ER/Golgi [35, 36]. We have previously shown that p120 can bind to cadherin that still retains the pro-region before it leaves the ER/Golgi [37]. For these studies we used a novel monoclonal antibody developed in our lab (10A10) that specifically recognizes the pro-region of immature N-cadherin [37]. Extracts of MiaPaCA-2 cells expressing N-cadherin were immunoprecipitated with the 10A10 antibody to pull down only the immature N-cadherin, or with the 13A9 antibody that recognizes the cytoplasmic domain of N-cadherin [19], to pull down all forms of N-cadherin. Since the majority of the N-cadherin is mature, and only a small fraction is recognized by the 10A10 antibody, we normalized the amount of N-cadherin in the immunoprecipitation reactions by using 7 times as much cell extract in the 10A10 immunoprecipitation reaction as in the 13A9 immunoprecipitation reaction. Immunoprecipitates were resolved by SDS-PAGE and immunoblotted for N-cadherin using the 13A9 antibody that recognizes all forms of the cadherin. Fig. 6C upper panel shows that the amount of N-cadherin in the two reactions was roughly equal. Immunoblotting with anti-p120 showed that the bands associated with mature N-cadherin, most of which is at the plasma membrane, were more diffuse than the bands associated with the immature N-cadherin, most of which is in the ER/Golgi. Immunoblotting with the phospho-specific antibodies showed that both T310 and T916 were phosphorylated when p120 was associated with either the mature cadherin or the immature cadherin, however the amount of phosphorylation was noticeably higher when the cadherin was mature and localized at the plasma membrane. There was barely any signal with the pT310 antibody in the 10A10 immunoprecipitation reaction, even though there was more p120 in this immunoprecipitation (compare the straight p120 immunoblots shown in lanes 2 and 3, second panel from the top). Likewise, the amount of phosphorylation on T916 when p120 was associated with immature cadherin was minimal. The data presented in Figs. 4-6 indicate that, although p120 can associate with cadherin regardless of its cellular localization, and likely does so as the cadherin is traveling through the ER/Golgi, its phosphorylation requires plasma membrane association but does not require extracellular cadherin-cadherin dimerization.
The phosphorylation domain of p120 regulates the dynamics of junction assembly
Phosphorylation of p120 has been extensively mapped, and it has been shown that the majority of the phosphorylated tyrosines, serines and threonines are in a 100 amino acid region in the amino terminus, termed the p120 regulatory domain that is important for signal integration from non-receptor kinases [14, 16, 38]. Threonine 310 is within the regulatory domain, while threonine 916 is in the carboxyl terminus outside of the regulatory domain. One goal of the current study was to further understand the role of phosphorylation of these two threonines in p120 function. p120 is expressed as a number of different isoforms, due to alternative start sites that determine the N-terminus of the protein and alternative splicing that influences the C-terminus of the protein [39]. There are 4 different start sites, termed 1-4, with 1 the longest and 4 the shortest. In addition, alternative splicing of exons 18 and 20 give rise to either A or B forms of the protein. The start site for isoform 4 is just carboxyl of the regulatory domain, resulting in proteins that lack this domain. Isoform 3A is the major isoform expressed in most epithelial cells, so we decided to use isoforms 3A and 4A to investigate the functions of T310 and T916, since T310 is included in isoform 3A, but not in isoform 4A and T916 is included in both isoforms.
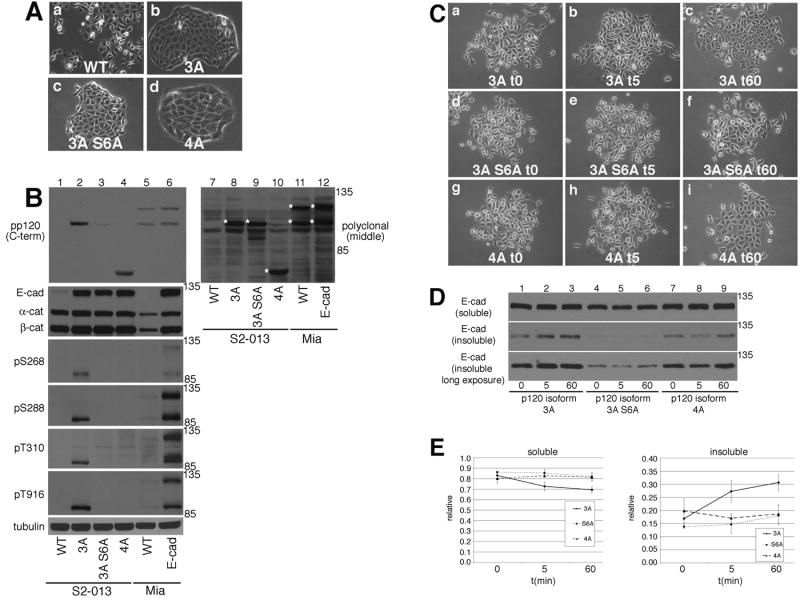
We found it difficult to completely knock down the endogenous expression of p120 in MiaPaCa-2 cells (data not shown). However, we identified a human pancreatic cancer cell line, S2-013, that is p120 deficient and is a derivative of the moderately-to-well differentiated SUIT-2 pancreatic cancer cell line (see Fig. 3C, panel a and Fig. 7B lane 1, top left panel) S2-013 expresses a very small amount of E-cadherin (Fig. 7B, lane 1, second panel). We retrovirally infected S2-013 cells with p120 isoform 3A (S2-013 3A), p120 isoform 3A with six S/T to alanine mutations (S2-013 3A S6A), or p120 isoform 4A (S2-013 4A) and confirmed expression by immunoblot analysis (Fig. 7B, top panel lanes 2-4). Cells lacking p120 typically do not have stable expression of E-cadherin. Isoform 3A (with or without the S/T mutations) and isoform 4A were able to stabilize E-cadherin expression (Fig. 7B, second panel, lanes 2-4). The pp120 antibody does not efficiently recognize p120 that is mutated in this region, so we also immunoblotted these extracts with a polyclonal antibody that recognizes each form of p120. This antibody has a high background in immunoblots, so we have pointed out the relevant bands with asterisks. Immunoblots using this antibody (Fig. 7B, top right panel) show that the p120 3A S6A construct is as highly expressed as the 3A and 4A constructs. S2-013 cells grow as rounded individual cells (Fig. 7A, panel a) and expression of p120 isoform 3A, 3A S6A, or 4A transformed the cells to an epithelial-like morphology with strong cell-cell contacts (Fig. 7A, panels b-d).
Fig. 7. Analysis of p120 isoforms 3A and 4A in p120-deficient S2-013 cells.
A: Phase contrast micrographs of parental S2-013 cells (a), S2-013 cells expressing p120 isoform 3A (b), S2-013 cells expressing p120 isoform 3A with six residues of S/T mutated to alanine 3AS6A(c), and S2-013 cells expressing p120 isoform 4A(d) were taken using a 10 X phase objective. Bar = 20 μm
B: TNE extracts of parental S2-013 cells (lane 1, WT), S2-013 cells expressing mouse p120 isoform 3A (lane 2), S2-013 cells expressing 3A S6A (lane 3), S2-013 cells expressing isoform 4A (lane 4), parental MiaPaCa-2 cells (lane 5), and MiaPaCa-2 cells expressing E-cadherin (lane 6) were resolved by SDS-PAGE and immunoblotted for p120 (top left, using pp120 antibody, which recognizes the C-terminus of p120; and top right, polyclonal antibody from Bethyl Labs, which recognizes a region between AA 625 and 681 of p120), E-cadherin, alpha-catenin, beta-catenin, p120 phosphorylated on S268, S288, T310, T916 residues, and tubulin. We used the polyclonal antibody to confirm the expression of p120 3A S6A because this mutated p120 is not efficiently recognized by the pp120 antibody. The polyclonal antibody has a high background on immunoblots, so we have pointed out the relevant bands by asterisks.
C: S2013 expressing p120 isoform 3A, the S6A mutant of 3A or isoform 4A were untreated (t0, a, d, and g), treated for 5 minutes (t5, b, e, and h), or treated for 1 hour (t60, c, f, and i) with 1.8 mM calcium. Photographs were taken using a 20 X phase objective.
D: Soluble (top panel) and insoluble (bottom two panels) fractions were prepared from S2-013 cells expressing p120 isoform 3A, the S6A mutant form of 3A or isoform 4A at time 0 (lanes 1, 4, 7), 5 minutes (lanes 2, 5, 8) or 60 minutes (lanes 3, 6, 9) after switching to 1.8 mM calcium. Each fraction was resolved by SDS-PAGE and immunoblotted for E-cadherin. The bottom panel is a long exposure of the middle panel to show that cells expressing the S6A mutant form of p120 do recover E-cadherin in this time frame.
E: Data from Fig. 7D (3 independent experiments) were quantified via densitometry using Adobe Photoshop histogram. Standard deviations are indicated.
S2-013 3A cells recover from a calcium switch more quickly than S2-013 3A S6A or S2-013 4A cells
Among the roles p120 plays in epithelial cells are regulating E-cadherin protein turnover and modulating the stability of junctional complexes. Adherens junctions are calcium dependent because the extracellular domain of the cadherin binds to calcium, which promotes protein folding into a domain structure that is capable of forming cadherin-cadherin dimers. Switching the culture medium from the normal 1.8 mM calcium concentration results in unfolding of the cadherin and dissolution of junctions. A switch back to normal calcium concentration allows the junction to reform. To investigate the functional significance of the p120 regulatory domain, we used S2-013 expressing the 3A or 4A isoform of p120 to examine the rate of E-cadherin recovery after calcium switch. To examine the role of S/T phosphorylation of p120, we used S2-013 expressing the S/T to alanine mutant form of the 3A isoform of p120. Subconfluent S2-013-3A, S2-013 3A S6A, and S2-013-4A cells were serum and calcium starved overnight, and then medium containing 1.8 mM calcium was added for 0 to 60 minutes. Phase contrast micrographs showed that cell-cell contacts in S2-013-3A cells recovered more rapidly than in S2-013-S6A or S2-013-4A cells (Fig. 7C). S2-013-3A cells showed clustering into relatively tight colonies even after 5 minutes of calcium recovery (Fig. 7C, panel b) when compared to S2-013 3A S6A cells (Fig. 7C, panel e) or S2-013 4A cells (Fig. 7C, panel h). The difference was even more evident at 60 minutes, when the S2-013-3A cells formed colonies with close cell-cell contact while the S2-013-3A-S6A cells and the S2-013-4A cells remained scattered (compare Fig. 7C, panels c, f. and i). Cells expressing the S6A mutant of isoform 3A were even more scattered than cells expressing isoform 4A.
Cells at 0 time, 5 minutes and 60 minutes were gently extracted with detergent-containing buffer and centrifuged to obtain a soluble fraction. The pellet (the insoluble fraction) was resuspended in sample buffer, and the detergent soluble and insoluble fractions were resolved by SDS-PAGE and immunoblotted for E-cadherin (Fig. 7D). S2-013 3A cells had relatively large amounts of E-cadherin incorporated into the insoluble fraction within 5 minutes of the calcium switch (Fig. 7D middle panel, lane 2), whereas S2-013-3A-S6A cells and S2-013 4A cells did not recover the insoluble fraction of E-cadherin even 1 hour after the addition of calcium (Fig. 7D, bottom panel, lanes 5-6 and middle panel, lanes 8-9). Quantification of immunoblots showed that E-cadherin recovery into the insoluble fraction, and presumably into junctions at sites of cell-cell contact, was higher in S2-013 3A cells relative to S2-013 3A-S6A cells or S2-013 4A cells in response to a calcium switch (Fig. 7E). These data suggest that cells expressing the 3A isoform of p120 can re-establish junctions more quickly than cells expressing the S/T mutation of the 3A isoform or the 4A isoform.
E-cadherin is more dynamic in S2-013 3A cells than in S2-013 4A
Since E-cadherin appears to be more available for re-establishing cell junctions after a calcium switch when cells express p120 isoform 3A compared to cells expressing p120 isoform 4A, we used pulse chase analysis to ask if E-cadherin protein was more stable in cells expressing p120 isoform 3A relative to cells expressing isoform 4A. The half life of E-cadherin was longer in S2-013 3A cells (Figs. 8A, B, lanes 5-8), compared to S2-013 4A cells (Figs. 8A, B, lanes 9-12). Quantitative analysis showed that the half life of E-cadherin in S2-013 3A cells was longer than 24 hours while the half life of E-cadherin in S2-013 4A cells was a little less than 18 hours (Fig. 8B). The difference was reproducible, but not statistically different. Although E-cadherin was more stable in cells expressing p120 isoform 3A than in cells expressing p120 isoform 4A, both isoforms were quite efficient at stabilizing E-cadherin, as its half life in mock transfected S2-013 cells was less than 6 hours (Figs. 8A, B, lanes 1-4).
Fig. 8. Junctional E-cadherin recovers more rapidly in cells expressing p120 isoform 3A than in cells expressing p120 isoform 4A.
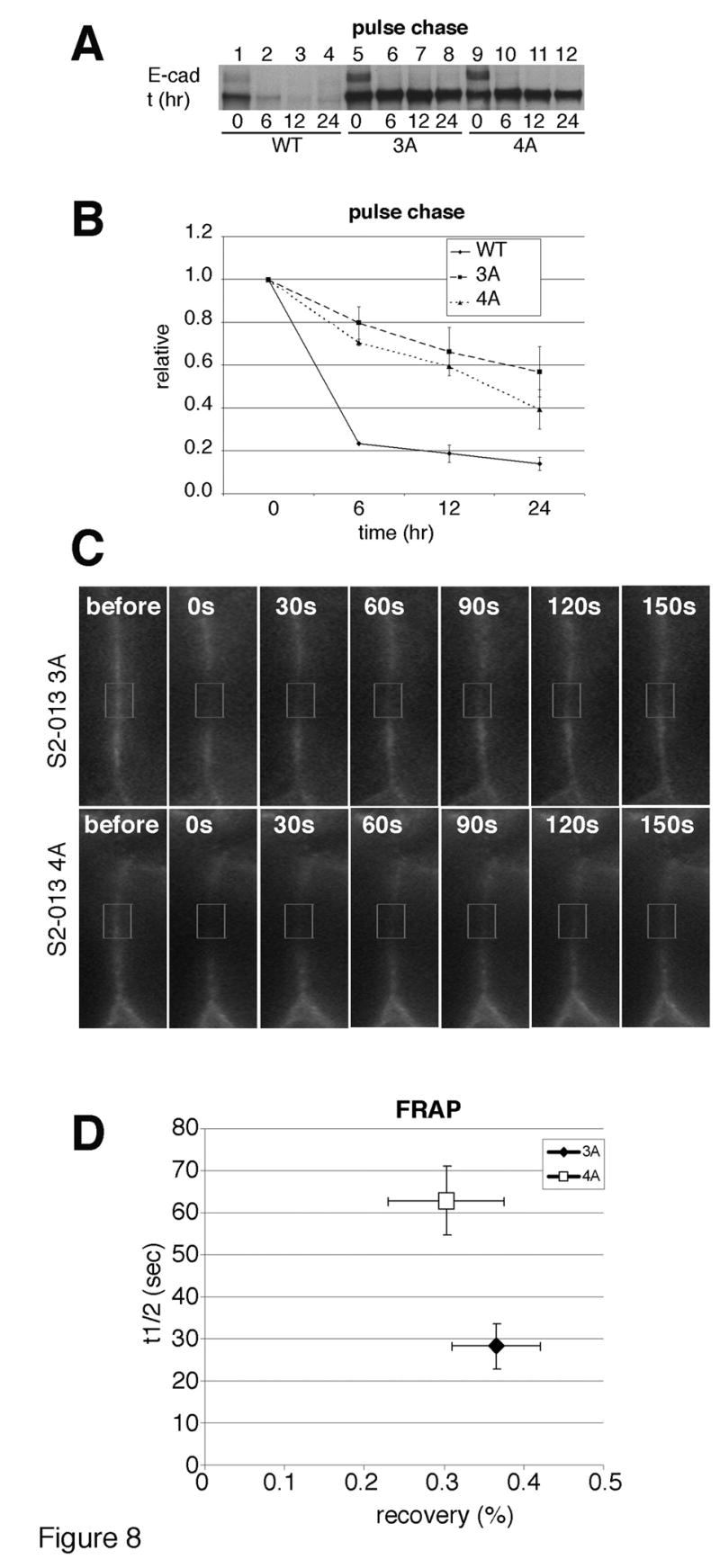
A: Parental S2-013 cells and S2-013 cells expressing p120 isoform 3A or isoform 4A were serum starved overnight in cysteine/methionine free medium and labeled with [35S]cysteine/methionine for 1 hour. Cells were extracted immediately (0 time; lanes 1, 5, and 9), or chased for 6 hours (lanes 2, 6, and 10), 12 hours (lanes 3, 7, and 11) or 24 hours (lanes 4, 8, and 12) in complete medium. TNE extracts at each time course were immunoprecipitated with anti-E-cadherin, resolved by SDS-PAGE and labeled protein was visualized by autoradiography.
B: E-cadherin expression levels from Fig. 8A were quantified by densitometry using Adobe Photoshop histogram from 2 independent experiments.
C: The recovery rate of junctional E-cadherin-GFP was monitored after photo-bleaching a small region (indicated in the box) at sites of cell-cell contact in S2-013 expressing p120 isoform 3A (upper panels) or isoform 4A (lower panels) cells. Cells were photographed before photobleaching and every 30 seconds after photobleaching.
D: Fluorescence recovery after photobleaching (FRAP) was quantified from ten independent measurements for the cells shown in Figure 8C. The X-axis indicates the mobile fraction of E-cadherin (recovery, %), and the y-axis indicates the half-life (t1/2) in seconds. The standard deviation is indicated.
To determine if there was any difference in the dynamics of cell-surface E-cadherin in cells expressing p120 isoform 3A vs. p120 isoform 4A, we performed fluorescence recovery after photobleaching (FRAP) using S2-013 3A and S2-013 4A cells stably expressing E-cadherin-GFP. The recovery rate of E-cadherin was monitored after bleaching a small region at sites of cell-cell contacts. There was no significant difference in the mobile fraction of membranous E-cadherin-GFP between S2-013 3A cells (recovery = 36.6% ± 5.5%) and S2-013 4A cells (recovery = 30.3% ± 7.2%). However, in the mobile fraction, the half-life of fluorescence recovery (t1/2) for E-cadherin-GFP in S2-013 3A cells was faster (t1/2 = 28.2 sec ± 5.5 sec) than that in S2-013 4A cells (t1/2 = 62.9 sec ± 8.2 sec) (Fig. 8C; and Supplemental Movies).
Together, the data presented in Figs. 7, 8 show that p120 isoform 3A is capable of stabilizing E-cadherin at the plasma membrane more dynamically than is p120 isoform 4A, and support our hypothesis that p120 phosphorylation in the regulatory domain plays a role in E-cadherin dynamics at the plasma membrane.
Discussion
The purpose of this study was to examine the role of the regulatory domain of p120 in cadherin function. Our study had three major findings. First, forced expression of cadherins in cadherin deficient MiaPaCa-2 cells increased cell-cell adhesion, localized p120 to the plasma membrane, and promoted serine/threonine phosphorylation of p120. Second, serine/threonine phosphorylation of p120 occurred only at the plasma membrane but was independent of cadherin dimerization or trafficking through the ER/Golgi. Finally, we showed that one potential function of serine/threonine phosphorylation of p120 is to modulate E-cadherin dynamics in cell-cell junctions.
The first finding is consistent with a report from Xia et al. showing that membrane-localization but not functional cadherin interaction was required for serine/threonine phosphorylation of p120 [32]. Adding to this story, we showed that cadherin-mediated serine/threonine phosphorylation of p120 was not specific to a single cadherin subtype but rather was promoted by any cadherin capable of binding to p120. In addition, we showed that the profile of phosphorylation was independent of cadherin subtype. This is in contrast to a study by Seidel et al., showing that the longer isoform of p120 binds to N-cadherin while the shorter isoform preferentially binds to E-cadherin [33]. Our study was done by infecting cadherins into MiaPaCa-2 cells using retro-viral infection, whereas Seidel et al. transfected cadherins into MiaPaCa-2 cells and selected clones. Thus, the discrepancy in our results may be due to our using a population of cells rather than clones.
Serine/threonine phosphorylation of p120
To date, multiple serine/threonine phosphorylation sites have been identified on p120. The majority of these sites are located in a small region called the phosphorylation domain or regulatory domain near the N-terminus. In addition, there are a few phosphorylation sites at the C-terminus [16]. Antibodies have been generated that specifically recognize p120 that is phosphorylated at a number of these sites [17]. Using these phospho-specific p120 antibodies, we showed that T310 and T916 of p120 are specifically phosphorylated when cadherins are expressed in cadherin deficient MiaPaCa-2 cells. This is in agreement with results from others showing that serine/threonine residues of p120 are constitutively phosphorylated in many different cell lines including MDCK, MCF-7, HCT-116, and A431 [16, 32, 40, 41]. Each of these cell lines expresses functional cadherins that are localized at the cell surface. In our study we showed that localizing p120 to internal cellular membranes, even the endoplasmic reticulum membrane did not promote its phosphorylation. Plasma membrane localization was essential for p120 to be phosphorylated on T310 and T916, however, p120 did not have to be associated with a functionally active cadherin. In fact, targeting to the membrane by association with a truncated cytoplasmic domain of a cadherin was sufficient to mediate phosphorylation.
Since cadherins must be at the plasma membrane to promote adhesion, it has been suggested that the serine/threonine kinase(s) involved in cadherin-dependent phosphorylation of p120 are membrane-associated, but the specific kinases have not been clearly identified [38, 39]. It would be nice to establish an experimental setting where one could identify the specific phosphorylation or dephosphorylation event for each individual residue within the regulatory domain of p120. This is complicated by the fact that not all phosphorylation and/or dephosphorylation events occur simultaneously, sequentially, or in a uniform direction. A stimulatory pathway might induce phosphorylation at one residue as well as dephosphorylation at another residue. For example, Xia et al. showed that activation of protein kinase C (PKC) induced dephosphorylation on all sites that were examined [32], but enhanced the phosphorylation of S879 [16]. Others reported that both inhibition and activation of PKC, or treatment of cells with the kinase inhibitor, staurosporine, reduced p120 serine phosphorylation [15, 42, 43]. These studies suggest that PKC influences phosphorylation of p120 on serine and/or threonine.
A role for p120 serine/threonine phosphorylation in E-cadherin function
Several recent studies addressing the role of p120 itself or its phosphorylation in regulating cadherin function have shown that p120 can both negatively and positively influence cadherin adhesive activity. Ireton, et al. reported that forced p120 expression in p120-defective SW48 cells reactivated E-cadherin function and rescued an epithelial-like morphology [31]. This is consistent with our data showing that p120-deficient S2-013 cells recovered epithelial cell-junctions upon forced expression of p120. The Ireton study illustrates the controversial nature of the role of p120 in regulating cadherin activity because this study showed that forced expression of the regulatory domain of p120 alone had a negative effect on cadherin adhesion in SW48 cells [31]. These data are consistent with a separate report using Colo205 cells, where expression of full-length p120 prevented E-cadherin activity, but expression of an N-terminally deleted version did not [15]. In addition, Ozawa's group reported that L cells expressing hyperphosphorylated p120 showed decreased adhesion, whereas L cells expressing N-terminally deleted p120 had increased cell aggregation and an epithelial morphology [11]. Interestingly, we observed that the effect of p120 serine/threonine phosphorylation on cadherin-mediated activity was to stabilize E-cadherin at the plasma membrane. Our third major finding stems from the observation that S2-013 3A cells showed more rapid assembly of E-cadherin into junctions following a calcium switch when compared to S2-013 3A-S6A and S2-013 4A cells. These data support the notion that phosphorylation of the regulatory domain of p120 (possibly the serine/threonine phosphorylation of p120) modulates the dynamics of assembly of E-cadherin into stable junctions.
A role for p120 regulatory domain in stabilizing E-cadherin at sites of cell-cell contacts
If p120 at the cell-surface regulates cadherin levels by controlling cadherin turnover [7], we could expect that modifications to p120 such as serine/threonine phosphorylation might influence cadherin stability at the cell-surface. Since increased E-cadherin trafficking occurs in response to weakening or disrupting cell-cell adhesion [44], we treated S2-013 3A and S2-013 4A cells with EGTA and found that E-cadherin underwent faster degradation in S2-013 4A cells than in S2-013 3A cells (not shown). At first glance, these data suggest more dynamic E-cadherin in cells expressing p120 isoform 4A. However, the trafficking patterns (i.e. the extent of re-cycling to the plasma membrane vs. targeting to lysosomes) may differ depending on expression of p120 isoform 3A vs. 4A. Furthermore, live cell imaging showed that junctional recovery was more rapid in cells expressing p120 3A, which contains the regulatory domain, than in cells expressing p120 4A, which does not contain the regulatory domain. These results suggest that serine/threonine phosphorylation of p120 may provide initial signals during junction assembly, possibly by either initiating rapid reorganization of E-cadherin at sites of cell-cell contact, by rescuing E-cadherin from degradation and prolonging its residence time at the cell surface, or by providing a plasma membrane retention signal. In conclusion, our study demonstrates that serine/threonine phosphorylation of p120 at the plasma membrane regulates the dynamics of E-cadherin at the cell-surface.
Supplementary Material
Acknowledgments
This work was supported by NIH R01-DE12308 and NIH R01-GM51188 and by NCI P30 CA36727 to the Eppley Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 2.Chen WC, Obrink B. Cell-cell contacts mediated by E-cadherin (uvomorulin) restrict invasive behavior of L-cells. J Cell Biol. 1991;114:319–327. doi: 10.1083/jcb.114.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 5.Thoreson MA, Reynolds AB. Altered expression of the catenin p120 in human cancer: implications for tumor progression. Differentiation. 2002;70:583–589. doi: 10.1046/j.1432-0436.2002.700911.x. [DOI] [PubMed] [Google Scholar]
- 6.Ohkubo T, Ozawa M. p120(ctn) binds to the membrane-proximal region of the E-cadherin cytoplasmic domain and is involved in modulation of adhesion activity. J Biol Chem. 1999;274:21409–21415. doi: 10.1074/jbc.274.30.21409. [DOI] [PubMed] [Google Scholar]
- 7.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mariner DJ, Davis MA, Reynolds AB. EGFR signaling to p120-catenin through phosphorylation at Y228. J Cell Sci. 2004;117:1339–1350. doi: 10.1242/jcs.01001. [DOI] [PubMed] [Google Scholar]
- 11.Ozawa M, Ohkubo T. Tyrosine phosphorylation of p120(ctn) in v-Src transfected L cells depends on its association with E-cadherin and reduces adhesion activity. J Cell Sci. 2001;114:503–512. doi: 10.1242/jcs.114.3.503. [DOI] [PubMed] [Google Scholar]
- 12.Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB. Inhibition of RhoA by p120 catenin. Nat Cell Biol. 2000;2:637–644. doi: 10.1038/35023588. [DOI] [PubMed] [Google Scholar]
- 13.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 14.Castano J, Solanas G, Casagolda D, Raurell I, Villagrasa P, Bustelo XR, Garcia de Herreros A, Dunach M. Specific phosphorylation of p120-catenin regulatory domain differently modulates its binding to RhoA. Mol Cell Biol. 2007;27:1745–1757. doi: 10.1128/MCB.01974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aono S, Nakagawa S, Reynolds AB, Takeichi M. p120(ctn) acts as an inhibitory regulator of cadherin function in colon carcinoma cells. J Cell Biol. 1999;145:551–562. doi: 10.1083/jcb.145.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia X, Mariner DJ, Reynolds AB. Adhesion-associated and PKC-modulated changes in serine/threonine phosphorylation of p120-catenin. Biochemistry. 2003;42:9195–9204. doi: 10.1021/bi034597h. [DOI] [PubMed] [Google Scholar]
- 17.Xia X, Brooks J, Campos-Gonzalez R, Reynolds AB. Serine and threonine phospho-specific antibodies to p120-catenin. Hybrid Hybridomics. 2004;23:343–351. doi: 10.1089/hyb.2004.23.343. [DOI] [PubMed] [Google Scholar]
- 18.Shimoyama Y, Hirohashi S, Hirano S, Noguchi M, Shimosato Y, Takeichi M, Abe O. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 1989;49:2128–2133. [PubMed] [Google Scholar]
- 19.Johnson KR, Lewis JE, Li D, Wahl J, Soler AP, Knudsen KA, Wheelock MJ. P- and E-cadherin are in separate complexes in cells expressing both cadherins. Exp Cell Res. 1993;207:252–260. doi: 10.1006/excr.1993.1191. [DOI] [PubMed] [Google Scholar]
- 20.Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci. 2005;118:873–887. doi: 10.1242/jcs.01634. [DOI] [PubMed] [Google Scholar]
- 21.Gimond C, van Der Flier A, van Delft S, Brakebusch C, Kuikman I, Collard JG, Fassler R, Sonnenberg A. Induction of cell scattering by expression of beta1 integrins in beta1-deficient epithelial cells requires activation of members of the rho family of GTPases and downregulation of cadherin and catenin function. J Cell Biol. 1999;147:1325–1340. doi: 10.1083/jcb.147.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JB, Islam S, Kim YJ, Prudoff RS, Sass KM, Wheelock MJ, Johnson KR. N-Cadherin extracellular repeat 4 mediates epithelial to mesenchymal transition and increased motility. J Cell Biol. 2000;151:1193–1206. doi: 10.1083/jcb.151.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi K, Yonemura S, Matsui T, Tsukita S. Immunofluorescence detection of ezrin/radixin/moesin (ERM) proteins with their carboxyl-terminal threonine phosphorylated in cultured cells and tissues. J Cell Sci. 1999;112(Pt 8):1149–1158. doi: 10.1242/jcs.112.8.1149. [DOI] [PubMed] [Google Scholar]
- 24.Salomon D, Ayalon O, Patel-King R, Hynes RO, Geiger B. Extrajunctional distribution of N-cadherin in cultured human endothelial cells. J Cell Sci. 1992;102(Pt 1):7–17. doi: 10.1242/jcs.102.1.7. [DOI] [PubMed] [Google Scholar]
- 25.Shimoyama Y, Yoshida T, Terada M, Shimosato Y, Abe O, Hirohashi S. Molecular cloning of a human Ca2+-dependent cell-cell adhesion molecule homologous to mouse placental cadherin: its low expression in human placental tissues. J Cell Biol. 1989;109:1787–1794. doi: 10.1083/jcb.109.4.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsunami H, Miyatani S, Inoue T, Copeland NG, Gilbert DJ, Jenkins NA, Takeichi M. Cell binding specificity of mouse R-cadherin and chromosomal mapping of the gene. J Cell Sci. 1993;106(Pt 1):401–409. doi: 10.1242/jcs.106.1.401. [DOI] [PubMed] [Google Scholar]
- 27.Okazaki M, Takeshita S, Kawai S, Kikuno R, Tsujimura A, Kudo A, Amann E. Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J Biol Chem. 1994;269:12092–12098. [PubMed] [Google Scholar]
- 28.Nieman MT, Kim JB, Johnson KR, Wheelock MJ. Mechanism of extracellular domain-deleted dominant negative cadherins. J Cell Sci. 1999;112(Pt 10):1621–1632. doi: 10.1242/jcs.112.10.1621. [DOI] [PubMed] [Google Scholar]
- 29.Rudiger M, Jockusch BM, Rothkegel M. Epitope tag-antibody combination useful for the detection of protein expression in prokaryotic and eukaryotic cells. Biotechniques. 1997;23:96–97. doi: 10.2144/97231bm20. [DOI] [PubMed] [Google Scholar]
- 30.Johnson E, Theisen CS, Johnson KR, Wheelock MJ. R-cadherin influences cell motility via Rho family GTPases. J Biol Chem. 2004;279:31041–31049. doi: 10.1074/jbc.M400024200. [DOI] [PubMed] [Google Scholar]
- 31.Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia X, Carnahan RH, Vaughan MH, Wildenberg GA, Reynolds AB. p120 serine and threonine phosphorylation is controlled by multiple ligand-receptor pathways but not cadherin ligation. Exp Cell Res. 2006;312:3336–3348. doi: 10.1016/j.yexcr.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Seidel B, Braeg S, Adler G, Wedlich D, Menke A. E- and N-cadherin differ with respect to their associated p120ctn isoforms and their ability to suppress invasive growth in pancreatic cancer cells. Oncogene. 2004;23:5532–5542. doi: 10.1038/sj.onc.1207718. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds AB, Roczniak-Ferguson A. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene. 2004;23:7947–7956. doi: 10.1038/sj.onc.1208161. [DOI] [PubMed] [Google Scholar]
- 35.Ozawa M, Kemler R. Correct proteolytic cleavage is required for the cell adhesive function of uvomorulin. J Cell Biol. 1990;111:1645–1650. doi: 10.1083/jcb.111.4.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Posthaus H, Dubois CM, Laprise MH, Grondin F, Suter MM, Muller E. Proprotein cleavage of E-cadherin by furin in baculovirus over-expression system: potential role of other convertases in mammalian cells. FEBS Lett. 1998;438:306–310. doi: 10.1016/s0014-5793(98)01330-1. [DOI] [PubMed] [Google Scholar]
- 37.Wahl JK, 3rd, Kim YJ, Cullen JM, Johnson KR, Wheelock MJ. N-cadherin-catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. J Biol Chem. 2003;278:17269–17276. doi: 10.1074/jbc.M211452200. [DOI] [PubMed] [Google Scholar]
- 38.Alema S, Salvatore AM. p120 catenin and phosphorylation: Mechanisms and traits of an unresolved issue. Biochim Biophys Acta. 2007;1773:47–58. doi: 10.1016/j.bbamcr.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Anastasiadis PZ, Reynolds AB. The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Sci. 2000;113(Pt 8):1319–1334. doi: 10.1242/jcs.113.8.1319. [DOI] [PubMed] [Google Scholar]
- 40.Kanner SB, Reynolds AB, Parsons JT. Tyrosine phosphorylation of a 120-kilodalton pp60src substrate upon epidermal growth factor and platelet-derived growth factor receptor stimulation and in polyomavirus middle-T-antigen-transformed cells. Mol Cell Biol. 1991;11:713–720. doi: 10.1128/mcb.11.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynolds AB, Herbert L, Cleveland JL, Berg ST, Gaut JR. p120, a novel substrate of protein tyrosine kinase receptors and of p60v-src, is related to cadherin-binding factors beta-catenin, plakoglobin and armadillo. Oncogene. 1992;7:2439–2445. [PubMed] [Google Scholar]
- 42.Ratcliffe MJ, Rubin LL, Staddon JM. Dephosphorylation of the cadherin-associated p100/p120 proteins in response to activation of protein kinase C in epithelial cells. J Biol Chem. 1997;272:31894–31901. doi: 10.1074/jbc.272.50.31894. [DOI] [PubMed] [Google Scholar]
- 43.Ratcliffe MJ, Smales C, Staddon JM. Dephosphorylation of the catenins p120 and p100 in endothelial cells in response to inflammatory stimuli. Biochem J. 1999;338(Pt 2):471–478. [PMC free article] [PubMed] [Google Scholar]
- 44.Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.