Ischemia reperfusion injury (IRI) is an increasingly important problem in clinical transplantation and is also implicated in a variety of non-transplant conditions, including myocardial ischemia, shock and stroke. Clinical and experimental data have established that IRI has both immediate and long-term effects on the allograft, contributing both to acute rejection and chronic allograft dysfunction [1]. However, at present there is no specific therapy available for prevention or for treatment of IRI.
The pathogenesis of IRI represents a complex interplay between biochemical, cellular, vascular endothelial and tissue specific factors, with inflammation being a common feature. There is considerable data implicating an important role for the innate immune response in IRI. According to the classic model, acute ischemia leads to production of oxygen free radicals, secretion of inflammatory cytokines/chemokines and activity of adherent molecules that are important initiators of the innate immune response which lead to tissue damage and endothelial cell activation [2–8]. Polymorphonuclear cells (PMNs) are the major leukocytes observed in tissue necrosis following ischemia. The neutrophil accumulation had been thought to be the prime cellular mediator of microvascular plugging and local tissue destruction in IRI. Monocyte/macrophage infiltration occurs later during IRI and likely contributes to extension of early injury as well as repair [9]. In addition, the role of the complement system in IRI has been firmly established, with activation demonstrated partially through the alternative pathway [10]. Both T and B cells constitute the primary arms of the adaptive immune response and were not felt to play a role in the acute phase of IRI. However, recent data have challenged this assumption and demonstrate an important modulatory role of T cells in IRI.
Evidence supporting a role of T cells as mediators in IRI
Numerous studies have identified T cells in both renal and non-renal organs after IRI. Initially considered as “innocent bystanders” in sites of inflammation, both indirect and direct evidence from several different animal models now support T cells may mediate in IRI. Indirect evidence in IRI came from studies of therapeutic agents targeting T cells. The calcineurin inhibitor, FK506, was found to attenuate experimental hepatic and mesenteric IRI in rats partially by reducing leukocyte adhesion [11, 12]. FK506 was also reported to reduce infarct size in rats after focal cerebral ischemia [13]. Neither rapamycin (which inhibits T cell activation), nor cyclosporine A (CsA) (a calcineurin inhibitor), was protective in the same experiment; both improved bowel IRI [14]. Mycophenolate mofetil (MMF), an anti-proliferative immunosuppressive agent, was demonstrated to be protective in IRI of cardiac transplantation, accompanied by decreased leukocyte infiltration [15]. Whereas some studies reported a protective effect of MMF in the kidney after experimental IRI [16, 17], one study found that MMF retarded repair due to toxic injury [18]. In more recent studies, a new immunosuppressant, FTY720 that modulates lymphocyte migration was found to attenuate renal and liver IRI in rodents [19–23]. FTY720 pretreatment resulted in improved renal function in association with reduced T cell infiltration in both cold and warm IRI models.
Reduced intragraft neutrophil infiltration was also observed with no change in expression of adhesion molecules. In the warm liver IRI model, the protection of FTY720 was partially attributed to the reduction of T lymphocyte infiltration and inhibition of MAPK (Raf-MEK-Erk) pathway [23]. However, FTY720 may have effects on endothelial cells that are independent of T cell function [24]. The activation of hepatocyte survival Akt signaling was also found in FTY720- treated normal and cirrhotic livers after IRI and may contribute to the improved liver function [21]. However, side effects of FTY720 have limited general use in the transplant population.
The protective effect of T cell costimulatory molecule blockade provides further indirect evidence for the role of T cells in the pathogenesis of IRI. Blockade of the T cell CD28-B7 costimulatory pathway with CTLA4Ig significantly attenuated renal dysfunction after cold IRI [25]. Anti-B7-1 antibody but not anti-B7-2, markedly reduced leukocyte accumulation in the kidney and improved renal function in rats after warm IRI, suggesting a predominant role of the B7-1 pathway in the protection of renal IRI through inhibition of T cell adherence and activation [26]. B7-1 expression was also identified on endothelial cells of the ascending vasa recta in postischemic human kidney (within 1 hour), suggesting a potential role of T cell co-stimulation in human renal IRI [8]. Recently, it has been reported that the CD154-CD40 T cell costimulatory pathway is active in the pathogenesis of hepatic and cerebral IRI [27, 28]. Gene therapy-mediated prolonged local CD154 blockade (Ad-CD40 Ig), antibody-induced systemic CD154 blockade (MR1 mAb), and genetically targeted CD154 absence (CD154 KO mice) all ameliorated liver injury after warm ischemia. This protection was accompanied by diminished intrahepatic T cell accumulation, decreased VEGF expression, reduced TNF type 1 cytokine production and inhibition of hepatocyte apoptosis.
It has been well established that leukocyte adhesion molecules, including CD11/CD18, ICAM-1, integrins and selectins play an important role in the initiation of IRI [3]. Studies have shown that blockade of CD11/CD18 and ICAM-1 protected experimental rodents from renal IRI, which was initially considered due to reduced infiltration of neutrophils [29, 30]. However, other groups subsequently demonstrated that induction of neutropenia did not lead to protection from IRI, suggesting that the interaction of T cells with these molecules may be operative [31]. P-selectin targeted treatment has been effective in liver and kidney models of IRI. A recent study demonstrated that P-selectin signaling had an important role in murine intestinal IRI in that either the blockade of or the genetic deficiency of P-selectin was protective. The protection was accompanied by a reduction of both T cell and PMN infiltration, and the induction of a Th2 dominant cytokine environment, implicating T cells as modulators in this inflammatory process [32].
T cells directly mediate IRI in liver (Table 1). The T cell deficient athymic nu/nu mice did not manifest warm hepatic IRI, but did so after adoptive transfer of wild type T cells[33]. Other researchers confirmed these results in nu/nu mice using a cold liver IRI model and found that donor pretreatment with IL-10 attenuated the liver injury induced by T cell reconstitution [34].
Table 1.
Direct evidence for a role of T cells in rodent IRI models
| Organ | Model | Finding | Ref. |
|---|---|---|---|
| Liver | IRI | Nu/nu mice & CD4 depleted mice protected; T cell transfer restores injury; CD4+ but not CD8+ T cell infiltration. | 33 |
| Liver | Cold IRI | Nu/nu mice protected; INF-γ and TNF-γ associated; IL-10 pre-treatment benefits. | 34 |
| Blood vessel | TNF-α treated | Reduced endothelial cell adhesion molecule expression in SCID & Rag KO mice, CD4+ but not CD8+ T cell transfer restores injury. | 35 |
| Gut, liver | IRI | SCID mice protected; T cell transfer restores injury, but not CD4-depleted T cells, or T cells from INF-γ KO mice. | 36 |
| Kidney | IRI | CD4/CD8 KO mice protected. | 37 |
| Kidney | IRI | Nu/nu mice protected from whole body IRI. | 38 |
| Kidney | IRI | T cell depletion (especially CD4+) protective. | 39 |
| Kidney | IRI | Nu/nu mice protected; CD4+ but not CD8+ T cell depletion protective; CD4+ T cell transfer restores injury, but not cells from IFN-γ or CD28 KO mice. | 40 |
| Kidney | IRI | CD4+ T cell infiltration in the late phase of IRI. | 41 |
| Liver | IRI | CD4+ T cell infiltration with binding of platelets; CD62P& CD4 deficiency, CD40/CD40L & B7/CD28 blockage protective. | 42 |
| Lung | Cold IRI | Nu/nu mice protected from syngeneic lung transplant; T cell transfer restores injury | 43 |
| Heart | IRI | RAG1 KO mice protected; CD4+ T cell transfer restores injury; A2AR activation protective by reducing CD4+ T cell infiltration. | 44 |
| Kidney | IRI | RAG1 KO mice protected; CD4+ T cell transfer restores injury; IFN-γ associated; A2AR activation protective. | 45 |
| Kidney | IRI | RAG1 KO mice protected; NKT cell transfer restores injury; IFN-γ associated; A2AR activation protective. | 46 |
| Liver | IRI | CD1d KO mice & nu/nu mice protected. | 48 |
| Kidney | IRI | TCR KO mice protected. | 60 |
Similar results were observed in severe combined immunodeficiency (SCID) mice. SCID mice were relatively resistant to liver IRI, while T cell adoptive transfer, particularly CD4+ T cells, restored the injury [35, 36]. T cells also directly mediate kidney IRI. Mice deficient in both CD4+ and CD8+ T cells had reduced functional and structural abnormalities after IRI [37]. Athymic nu/nu mice were also protected from renal IRI, and adoptive transfer of wild type T cells abolished these effects both in clamp and whole body IRI models [38,39]. Interestingly, neither neutrophil nor macrophage infiltration was changed in this model, suggesting a neutrophil/macrophage-independent effect of T cells in IRI.
Different roles of T cell subsets in IRI
Differential roles of CD4+ and CD8+ T cell subsets have been elucidated in various IRI models. In nu/nu mice, adoptive transfer of CD4+ but not CD8+ T cells restored injury in renal IRI [40]. To determine the effect of an intervention in wild type mice, T cell depleting antibodies to CD4 (GK1.5) and CD8 (2.43) were administered to mice prior to IRI. This combination depleted T cells but did not protect renal function after ischemia. However, the addition of a third antibody (30.H12) that targets Th1.2 antigen decreased T cells further, in particular the CD4+ subset, and led to renal protection [39]. In the late phase (6 weeks) of severe renal IRI, there was a marked infiltration of CD4+ T cells throughout the injured kidney, which may promote the development of chronic renal disease [41]. The predominant role of CD4+ T cells in IRI was observed in other organs. Depletion of CD4+ T cells but not CD8+ T cells reduced subacute injury and inflammation in mice after hepatic IRI [33]. Kinetic analysis of T cell infiltration in the liver in IRI mice demonstrated a marked accumulation of CD4+ T cells in the early phase of reperfusion (within 1 hour) with no change in the number of CD8+ T cells [33]. In a warm hepatic IRI rodent model, an accumulation and transendothelial migration of CD4+ but not CD8+ T cells 30% of which were colocalized with platelets was found during early reperfusion. CD62P and CD4 deficiency, as well as CD40-CD40L and CD28-B7 disruption, attenuated postischemic platelet adherence, suggesting a CD62P-mediated, costimulatory pathway-dependent effect of T cells on IRI [42]. In a syngeneic rat lung transplant model, recipient CD4+ T cell infiltration in the lung graft was observed within 1 hour of reperfusion followed by up-regulation of the T cell activation marker, CD25, over the ensuing 12 hours [43]. In vivo depletion of CD4+ but not CD8+ T cells significantly reduced infarct size in a heart IRI model [44]. Activation of the adenosine A2A receptor (A2AR) by CGS-21680 or ATL146e reduced IRI in liver, kidney and heart models [44–46]. Accumulation of CD4+ T cells in the heart after myocardial infarction was markedly reduced by ATL146e treatment. ATL146e administration also reduced injury after renal and heart ischemia in RAG1 KO mice reconstituted with wild type but not A2AR KO CD4+ T cells, implicating CD4+ T cells as the primary targets of A2AR agonists [44, 45]. CXCR3+CD4+ T cells were also demonstrated to play a critical role in the innate immune response in cold liver IRI model [47].
Recently, NKT cells were also reported to play an important role in liver IRI. NKT cells were found to be the major expander cell in the liver after IRI, and CD1d KO mice were protected from IRI [48]. The protective effect of A2AR activation in liver IRI was also associated with reduced activity of NKT cells [46].
IFN-γ-producing Th1 cells may be important in the pathogenesis of IRI. Increased serum or tissue IFN-γ level was observed in IRI animal models [45, 46, 49, 50], as well as in patients undergoing hepatic resection following intermittent portal clamping [51]. Flow cytometric analysis of infiltrated lymphocytes in ischemic kidney revealed increased IFN-γ and TNF-α producing T cells 24 hours after renal IRI [52]. Increased IFN-γ production was not only found in ischemic organs, but also in splenic T cells in the late phase of severe renal IRI without any change in CD4+ and CD8+ T cells numbers [41]. Adoptive transfer of splenic lymphocytes from mice with severe renal IRI induced albuminuria in naïve mice accompanied by migration of donor lymphocytes to recipient kidney and increase of activated and memory T cells in recipient spleen [53]. CD4+ T cells deficient in CD28 or IFN-γ did not restore ischemic injury in nu/nu mice, CD4 KO mice or RAG1 KO mice [40]. Furthermore, activation of A2AR on CD4+ T cells reduced IFN-γ production by 98%, while this inhibitory effect was blocked by 100% in CD4+ T cells from A2AR KO mice [54]. ATL146e significantly reduced plasma IFN-γ levels in mice with myocardial reperfusion injury (44). These data suggest that the protection of A2AR agonist in IRI may reflect suppression of IFN-γ production. STAT signaling is essential for the differentiation of Th1 (STAT4)/Th2 (STAT6) cells and their cytokine production. STAT6 deficient mice developed more severe renal injury after ischemia, while STAT4 deficient mice had mildly improved function, suggesting the Th2 cells protect against renal IRI [55]. STAT6 may also protect against liver IRI [56]. Recent data showed that IL-4 but not IL-12 deficient mice developed more severe renal injury after ischemia, also suggesting a protective effect of Th2 cells in IRI [57]. However, the significance of the Th1 and Th2 cytokine environment remains controversial, since both protective and deleterious effects of the Th2-type cytokine IL-10 has been reported in various IRI models [58, 59].
The role of the T cell receptor (TCR) in renal IRI was recently demonstrated. TCRαβ deficient mice had significantly improved renal function with reduced TNF-α and IL-6 production after IRI, while TCRγδ deficient mice only presented slight amelioration [60]. While initially another group failed to observe a role of TCR in renal IRI [61], the importance of TCR in kidney IRI was confirmed by careful knockout and antibody depletion studies [62].
Several recent studies have demonstrated a more complex role of T cells in IRI in that T cells appear to exhibit protective effects (Table 2). To explore the functional role of kidney-infiltrating lymphocytes after IRI, these cells were transferred into T cell deficient nu/nu mice at 24 hours after ischemia [52]. Unexpectedly, transfer of these cells led to attenuated renal dysfunction after IRI in the recipient mice, suggesting a protective effect of ischemic kidney infiltrating lymphocytes in IRI. Kinetic analysis of lymphocyte infiltration revealed a marked increase in T cell accumulation in post-ischemic kidney both in sham-operated and IRI mice 3 hours after renal IRI, that may represent the effects of laparotomy and anesthesia alone, but also a marked reduction 24 hours after surgery. T cell numbers in the kidney 24 hours after surgery were significantly lower in renal IRI mice compared with sham. These data suggest infiltrating T cells have a complex role in the different phases of IRI. The protective role of splenic lymphocytes 5 days after renal IRI was also demonstrated when transferred to wild type mice with IRI [63]. However, the particular cell type responsible for the aforementioned protective effects has not been identified yet.
Table 2.
Divergent role of lymphocytes in warm IRI models
| Organ | Finding | Ref. |
|---|---|---|
| Kidney | Transfer of ischemic kidney infiltrated lymphocytes into nu/nu mice protective. | 52 |
| Kidney | CD4 depletion or TCRα KO not protected. | 61 |
| Kidney | Transfer of splenic lymphocytes from IRI protective. | 63 |
| Liver | CD4 KO mice with greater IRI but less neutrophil infiltration. | 64 |
| Kidney | RAG1 KO mice not protected; Transfer of T or B cells protective. | 65 |
| Kidney | RAG1 KO mice not protected. | 66 |
CD4+ T cells have divergent functions in liver IRI [64]. CD4 KO mice had significantly greater liver injury but far less neutrophil infiltration. Adoptive transfer of CD4+ T cells restored the wild type response. The increased activation of liver infiltrating neutrophils was observed in CD4 KO mice, suggesting CD4+ T cells might suppress activation of neutrophils. Interestingly, RAG-1 KO mice with deficiency of both B and T cells were not protected from IRI, and in fact, appeared to have worse injury than wild type mice [65, 66]. However, adoptive transfer of either B or T cells into RAG-1 KO mice significantly improved the function of ischemic kidney without any change in neutrophil infiltration. The RAG-1 KO, although lacking mature T and B cells, can have an increase in other immune mediators such as NK cells, complement and macrophages that may promote early injury responses after IRI. Although numerous data have demonstrated a critical role of regulatory T cells in autoimmune disease, little is known so far as to whether these cells play a role in IRI. One preliminary study reported that depletion of CD4+CD25+ regulatory T cells by PC61 (anti-CD25) had little impact on the early phase of IRI, but led to higher necrosis indices 3d after IRI, compared with IgG-treated control mice. Thus, CD4+CD25+ cells may facilitate recovery from IRI [67].
Mechanisms of T cell activation in IRI
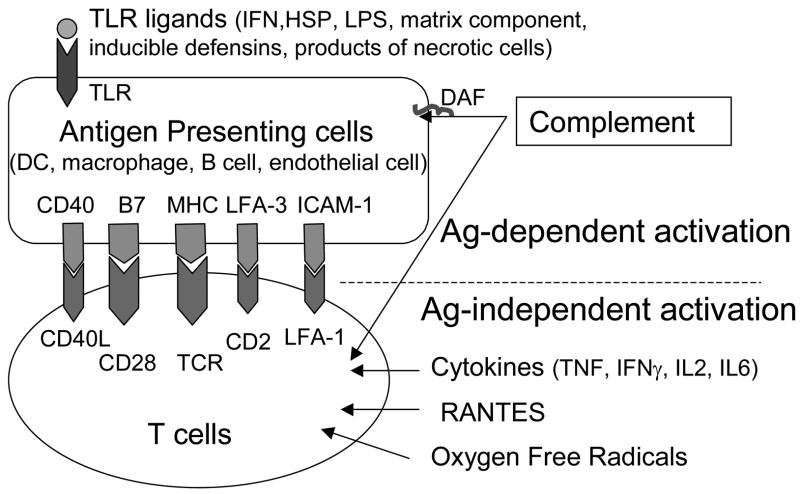
T cell activation involves antigen-dependent and -independent pathways (Figure 1) [2–8]. Antigen-independent T cells activation probably plays a pivotal role in IRI. Oxygen free radicals, regulated upon activation: normal T cell expressed/secreted (RANTES), and cytokines, including TNF-α, IFN-γ, IL-2 and IL-6 have all been demonstrated to activate T cells directly. Chemokines such as growth-related oncogene (GRO/CXCL1), monocyte chemoattractant protein (MCP1), macrophage inflammatory protein (MIP-1), and interferon-inducible protein 10 (IP-10) have also been implicated in the early inflammatory responses in transplanted organs.
Figure 1. Antigen-dependent and -independent T cell activation.
Two signals are provided by APCs to activate T cells, including antigen-specific signal received as a result of binding of the T cell receptor to peptide presented by MHC molecule, and the second signal provided by costimulatory molecules such as B7-1 (CD80), B7-2 (CD86) and CD40. T cells can also be directly activated through Ag-independent pathways by cytokines, RANTES and oxygen free radicals. Complement system serves both during innate immunity, as well as a mediator of adaptive immunity by directly activating APCs and T cells. In IRI, Ag-independent pathways play an important role in T cell activation. Moreover, several proteins induced by IRI, including HSP, matrix component, inducible defensins, products of necrotic cells, as well as cytokines can activate APCs through TLR contributing to the T cells proliferation. Dendritic cells, macrophages, B cells and endothelial cells are involved in Ag presentation to T cells.
Recently, toll-like receptor (TLR)-mediated pathways have been demonstrated to be activated by non-infectious stimuli in IRI. TLRs are primarily expressed in antigen presenting cells (APCs) and are also present on endothelial and stromal cells [68–70]. TLR4 deficient mice were protected from IRI of lung, liver, heart and brain, implicating the role of TLR4 in the initiation of IRI [71–75]. In the kidney, both TLR2 and TLR4 expression were increased after IRI [76], and TLR2 KO mice exhibited attenuated renal injury after IRI [77]. Heat shock proteins, as well as other molecules induced by IRI, including matrix components and inducible defensins, are putative agonists of TLRs [6, 68]. Activation of TLRs induces dendritic cell maturation leading to the proliferation and differentiation of T cells.
It is well known that the complement system plays an important role in IRI as a manifestation of innate immunity. Complement also regulates adaptive immune responses. C3a and C5a played opposing roles for T cells polarization in allergic asthma where CD3a promoted Th2 polarization while CD5a prevented a Th2 response [78]. In addition, CD46, a member of the complement regulatory receptor family, widely expressed on human cells [79], induced regulatory T cell function (IL-10 production) when cross-linking with CD3 on human CD4+ T cells. More recently, decay-accelerating factor (Daf, or CD55) was reported to regulate T cells responses through C3/C5 dependent pathway [80].
Dendritic cells and B cells in IRI
Dendritic cells (DCs) are the major professional antigen-presenting cells of the immune system, with the unique capacity to trigger na ve T cell responses. Recent studies have provided novel insights into the potential role of DCs in mediating immune responses in IRI. Increased numbers of DCs expressing a more mature phenotype were identified in ischemic kidney in the early phase (within 24 hours) after IRI, while these activated DCs with a strong capacity to induce T cell proliferation migrated from the kidney into the renal lymph node 1 day after IRI [81]. Accumulation of DCs in liver and kidney was observed within 1 hour and peaked at 24 hours after IRI [82]. Transplantation of a syngeneic renal graft also increased DC accumulation in the kidney [83]. DCs freshly isolated from liver after IRI exhibited a mature phenotype with an inhibitory profile of increased IL-10 and reduced IL-12 production [84].
Endothelial cell activation might be involved in DC trafficking, partially by production of chemokines and NOS, suggesting that endothelial dysfunction may accelerate DC-mediated immune activation [85]. Endothelial cells also express costimulatory molecules and can serve as APCs for T cell activation [86]. The important role of DCs in mediating immune responses in hepatic IRI was also reported in part by enhanced TLR4 reactivity [87]. TLRs are expressed abundantly on DCs and play a pivotal role in DC activation [68], which suggests a potential involvement of DC in the immune responses in IRI.
B cells may also participate in IRI. B cell deficient mice were relatively protected from renal IRI [88]. Transfer of serum from wild type mice restored the injury, suggesting a role of a soluble mediator such as antibody. However, not all B cell deficient mice are protected from IRI. As mentioned above, RAG-1 KO mice deficient in both B and T cells were not protected from IRI [65], and adoptive transfer of either B or T cells attenuated kidney injury. Only small numbers of B cells (4%~10% reconstitution) were sufficient to yield major changes in kidney injury. B cells might also be involved in tissue repair after acute injury [89]. These data suggest that immune cells mediate a contribution to the pathogenesis of IRI. How exactly immune cells do so warrants further investigation.
Conclusion
T cells contribute to the pathogenesis of IRI. Thus, the function of T cells extends beyond the conventional dogma. T cells function in IRI of kidney, liver, lung, brain and intestinal in both alloantigen-dependent and -independent tissue injury but also possibly in putative repair mechanisms. These discoveries open up the exciting possibility of harnessing immunotherapeutic agents previously developed for more classic immune diseases to test their efficacy in the prevention and treatment of IRI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tilney NL, Guttmann RD. Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation. 1997;64:945–947. doi: 10.1097/00007890-199710150-00001. [DOI] [PubMed] [Google Scholar]
- 2.Rabb H. The T cell as a bridge between innate and adaptive immune systems: Implication for the kidney. Kidney Int. 2002;61:1935–1946. doi: 10.1046/j.1523-1755.2002.00378.x. [DOI] [PubMed] [Google Scholar]
- 3.Burne-Taney MJ, Rabb H. The role of adhesion molecules and T cells in ischemic renal injury. Curr Opin Nephrol Hypertens. 2003;12:85–90. doi: 10.1097/00041552-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Kieran NE, Rabb H. Immune responses in kidney preservation and reperfusion injury. J Investig Med. 2004;52:310–314. doi: 10.1136/jim-52-05-30. [DOI] [PubMed] [Google Scholar]
- 5.Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–491. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- 6.Boros P, Bromberg JS. New cellular and molecular immune pathways in ischemia/reperfusion injury. Am J Transplant. 2006;6:652–658. doi: 10.1111/j.1600-6143.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- 7.Kupiec-Weglinski JW, Busuttil RW. Ischemia and reperfusion injury in liver transplantation. Transplant Proc. 2005;37:1653–1656. doi: 10.1016/j.transproceed.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 8.Ysebaert DK, De Greef KE, De Beuf A, Van Rompay AR, Vercauteren S, Persy VP, De Broe ME. T cells as mediators in renal ischemia/reperfusion injury. Kidney Int. 2004;66:491–496. doi: 10.1111/j.1523-1755.2004.761_4.x. [DOI] [PubMed] [Google Scholar]
- 9.Ysebaert DK, De Greef KE, Vercauteren SR, Ghielli M, Verpooten GA, Eyskens EJ, De Broe ME. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant. 2000;15:1562–1574. doi: 10.1093/ndt/15.10.1562. [DOI] [PubMed] [Google Scholar]
- 10.Seelen MA, Roos A, Daha MR. Role of complement in innate and autoimmunity. J Nephrol. 2005;18:642–53. [PubMed] [Google Scholar]
- 11.Garcia-Criado FJ, Lozano-Sanchez F, Fernandez-Regalado J, Valdunciel-Garcia JJ, Parreno-Manchado F, Silva-Benito I, Zambrano-Cuadrado Y, Gomez-Alonso A, Garcia-Criado FJ, Lozano-Sanchez F, Fernandez-Regalado J. Possible tacrolimus action mechanisms in its protector effects on ischemia-reperfusion injury. Transplantation. 1998;66:942–943. doi: 10.1097/00007890-199810150-00028. [DOI] [PubMed] [Google Scholar]
- 12.Yang B, Jain S, Pawluczyk IZ, Imtiaz S, Bowley L, Ashra SY, Nicholson ML. Inflammation and caspase activation in long-term renal ischemia/reperfusion injury and immunosuppression in rats. Kidney Int. 2005;68:2050–67. doi: 10.1111/j.1523-1755.2005.00662.x. [DOI] [PubMed] [Google Scholar]
- 13.Sharkey J, Butcher SP. Immunophilins mediate the neuroprotective effects of FK506 in focal cerebral ischaemia. Nature. 1994;371:336–339. doi: 10.1038/371336a0. [DOI] [PubMed] [Google Scholar]
- 14.Puglisi RN, Strande L, Santos M, Schulte G, Hewitt CW, Whalen TV. Beneficial effects of cyclosporine and rapamycin in small bowel ischemic injury. J Surg Res. 1996;65:115–118. doi: 10.1006/jsre.1996.0352. [DOI] [PubMed] [Google Scholar]
- 15.Ysebaert DK, De Greef KE, Vercauteren SR, Verhulst A, Kockx M, Verpooten GA, De Broe ME. Effect of immunosuppression on damage, leukocyte infiltration, and regeneration after severe warm ischemia/reperfusion renal injury. Kidney Int. 2003;64:864–873. doi: 10.1046/j.1523-1755.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- 16.Valentin JF, Bruijn JA, Paul LC. Donor treatment with mycophenolate mofetil: protection against ischemia-reperfusion injury in the rat. Transplantation. 2000;69:344–350. doi: 10.1097/00007890-200002150-00006. [DOI] [PubMed] [Google Scholar]
- 17.Ventura CG, Coimbra TM, de Campos SB, de Castro I, Yu L, Seguro AC. Mycophenolate mofetil attenuates renal ischemia/reperfusion injury. J Am Soc Nephrol. 2002;13:2524–2533. doi: 10.1097/01.asn.0000030143.73830.3c. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez N, Alvarez V, Pons H, Parra G, Quiroz Y, Rodriguez-Iturbe B. Mycophenolate mofetil aggravates postischemic acute renal failure in rats. Transplant Proc. 2002;34:43–44. doi: 10.1016/s0041-1345(01)02658-6. [DOI] [PubMed] [Google Scholar]
- 19.Kaudel CP, Schmiddem U, Frink M, Bergmann S, Pape HC, Krettek C, Klempnauer J, Winkler M. FTY720 for treatment of ischemia-reperfusion injury following complete renal ischemia in C57/BL6 mice. Transplant Proc. 2006;38:679–681. doi: 10.1016/j.transproceed.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 20.Suleiman M, Cury PM, Pestana JO, Burdmann EA, Bueno V. FTY720 prevents renal T-cell infiltration after ischemia/reperfusion injury. Transplant Proc. 2005;37:373–374. doi: 10.1016/j.transproceed.2004.12.280. [DOI] [PubMed] [Google Scholar]
- 21.Man K, Ng KT, Lee TK, Lo CM, Sun CK, Li XL, Zhao Y, Ho JW, Fan ST. FTY720 attenuates hepatic ischemia-reperfusion injury in normal and cirrhotic livers. Am J Transplant. 2005;5:40–49. doi: 10.1111/j.1600-6143.2004.00642.x. [DOI] [PubMed] [Google Scholar]
- 22.Dragun D, Bohler T, Nieminen-Kelha M, Waiser J, Schneider W, Haller H, Luft FC, Budde K, Neumayer HH. FTY720-induced lymphocyte homing modulates post-transplant preservation/reperfusion injury. Kidney Int. 2004;65:1076–1083. doi: 10.1111/j.1523-1755.2004.00478.x. [DOI] [PubMed] [Google Scholar]
- 23.Anselmo DM, Amersi FF, Shen XD, Gao F, Katori M, Lassman C, Ke B, Coito AJ, Ma J, Brinkmann V, Busuttil RW, Kupiec-Weglinski JW, Farmer DG. FTY720 pretreatment reduces warm hepatic ischemia reperfusion injury through inhibition of T-lymphocyte infiltration. Am J Transplant. 2002;2:843–849. doi: 10.1034/j.1600-6143.2002.20906.x. [DOI] [PubMed] [Google Scholar]
- 24.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 25.Takada M, Chandraker A, Nadeau KC, Sayegh MH, Tilney NL. The role of the B7 costimulatory pathway in experimental cold ischemia/reperfusion injury. J Clin Invest. 1997;100:1199–1203. doi: 10.1172/JCI119632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Greef KE, Ysebaert DK, Dauwe S, Persy V, Vercauteren SR, Mey D, De Broe ME. Anti-B7-1 blocks mononuclear cell adherence in vasa recta after ischemia. Kidney Int. 2001;60:1415–1427. doi: 10.1046/j.1523-1755.2001.00944.x. [DOI] [PubMed] [Google Scholar]
- 27.Ke B, Shen XD, Gao F, Tsuchihashi S, Farmer DG, Briscoe D, Busuttil RW, Kupiec-Weglinski JW. The CD154-CD40 T-cell co-stimulation pathway in liver ischemia and reperfusion inflammatory responses. Transplantation. 2005;79:1078–1083. doi: 10.1097/01.tp.0000161248.43481.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishikawa M, Vowinkel T, Stokes KY, Arumugam TV, Yilmaz G, Nanda A, Granger DN. CD40/CD40 ligand signaling in mouse cerebral microvasculature after focal ischemia/reperfusion. Circulation. 2005;111:1690–1696. doi: 10.1161/01.CIR.0000160349.42665.0C. [DOI] [PubMed] [Google Scholar]
- 29.Rabb H, Mendiola CC, Dietz J, Saba SR, Issekutz TB, Abanilla F, Bonventre JV, Ramirez G. Role of CD11a and CD11b in ischemic acute renal failure in rats. Am J Physiol. 1994;267:F1052–1058. doi: 10.1152/ajprenal.1994.267.6.F1052. [DOI] [PubMed] [Google Scholar]
- 30.Rabb H, O’Meara YM, Maderna P, Coleman P, Brady HR. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. 1997;51:1463–1468. doi: 10.1038/ki.1997.200. [DOI] [PubMed] [Google Scholar]
- 31.Beray-Berthat V, Palmier B, Plotkine M, Margaill I. Neutrophils do not contribute to infarction, oxidative stress, and NO synthase activity in severe brain ischemia. Exp Neurol. 2003;182:446–54. doi: 10.1016/s0014-4886(03)00106-7. [DOI] [PubMed] [Google Scholar]
- 32.Farmer DG, Anselmo D, Da Shen X, Ke B, Carmody IC, Gao F, Lassman C, McDiarmid SV, Shaw G, Busuttil RW, Kupiec-Weglinski JW. Disruption of P-selectin signaling modulates cell trafficking and results in improved outcomes after mouse warm intestinal ischemia and reperfusion injury. Transplantation. 2005;80:828–835. doi: 10.1097/01.tp.0000174337.53658.b0. [DOI] [PubMed] [Google Scholar]
- 33.Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4+ T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Moine O, Louis H, Demols A, Desalle F, Demoor F, Quertinmont E, Goldman M, Deviere J. Cold liver ischemia-reperfusion injury critically depends on liver T cells and is improved by donor pretreatment with interleukin 10 in mice. Hepatology. 2000;31:1266–1274. doi: 10.1053/jhep.2000.7881. [DOI] [PubMed] [Google Scholar]
- 35.Horie Y, Chervenak RP, Wolf R, Gerritsen ME, Anderso DC, Komatsu S, Granger DN. Lymphocytes mediate TNF-alpha-induced endothelial cell adhesion molecule expression: studies on SCID and RAG-1 mutant mice. J Immunol. 1997;159:5053–5062. [PubMed] [Google Scholar]
- 36.Horie Y, Wolf R, Chervenak RP, Jennings SR, Granger DN. T-lymphocytes contribute to hepatic leukostasis and hypoxic stress induced by gut ischemia-reperfusion. Microcirculation. 1999;6:267–280. [PubMed] [Google Scholar]
- 37.Rabb H, Daniels F, O’Donnell M, Haq M, Saba SR, Keane W, Tang WW. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2000;279:F525–531. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- 38.Burne-Taney MJ, Kofler J, Yokota N, Weisfeldt M, Traystman RJ, Rabb H. Acute renal failure after whole body ischemia is characterized by inflammation and T cell-mediated injury. Am J Physiol Renal Physiol. 2003;285:F87–94. doi: 10.1152/ajprenal.00026.2003. [DOI] [PubMed] [Google Scholar]
- 39.Yokota N, Daniels F, Crosson J, Rabb H. Protective effect of T cell depletion in murine renal ischemia-reperfusion injury. Transplantation. 2002;74:759–763. doi: 10.1097/00007890-200209270-00005. [DOI] [PubMed] [Google Scholar]
- 40.Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O’Donnell MP, Rabb H. Identification of the CD4+ T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest. 2001;108:1283–1290. doi: 10.1172/JCI12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burne-Taney MJ, Yokota N, Rabb H. Persistent renal and extrarenal immune changes after severe ischemic injury. Kidney Int. 2005;67:1002–1009. doi: 10.1111/j.1523-1755.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- 42.Khandoga A, Hanschen M, Kessler JS, Krombach F. CD4+ T cells contribute to postischemic liver injury in mice by interacting with sinusoidal endothelium and platelets. Hepatology. 2006;43:306–315. doi: 10.1002/hep.21017. [DOI] [PubMed] [Google Scholar]
- 43.de Perrot M, Young K, Imai Y, Liu M, Waddell TK, Fischer S, Zhang L, Keshavjee S. Recipient T cells mediate reperfusion injury after lung transplantation in the rat. J Immunol. 2003;171:4995–5002. doi: 10.4049/jimmunol.171.10.4995. [DOI] [PubMed] [Google Scholar]
- 44.Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, French BA, Linden J. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056–2064. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- 45.Day YJ, Huang L, Ye H, Li L, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J Immunol. 2006;176:3108–3114. doi: 10.4049/jimmunol.176.5.3108. [DOI] [PubMed] [Google Scholar]
- 46.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhai Y, Shen XD, Hancock WW, Gao F, Qiao B, Lassman C, Belperio JA, Strieter RM, Busuttil RW, Kupiec-Weglinski JW. CXCR3+CD4+ T cells mediate innate immune function in the pathophysiology of liver ischemia/reperfusion injury. J Immunol. 2006;176:6313–6322. doi: 10.4049/jimmunol.176.10.6313. [DOI] [PubMed] [Google Scholar]
- 48.Shimamura K, Kawamura H, Nagura T, Kato T, Naito T, Kameyama H, Hatakeyama K, Abo T. Association of NKT cells and granulocytes with liver injury after reperfusion of the portal vein. Cell Immunol. 2005;234:31–38. doi: 10.1016/j.cellimm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 49.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 50.Braun F, Hosseini M, Wieland E, Sattler B, Muller AR, Fandrich F, Kremer B, Ringe B. Kinetics and localization of interleukin-2, interleukin-6, heat shock protein 70, and interferon gamma during intestinal-reperfusion injury. Transplant Proc. 2004;36:267–269. doi: 10.1016/j.transproceed.2004.01.082. [DOI] [PubMed] [Google Scholar]
- 51.Pulitano C, Sitia G, Aldrighetti L, Finazzi R, Arru M, Catena M, Guidotti LG, Ferla G. Reduced severity of liver ischemia/reperfusion injury following hepatic resection in humans is associated with enhanced intrahepatic expression of Th2 cytokines. Hepatol Res. 2006;36:20–26. doi: 10.1016/j.hepres.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Ascon DB, Lopez-Briones S, Liu M, Ascon M, Savransky V, Colvin RB, Soloski MJ, Rabb H. Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J Immunol. 2006;177:3380–3387. doi: 10.4049/jimmunol.177.5.3380. [DOI] [PubMed] [Google Scholar]
- 53.Burne-Taney MJ, Liu M, Ascon D, Molls RR, Racusen L, Rabb H. Transfer of lymphocytes from mice with renal ischemia can induce albuminuria in naive mice: a possible mechanism linking early injury and progressive renal disease? Am J Physiol Renal Physiol. 2006;291:F981–986. doi: 10.1152/ajprenal.00229.2005. [DOI] [PubMed] [Google Scholar]
- 54.Lappas MC, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 55.Yokota N, Burne-Taney M, Racusen L, Rabb H. Contrasting roles for STAT4 and STAT6 signal transduction pathways in murine renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2003;285:F319–325. doi: 10.1152/ajprenal.00432.2002. [DOI] [PubMed] [Google Scholar]
- 56.Shen XD, Ke B, Zhai Y, Gao F, Anselmo D, Lassman CR, Busuttil RW, Kupiec-Weglinski JW. Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology. 2003;37:296–303. doi: 10.1053/jhep.2003.50066. [DOI] [PubMed] [Google Scholar]
- 57.Marques VP, Goncalves GM, Feitoza CQ, Cenedeze MA, Fernandes Bertocchi AP, Damiao MJ, Pinheiro HS, Antunes Teixeira VP, dos Reis MA, Pacheco-Silva A, Saraiva Camara NO. Influence of TH1/TH2 switched immune response on renal ischemia-reperfusion injury. Nephron Exp Nephrol. 2006;104:e48–56. doi: 10.1159/000093676. [DOI] [PubMed] [Google Scholar]
- 58.Nussler NC, Muller AR, Weidenbach H, Vergopoulos A, Platz KP, Volk HD, Neuhaus P, Nussler AK. IL-10 increases tissue injury after selective intestinal ischemia/reperfusion. Ann Surg. 2003;238:49–58. doi: 10.1097/01.sla.0000074962.26074.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60:2118–2128. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 60.Savransky V, Molls RR, Burne-Taney M, Chien CC, Racusen L, Rabb H. Role of the T-cell receptor in kidney ischemia-reperfusion injury. Kidney Int. 2006;69:233–238. doi: 10.1038/sj.ki.5000038. [DOI] [PubMed] [Google Scholar]
- 61.Faubel S, Ljubanovic D, Poole B, Dursun B, He Z, Cushing S, Somerset H, Gill RG, Edelstein CL. Peripheral CD4 T-cell depletion is not sufficient to prevent ischemic acute renal failure. Transplantation. 2005;80:643–649. doi: 10.1097/01.tp.0000173396.07368.55. [DOI] [PubMed] [Google Scholar]
- 62.Hochegger K, Schätz T, Tagwerker A, Heininger D, Mayer G, Rosenkranze AR. Regulatory interaction of α/β- and γ/δ-T cells in renal ischemia reperfusion injury. J Am Soc Nephrol. 2006;17:709A. [Abstract] [Google Scholar]
- 63.Burne-Taney MJ, Liu M, Baldwin WM, Racusen L, Rabb H. Decreased capacity of immune cells to cause tissue injury mediates kidney ischemic preconditioning. J Immunol. 2006;176:7015–7020. doi: 10.4049/jimmunol.176.11.7015. [DOI] [PubMed] [Google Scholar]
- 64.Caldwell CC, Okaya T, Martignoni A, Husted T, Schuster R, Lentsch AB. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2005;289:G969–976. doi: 10.1152/ajpgi.00223.2005. [DOI] [PubMed] [Google Scholar]
- 65.Burne-Taney MJ, Yokota-Ikeda N, Rabb H. Effects of combined T- and B-cell deficiency on murine ischemia reperfusion injury. Am J Transplant. 2005;5:1186–1193. doi: 10.1111/j.1600-6143.2005.00815.x. [DOI] [PubMed] [Google Scholar]
- 66.Park P, Haas M, Cunningham PN, Bao L, Alexander JJ, Quigg RJ. Injury in renal ischemia-reperfusion is independent from immunoglobulins and T lymphocytes. Am J Physiol Renal Physiol. 2002;282:F352–357. doi: 10.1152/ajprenal.00160.2001. [DOI] [PubMed] [Google Scholar]
- 67.Midory R, Camara NOS, Cenedeze M, Rodrigues MM, Pacheco-Silva A. Regulatory T cells (CD4+CD25+) in kidney ischemia reperfusion injury. Transplantation. 2006;82:1042–1043. [Abstract] [Google Scholar]
- 68.Tsan MF, Gao B. Endogenous ligands of Toll like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 69.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 70.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 71.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 72.Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353:509–514. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 73.Shimamoto A, Pohlman TH, Shomura S, Tarukawa T, Takao M, Shimpo H. Toll-like receptor 4 mediates lung ischemia-reperfusion injury. Ann Thorac Surg. 2006;82:2017–2023. doi: 10.1016/j.athoracsur.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 74.Wu HS, Zhang JX, Wang L, Tian Y, Wang H, Rotstein O. Toll-like receptor 4 involvement in hepatic ischemia/reperfusion injury in mice. Hepatobiliary Pancreat Dis Int. 2004;3:250–253. [PubMed] [Google Scholar]
- 75.Stapel H, Kim SC, Osterkamp S, Knuefermann P, Hoeft A, Meyer R, Grohe C, Baumgarten G. Toll-like receptor 4 modulates myocardial ischaemia-reperfusion injury: Role of matrix metalloproteinases. Eur J Heart Fail. 2006;8:665–672. doi: 10.1016/j.ejheart.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 76.Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, Han SW, Kim J, Yang CW. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation. 2005;79:1370–1377. doi: 10.1097/01.tp.0000158355.83327.62. [DOI] [PubMed] [Google Scholar]
- 77.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Free in PMC Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hawlisch H, Wills-Karp M, Karp CL, Kohl J. The anaphylatoxins bridge innate and adaptive immune responses in allergic asthma. Mol Immunol. 2004;41:123–131. doi: 10.1016/j.molimm.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 79.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 80.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, Xu Y, Medof ME. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201:1523–1530. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD. Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int. 2005;68:1096–1108. doi: 10.1111/j.1523-1755.2005.00502.x. [DOI] [PubMed] [Google Scholar]
- 82.Zhou T, Sun GZ, Zhang MJ, Chen JL, Zhang DQ, Hu QS, Chen YY, Chen N. Role of adhesion molecules and dendritic cells in rat hepatic/renal ischemia-reperfusion injury and anti-adhesive intervention with anti-P-selectin lectin-EGF domain monoclonal antibody. World J Gastroenterol. 2005;11:1005–1010. doi: 10.3748/wjg.v11.i7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Penfield JG, Dawidson IA, Ar’Rajab A, Kielar MA, Jeyarajah DR, Lu CY. Syngeneic renal transplantation increases the number of renal dendritic cells in the rat. Transpl Immunol. 1999;7:197–200. doi: 10.1016/s0966-3274(99)80002-1. [DOI] [PubMed] [Google Scholar]
- 84.Loi P, Paulart F, Pajak B, Nagy N, Salmon I, Moser M, Goldman M, Flamand V. The fate of dendritic cells in a mouse model of liver ischemia/reperfusion injury. Transplant Proc. 2004;36:1275–1279. doi: 10.1016/j.transproceed.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 85.Schlichting CL, Schareck WD, Weis M. Renal ischemia-reperfusion injury: new implications of dendritic cell-endothelial cell interactions. Transplant, Proc. 2006;38:670–673. doi: 10.1016/j.transproceed.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 86.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 87.Tsung A, Zheng N, Jeyabalan G, Izuishi K, Klune JR, Geller DA, Lotze MT, Lu L, Billiar TR. Increasing numbers of hepatic dendritic cells promote HMGB1-mediated ischemia-reperfusion injury. J Leukoc Biol. 2007;81:119–128. doi: 10.1189/jlb.0706468. [DOI] [PubMed] [Google Scholar]
- 88.Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H. B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol. 2003;171:3210–3215. doi: 10.4049/jimmunol.171.6.3210. [DOI] [PubMed] [Google Scholar]
- 89.Novobrantseva TI, Majeau GR, Amatucci A, Kogan S, Brenner I, Casola S, Shlomchik MJ, Koteliansky V, Hochman PS, Ibraghimov A. Attenuated liver fibrosis in the absence of B cells. J Clin Invest. 2005;115:3072–3082. doi: 10.1172/JCI24798. [DOI] [PMC free article] [PubMed] [Google Scholar]