Abstract
Sharks can sense bioelectric fields of prey and other animals in seawater using an extraordinary system of sense organs (ampullae of Lorenzini) [9]. A recent study reported that these sense organs also enable sharks to locate prey-rich thermal fronts using a novel mode of temperature reception without ion channels. The study reported that gel extracted from the organs operates as a thermoelectric semiconductor, generating electricity when it is heated or cooled [2]. Here we report biophysical studies that call into question this mechanism of sensory transduction. Our experiments indicate that the material exhibits no unusual thermoelectric or electromechanical properties, and that the thermoelectric response is an artifact caused by temperature effects on the measurement electrodes. No response is seen when non-metallic electrodes (carbon or salt bridges) are used, and ordinary seawater produces the same effect as shark organ gel when silver wire electrodes are used. These data are consistent with the voltages arising from electrochemical electrode potentials rather generated intrinsically within the sample. This new evidence, together with the anatomy of the organs and behavioral studies in the literature, best support the conclusion that the biological function of these sense organs is to detect electric fields.
Keywords: Ampullae of Lorenzini, electroreception, temperature receptors, sharks, rays, chimaeras, thermoelectric semiconduction, sensory reception, lateral line, electrode potential, sensory transduction, ion channels
Introduction
Peculiar structures on the head of sharks, rays, and chimaeras have been the source of scientific controversy since their discovery 350 years ago. In 1678 Stefano Lorenzini tentatively proposed that the conspicuous pores on the head of these animals were mucus ducts (Fig. 1a), but he immediately cast doubt on his own conclusion, writing that their unusual anatomy made him suspect that "they are intended for another, more hidden function" [19]. An astonishing range of sensory functions have been ascribed to these organs (ampullae of Lorenzini), including sensory receptors for touch [20], pressure [20], salinity [21], temperature [23], electric [10, 16, 21] and magnetic fields [18]. The most recent studies report that gel extracted from these organs is a thermoelectric semiconductor, enabling sharks to locate prey-rich thermal fronts using a novel mechanism of sensory transduction without ion channels [2]. This thermosensory function contradicts the accepted view that ampullae of Lorenzini are organs for detecting weak electric fields generated by many physical and biological processes in the marine environment [17]. Here we analyze biophysical properties of the gel inside these sense organs in light of this controversy.
Fig. 1.
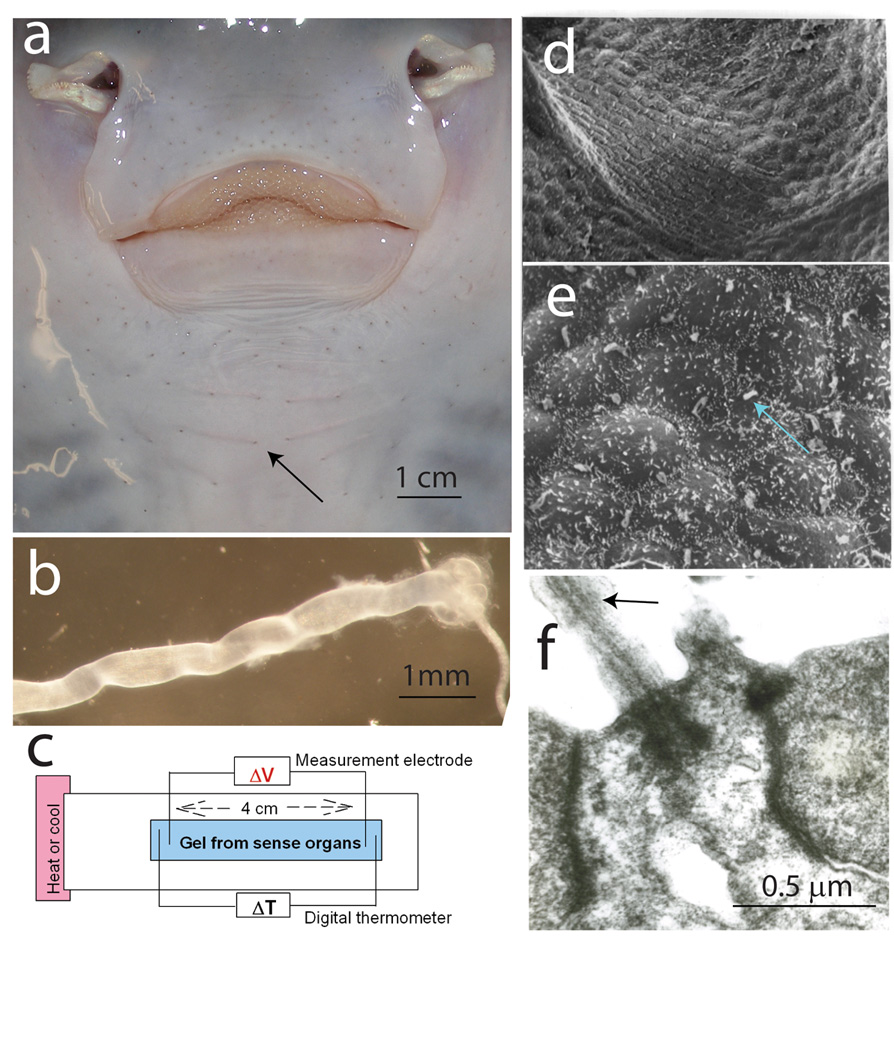
Ampullae of Lorenzini [9] are sense organs on the head of sharks [21], rays [5, 12], and chimaeras [10], containing a gel reported to have unique thermoelectric semiconductor properties [2]. (a) Visible as small pores around the oral surface of a skate (Raja erinacea) (arrow), the tubular organs, with an alveolus-shaped ending containing sense cells (b), are filled with an electrically conductive gel [24]. (c) Changes in voltage between two silver wire electrodes inserted in gel extracted from the organs were measured when a temperature gradient was imposed by heating or cooling one end of the apparatus. (d) Scanning electron microscopy of the sensory epithelium shows two types of cells: (e) sense cells, bearing a non-motile cilium (arrow), and mound-shaped support cells. (f) A cilium shown by transmission electron microscopy. (d–f) from Hydrolagus colliei. Previous studies show no ultrastructural differences in the sensory epithelium of chimaeras and elasmobranchs [11–14].
Each pore in the skin (Fig. 1a) leads through a gel-filled tube, ending in an alveolus-shaped sensory ending (Fig. 1b). Sense cells in the ampulla (Fig. 1d,e) bear a single non-motile cilium (Fig. 1e,f) and form synapses with the anterior lateral line nerve (Fig. 1b) [13]. Minute voltages (<0.05 µV/cm) applied to the openings alter impulse firing [13, 21] and evoke responses in medulla and midbrain [1, 13]. The gel inside the tubes has a similar ionic composition to seawater and it was previously thought to serve as a passive electrical conductor, carrying the voltage to sense cells in the alveolus ending [23].
This was challenged by Brown [2], who concluded that the gel generates electricity in response to temperature changes. This was supported by measurements of voltage changes between two silver wires inserted into gel extracted from the organs when one end of the sample holder was heated or cooled (Fig. 1c).
Materials and Methods
Thermoelectric response
Ampullae of Lorenzini were dissected from the ventral region adjacent to the first gill slit of skates, Raja erinacea, following anesthesia by MS222 and decapitation. Gel was extracted from several organs and placed on a glass slide in contact with electrodes separated by 40 mm. A 1 mm thick layer of gel was sealed beneath a 50 mm × 24 mm # 1 coverslip and miniature thermocouple sensors near each electrode recorded temperature changes. One end of the apparatus was heated or cooled using a piezoelectric temperature controller (Harvard Apparatus, TC-202A, Greenvail NY), creating a temperature gradient across the gel with respect to the opposite end held at ambient temperature. In some experiments, an electrical heating element was brought near one end of the slide to duplicate the method used by Brown [2], or ice was used to cool one end. All of these methods produced similar responses. Carbon fiber, silver wire, and salt bridge electrodes were used for recording. Salt bridges were made by filling fine 5 cm long plastic tubes with seawater in 2% agar. Electrical contact was made with the seawater/agar using silver wires. Electrical potentials between the electrodes in the gel were amplified using an Axoclamp 2B microelectrode amplifier (Axon Instruments, Foster City, CA) with DC-10 KHz bandwidth, using an HSA 1XLU headstage.
Temperature-dependent displacement
Temperature-dependent changes in gel volume were measured using an MTI-2000, Fotonic Sensor (MTI, Instruments, Inc., Albany, NY). The device uses a miniature fiber optic probe to illuminate and record light reflected from the sample. Calibrating the device according to manufacturer instructions indicated sensitivity to displacements of 0.0147 µm/mV. A piece of thin mylar was placed on the gel to enhance reflectance to the sensor.
Voltage-dependent changes in volume
Possible swelling or contraction of the gel in response to applied voltages was tested using the MTI-2000 Fotonic Sensor (above). Voltages up to 0.1V, DC-10Hz (square wave), and ramped voltage changes of both positive and negative polarities were tested. A square-wave stimulator was used to apply DC or voltage pulses through a 100:1 voltage divider, and voltage ramps were delivered using Clampex software (described below). Silver stimulus electrodes were used routinely to apply voltages to the gel, but carbon and salt bridge electrodes were also tested. These experiments were performed on samples of gel extracted from the organs and on intact organs to test if a possible voltage-dependent volume change was disrupted by extracting the gel. (No differences were observed.) Measurements were made on the ampullar region of the organ in response to voltages applied inside the canal of the ampullae of Lorenzini using a microelectrode, and on intact organs sectioned through the ampullar region of the organ, with the light beam from the Fotonic Sensor reflected off the gel inside intact organs. Stimulation of organs suspended in Ringer solution with electric fields applied to the solutions was also used to eliminate inserting electrodes into the canal.
A Michelson interferometer was also used to monitor changes in displacement or index of refraction of the gel in response to applied electric fields. A helium-neon laser (633nm wavelength) was used for illumination on an airtable. A sample of the gel was placed in one arm of the interferometer, and changes in intensity of interference bands were monitored with a photodiode at the point where the sample beam and reference beam converged through a 50 % beam splitter. The gel sample was held in the sample beam on a glass slide covered with a 24 × 24 mm #1 coverslip, with voltages applied to the gel with silver or carbon electrodes.
Recording and analysis
Voltage, temperature, displacement, and light intensity from the photodiode in interferometry, were digitized with a Digidata 1322A A/D converter (Axon Instruments, Foster City, CA), and displayed on a Gould 740 digital display oscilloscope. Clampex 8.2 software (Axon Instruments, Foster City, CA) was used for recording and analysis, and for delivering ramp voltages to the gel in the piezoelectric experiments. Results are reported as mean ± SEM, and statistical analysis was by ANOVA performed using Minitab 11 software.
Electron microscopy
Hydrolagus colliei were anesthetized with MS222 and fixed by cardiac perfusion using 2% glutaraldehyde in 0.1M cacodylate, pH 7.35 [12]. Samples were embedded in Epon/Araldite for transmission microscopy, stained with lead citrate and uranyl acetate. For scanning electron microscopy, samples were dried by critical point drying with liquid CO2 and coated with carbon and gold.
Results
Using the same methods that showed the thermoelectric semiconductor properties of the gel extracted from ampullae of Lorenzini (Fig. 1c), we confirm the widely varying standing potential, reported as varying between − 8 mV to + 8 mV [2], which changed when a temperature gradient was imposed on the gel (Fig. 2a). The voltage was sensitive to small changes in temperature, −281 ± 81.9 µV/ °C, (mean & SEM; n = 6), which is similar to the 370 and 240 µV/°C values reported by Brown [2]. (The sign of the response was erroneously reported as positive in the study published in Nature [2], but the data plotted in that paper have a negative slope.) Hysteresis to heating and cooling was observed (Fig. 2b), which varied with the rate of temperature change, as described previously [2].
Fig. 2.
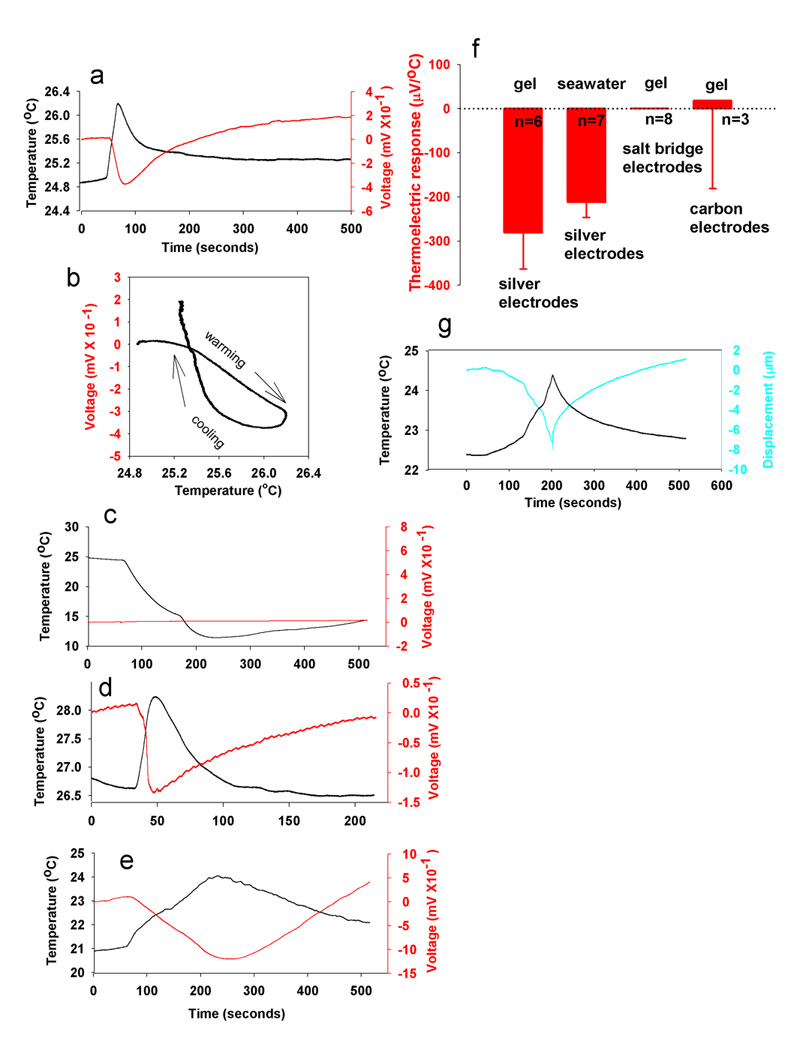
The reported thermoelectric semiconductor properties of gel in ampullae of Lorenzini [2] are measurement artifacts arising from electrochemical reactions at the measurement electrodes. (a) Thermal gradients changed the voltage between two silver electrodes in the gel. (b) The thermoelectric response was sensitive to small temperature changes and showed hysteresis. (c) No thermoelectric response was detected when nonmetallic measurement electrodes (salt bridges) were used. (d) Heating or cooling the point where the silver wire contacts the salt bridge mimicked the thermoelectric response, even though this does not cause a temperature gradient in the gel. (e) The thermoelectric response can be measured with silver electrodes in ordinary seawater with 2% agar. (f) The thermoelectric response (maximal slope from voltage/temperature plots), is not significantly different in seawater and extracellular gel when measured with silver electrodes. (g) Temperature-dependent changes in gel viscosity can be measured as swelling and shrinking of the gel beneath a photonic displacement sensor (MTI 2000), but this is not likely to be a biologically relevant mode of thermoreception as similar changes are seen in seawater/agar.
Classically, semiconduction results from quantum mechanical thermodynamics of electrons in atoms of solid state materials [6, 8], but the gel from the ampullae of Lorenzini is an electrolyte. In electrolytes, electricity is typically conducted by dissolved ions, and a voltage would develop from electrochemical reactions between silver wire and electrolytes in the gel. This voltage would depend on temperature, in accordance with the Nernst equation, and a potential difference would necessarily develop between two silver electrodes at different temperature immersed in an electrolyte containing chloride ions.
To determine whether the voltage response was a thermoelectric property of the gel or instead was generated by electrochemical action at the metal electrodes, we performed the same experiment using non-metallic measurement electrodes. We found that when carbon or salt bridge electrodes were used a thermoelectric effect was not measurable, even in response to extreme temperature changes (Fig. 2c). Salt bridge electrodes are slender tubes filled with seawater in 2% agar placed in electrical contact with the extracellular gel. Silver wires connect to the distal end of the salt bridge tube, isolating them from the thermal gradient in the sample. However, heating or cooling the point near the salt bridge where the silver wire contacts it mimicked the thermoelectric response, even though this does not cause a temperature gradient in the gel (Fig. 2d). This is consistent with the temperature-dependent voltages arising from electrochemical reaction between the silver wire and electrolytes in seawater, rather than arising intrinsically from within the sample.
Finally, similar thermoelectric responses to those previously reported in shark sense organ gel were measured with silver electrodes when the gel sample was replaced with plain seawater in 2% agar (Fig. 2e). The values in seawater (−212 ± 34.5 µV/°C) and gel samples (−281 ± 81.9µV/°C) were not significantly different (p>0.47, n = 13). This indicates that the gel inside the ampullae of Lorenzini lacks any remarkable thermoelectric semiconductor property beyond that of normal seawater (Fig. 2f).
The mechanism of sensory transduction in the sense cells of the ampullae of Lorenzini is known to involve calcium-activated potassium channels in the apical membrane [5]. The sensitivity of the organs to nanovolt/cm gradients is remarkable, and we also explored an alternative hypotheses that the gel might transduce thermal or electrical signals by swelling or shrinking, and thus displace cilia on the sense cells (Fig. 1e,f). Displacement of the cilium might activate mechanosensitive ion channels in the sense cell in response to pressure applied to or generated by the gel. No displacement of the gel was detected in response to applied voltages, using the photonic displacement sensor or Michelson interferometry with laser illumination at 633 nm. Ramped and pulsed voltages were tested at various frequencies up to the extreme of 0.1V, well beyond the range of electroreception.
Temperature-dependent changes in gel volume were also measured using a photonic displacement sensor (Fig. 2g), but these responses were similar to normal seawater in 2% agar (data not shown). These measurements fail to support the hypotheses that the gel has unusual thermoelectric or electromechanical properties. Other types of electrosensory receptors on other animals lack gel-filled tubes and operate by an ion channel sensory transduction mechanism [3, 21].
Discussion
The previous study reporting thermoelectric semiconductor properties of the gel in the ampullae of Lorenzini used only metal electrodes for measurement, which would have been subject to temperature-dependent electrochemical junction potentials of the same magnitude as those attributed to thermoelectric semiconduction [2]. We find that when the temperature-dependent electrode potentials are eliminated by using nonmetallic electrodes no voltage changes are induced by heating or cooling the gel. Control experiments show that the temperature-dependent voltage changes are generated in normal seawater using silver wire measurement electrodes, indicating no special properties of the gel are necessary for the response, other than electrochemical temperature-dependent reaction with the metal measurement electrodes.
We conclude that the proposed temperature reception without ion channels is an erroneous conclusion arising from a measurement artifact, and that the ampullae of Lorenzini are electroreceptors. This is most consistent with the anatomy of the organs [13, 24], the cellular [12] and electrophysiological [5] responses to applied electric fields, and behavioral studies showing that pelagic sharks use electroreception in their natural feeding behavior to locate prey in the open ocean [15]. Although temperature [23], and pressure [20] can cause responses in ampullae of Lorenzini, we know of no behavioral evidence that either one is a biologically relevant mode of sensory reception, and these effects can be explained by conventional action of temperature and pressure on cellular processes and materials. Thermal stimulation of other sense organs is not uncommon. For example, electrophysiological responses to temperature changes are observed in taste buds [7] and cochlea [4], but temperature is not considered a biologically relevant sensory modality for these organs. We note that not all fish with ampullae of Lorenzini feed on prey-rich thermal fronts, and many, including skates, rays, and chimaeras, are bottom dwellers, feeding on organisms buried in the sand. Electroreception provides an effective means of locating such hidden prey by sensing their bioelectric fields [9, 17]. Finally, these fish have sensitive thermoreceptive nerve endings in their skin from dorsal root ganglion neurons, which could enable them to locate thermal fronts without such specialized organs, and the central connections of the nerves from the ampullae of Lorenzini terminate in regions of the brain involved in spatial analysis, not temperature sensation [1, 13].
Acknowledgements
Supported by NIH, NICHD intramural funds. We thank Michael VL Bennett for helpful comments on an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bodznick D, Montgomery JC, Carey M. Adaptive mechanisms in the elasmobranch hindbrain. J. Exp. Biol. 1999;202:1357–1364. doi: 10.1242/jeb.202.10.1357. [DOI] [PubMed] [Google Scholar]
- 2.Brown BR. Neurophysiology: Sensing temperature without ion channels. Nature. 2003;495:495. doi: 10.1038/421495a. [DOI] [PubMed] [Google Scholar]
- 3.Bullock TH, Hopkins CD, Popper AN, Fay RR. Electroreception. New York: Springer Press; 2005. [Google Scholar]
- 4.Butler RA, Konishi T, Fernandez C. Temperature coefficient of cochlear potentials. Am. J. Physiol. 1960;199:688–692. doi: 10.1152/ajplegacy.1960.199.4.688. [DOI] [PubMed] [Google Scholar]
- 5.Clusin WT, Bennett MVL. Calcium-activated conductance in skate electroreceptors: current clamp experiments. J. Gen. Physiol. 1977;69:121–143. doi: 10.1085/jgp.69.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cope FW. Evidence for semiconduction in Aplysia nerve membrane. Proc. Natl. Acad. Sci. USA. 1968;61:905–908. doi: 10.1073/pnas.61.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz A, Green BG. Thermal stimulation of taste. Nature. 2000;403:889–892. doi: 10.1038/35002581. [DOI] [PubMed] [Google Scholar]
- 8.Eley DD. Biological semiconduction. Nature. 1979;280:520–521. [Google Scholar]
- 9.Fields RD. The shark's electric sense. Scientific American August. 2007;297:74–81. doi: 10.1038/scientificamerican0807-74. [DOI] [PubMed] [Google Scholar]
- 10.Fields RD, Lange GD. Electroreception in the ratfish (Hydrolagus colliei) Science. 1980;207:547–548. doi: 10.1126/science.7352266. [DOI] [PubMed] [Google Scholar]
- 11.Fields RD, Ellisman MH. Functionally significant plasticity of synaptic morphology: Studies of the ribbon synapse of the ampullae of Lorenzini. Neuroscience. 1988;25:705–720. doi: 10.1016/0306-4522(88)90271-0. [DOI] [PubMed] [Google Scholar]
- 12.Fields RD, Ellisman MH. Synaptic morphology and differences in sensitivity. Science. 1984;228:197–199. doi: 10.1126/science.3975637. [DOI] [PubMed] [Google Scholar]
- 13.Fields RD, Bullock TH, Lange GD. Ampullary sense organs, peripheral, central and behavioral electroreception in chimeras (Hydrolagus, Holocephali, Chondrichthyes) Brain Behav. Evol. 1993;41:269–299. doi: 10.1159/000113849. [DOI] [PubMed] [Google Scholar]
- 14.Fields RD, Ellisman MH, Waxman SG. Changes in synaptic morphology associated with presynaptic and postsynaptic activity: An in vitro study of the electrosensory organ of the thornback ray. Synapse. 1987;1:335–346. doi: 10.1002/syn.890010407. [DOI] [PubMed] [Google Scholar]
- 15.Heyer GW, Fields MC, Fields RD, Kalmijn AJ. Field Experiments on electrically evoked feeding responses in the pelagic blue shark, Prionace glauca. Biol. Bull. 1981;161:344. [Google Scholar]
- 16.Kalmijn AJ. Electro-perception of sharks and rays. Nature. 1966;212:1232–1233. [Google Scholar]
- 17.Kalmijn AJ. The electric sense of sharks and rays. J. Exp Biol. 1971;55:371–383. doi: 10.1242/jeb.55.2.371. [DOI] [PubMed] [Google Scholar]
- 18.Kalmijn AJ. Electric and magnetic field detection in elasmobranch fishes. Science. 1982;218:916–918. doi: 10.1126/science.7134985. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzini S. Osservazioni Intorno Alle Torpedini. Firenze; 1678. [Google Scholar]
- 20.Murray RW. Evidence for a mechanoreceptive function of the ampullae of Lorenzini. Nature. 1957;179:406–407. doi: 10.1038/179106a0. [DOI] [PubMed] [Google Scholar]
- 21.Murray RW. Electrical sensitivity of the ampullae of Lorenzini. Nature. 1960;187:957. doi: 10.1038/187957a0. [DOI] [PubMed] [Google Scholar]
- 22.Pettigrew JD. Electroreception in Monotremes. J. Experimental Biology. 1999;202:1447–1454. doi: 10.1242/jeb.202.10.1447. [DOI] [PubMed] [Google Scholar]
- 23.Sand A. The function of the ampullae of Lorenzini with some observations on the effect of temperature on sensory rhythms. Proc. R. Soc. Lond. B. 1938;125:524–533. [Google Scholar]
- 24.Waltman B. Electrical properties and fine structure of the ampullary canals of Lorenzini. Acta Physiol. Scand. 1966;66 Suppl. 264:1–60. [PubMed] [Google Scholar]


