Abstract
Objective
To evaluate for direct toxic effects of high glucose concentrations on cellular physiology of GnRH secreting immortalized GT1-1 neurons.
Design
Prospective experimental design.
Setting
In vitro experimental model utilizing cell culture system.
Interventions
GT1-1 cells were cultured in replicates in media with two different glucose concentrations (450mg/dl & 100mg/dl respectively) for varying time intervals (24, 48 and 72 hours).
Main Outcome Measures
Effects of glucose concentrations on GnRH secretion by the GT1-1 neurons were evaluated using a static culture model. Cell viability, cellular apoptosis and cell cycle events in GT1-1 neurons maintained in two different glucose concentrations were assessed by flow cytometry (FACS) using Annexin V-PI staining.
Results
Adverse influences of high glucose concentrations on GnRH secretion and cell viability were noted in cultures maintained in high glucose concentration (450mg/dl) culture medium for varying time intervals. A significantly higher percentage of cells maintained in high glucose concentration medium demonstrated evidence of apoptosis by FACS.
Conclusion
We provide in vitro evidence of glucose induced cellular toxicity in GnRH secreting GT1-1 neurons. Significant alterations in GnRH secretion, reduced cell viability and a higher percentage of apoptotic cells were observed in GT1-1 cells maintained in high (450mg/dl) compared to low (100mg/dl) glucose concentration culture medium.
Keywords: Apoptosis, GnRH, GT1, Neuron, Glucotoxicity, In-vitro
Introduction
Poorly controlled diabetes mellitus is associated with a spectrum of reproductive deficits ranging from delayed menarche, menstrual irregularities and infertility (1–5). Menstrual irregularities may be seen in up to 20–30% of patients (3) and a pre-ponement of menopause by 6 years has also been reported (4). Amongst type 1 diabetics, those in whom the onset of metabolic dysfunction manifested in pre-pubertal years, and those with poorly controlled diabetes are identified as being at the greatest risk for reproductive dysfunction (5). An association between menstrual irregularities and hemoglobin A1c levels is described and reproductive deficits are especially notable in the setting of poorly controlled diabetes. Pathophysiological mechanisms influencing the reproductive behavior in type I diabetes have been extensively studied in pharmacologically induced "insulinopenic" diabetic animal models (6–7).
The reproductive axis is driven by a synchronous functioning of the hypothalamo-pituitary-ovarian (HPO) axis. Each of the levels of the HPO axis, i.e. the hypothalamic pulse generator releasing the gonadotropin releasing hormone (GNRH), the pituitary gonadotrophs secreting gonadotropic hormones (follicle stimulating hormone and luteinizing hormone) and the ovarian oocyte-granulosa cell units responsible for secreting the reproductive hormones estradiol and progesterone have all been explored to varying degrees in attempts at understanding mechanisms underlying the reproductive dysfunction of poorly controlled diabetes.
The importance of insulin homeostasis in the regulation of GnRH secretion is established in studies examining LH secretion in diabetic models (8–10). Serum levels of luteinizing hormone (LH) are commonly utilized as a surrogate for GnRH release due to the short half life of 2–4 minutes of the latter. Peripheral insulin administration in addition to lowering serum glucose levels has been demonstrated to restore LH Pulsatility in the diabetic animal models (11). Interestingly, central (ICV) insulin administration has recently been shown to restore the LH surge (9–10) in diabetic rat and sheep models, despite persisting peripheral hyperglycemia. These latter finding imply a key role for central insulin in the functioning of the GnRH pulse generator.
Chronically elevated glucose concentrations are purported as a major pathogenic mechanism underlying the end organ damage associated with poor metabolic control of diabetes (12–15). Toxic effects of high glucose concentrations on a variety of cells including neurons are well described. Apoptosis has been substantiated as an underlying pathogenic mechanism in states of end organ damage associated with poor glycemic control, e.g. diabetic neuropathy and cardiomyopathy, as well as in experimental models (16–19). Although limited data suggest that the number of LHRH neurons in the septal and pre-optic areas are preserved in rats following experimental diabetes of short duration (7), the actual state of viability of these neurons remains unaddressed and direct toxic effects of chronic exposures to high glucose concentrations on the hypothalamic GnRH secreting neurons remain relatively unexplored.
We utilized an in vitro cell culture model of GnRH secreting GT1-1 neurons as a surrogate for the assessment of toxic effects of high glucose concentrations on cell viability and on the secretory function of these neurons. GT1 cells belong to an immortalized line of mouse hypothalamic GnRH secreting neurons that exhibit a pulsatile secretion of GnRH; the secreted GnRH can be easily quantified and this system has been widely utilized in attempts to improve our understanding of the functioning of the HPO pulse generator. We hypothesized that high glucose concentrations have direct adverse influences on the GnRH secreting neurons, will induce cellular apoptosis and impair GnRH secretion by the GT1-1 neurons.
Design
Prospective, controlled experimental design.
Materials & Methods
Cell Culture
Immortalized GnRH secreting GT1-1 neuronal cells were provided courtesy of Dr. J. Pollard, having originated from Dr. P. Mellon's Laboratory (University of California, San Diego). GT1-1 cells were cultured in polystyrene culture plates in density of 106 in equi-osmolar culture media containing two different glucose concentrations (DMEM high glucose-450mg/dl and DMEM low glucose, 100mg/dl, Gibco, life technologies), and supplemented with 10% fetal bovine serum (FBS) and antibiotics Penicillin 1mg/ml and Streptomycin 0.5mg/ml. Cells were cultured for varying time intervals (24,48 hours and 72 hours) at a humidified atmosphere with 5% CO² at 37° C.
Secreted and cellular GnRH assays
A static culture model was employed. Cells were plated in density of 106 per 60mm culture dishes and exposed to different glucose concentrations, in two separate experiments (each performed in six replicates per condition) utilizing varying durations of exposure to the respective glucose concentrations (48 hours and 72 hours respectively). GnRH levels in the culture medium (secreted) and in the lysed cells (cellular) were assayed using RIA and are reported as normalized per million cells. (LHRH assay kit, Peninsula laboratories, PA, USA, limit of detection of 2pg/ml and inter-assay CV of 12%). In a separate experiment (six replicates), to confirm appropriate cell function and adequacy of culture technique, the effect of a known secretagogue (8-bromo cAMP 2.5mM) on GnRH secretion by GT1-1 cells was analyzed.
Pulsatility of GnRH secretion
Adherent cells at 70% confluence were washed with PBS, allowed to acclimatize in 1ml PBS with respective glucose concentrations (100mg and 450mg/dl) for 60 minutes; culture medium was sequentially aspirated and replaced with 1ml aliquots of PBS at 15 minute intervals over a one hour period. Aspirated culture medium was centrifuged at 3500 rpm at −4° C for 5 minutes; pellet was discarded and the supernatant stored at −20° C for subsequent assay for baseline secreted GnRH.
Cellular GnRH
At the end of experiment, adherent cell monolayers were trypsinized; cell counts were performed and cell viability was assessed. Harvested cells were lysed (0.1N HCl followed by brief sonicade for 10 seconds), centrifuged as described and supernatant stored at −20° C for analysis of total cellular GnRH. Concentrations of secreted and cellular GnRH were normalized per million cells.
Cell Viability Assays
ia. Trypan blue (2%) exclusion assay
Living cell membranes are impervious to trypan blue dye penetration, whereas dead cells stain blue. Percentages of nonviable cells were calculated for cultures exposed to media with different glucose concentration. Effects of glucose concentration on cell viability were assessed in eight different experiments (all in replicates of three cell cultures per condition) utilizing varying durations of exposure to the respective glucose concentrations. In 3/8 experiments, cells were cultured for 24 hours; additional 3/8 involved cultures over 48 hours and 2/8 utilized cell cultures for 72 hours in the respective media. Cell counts were performed under light microscopy (Olympus, BX60) at 20× magnification using a hemocytometer and percentage of non-viable (blue) cells were calculated.
Apoptosis
Fluorescent activated cell sorting (FACS)
The capacity of flow cytometry for rapid and individual analysis of a large number of cells makes this an ideal tool for assessment of cellular events. The apoptotic cells maintain membrane integrity and can be recognized by their diminished stainability with DNA specific fluorochromes (e.g. PI). The poor uptake of DNA avid dyes is a combination of both membrane preservation and degradation of DNA and subsequent loss from the cell (20). In a single experiment, following 72 hours of culture in respective glucose concentrations (six replicates per condition), cells were harvested and subjected to FACS analysis. FACS was performed using the Becton Dickinson FACS Scan Flow Cytometer (488nM blue laser). Utilizing nuclear/DNA fluorochromes, FACS provides a reliable interpretation of cell cycle events (G1, G2 and S) based on DNA histogram to detect cell cycle distribution (21). Analysis was performed using dual staining with annexin V and propidium iodide (PI) using a commercially available kit (The MBL MEBCYT ® Apoptosis Kit, Woburn, MA). Annexin V staining is based on an early event in apoptosis whereby translocation of phosphatidyl serine (PS) occurs from the inner to the outer layer of plasma membrane (22). Annexin V is a Ca2+ -dependent, phospholipid-binding protein with high affinity for PS, and is useful for identifying apoptotic cells with exposed PS. Apoptotic cells stain positive for Annexin V, whereas necrotic cells exhibit dual staining with Propidium Iodide (PI) as well as Annexin V. The combination of Annexin V and PI discriminates between early apoptotic events from late apoptotic and necrotic cells.
Statistics
The differences in cell count in cultures exposed to the two different glucose concentrations were evaluated using two tailed t-tests. Area under the curve was calculated for secreted GnRH in cultures maintained under two different concentrations of glucose. Two tailed Student's t test was employed to assess significance of differences in secreted and cellular GnRH between two glucose concentrations at the individual time points and ANOVA was employed to to determine an interaction between glucose concentration and time interval. Linear regression analysis was utilized to assess the contribution of glucose concentration in the medium to the percentage of apoptotic cells. STATA Intercooled 8.2 (STATACorps, TX, USA) was employed for statistical analysis and two tailed p<0.05 was considered statistically significant.
Results
GnRH secretion
Figure 1 demonstrates results of a single experiment (performed in six replicates for each glucose concentration) evaluating GnRH secretion by cells cultured for 72 hours in the specified media. During the 60 minutes of secretory assessment in static culture, cells maintained in high glucose concentrations demonstrated lower amplitude of secretory pulses at 0 (baseline), 15, 30 and 45 minutes (Figure 1). ANOVA demonstrated an interaction between time interval and glucose concentration (p 0.007). The difference in GnRH secretion in cultures maintained in the two different glucose concentrations was of statistical significance at 30 minutes sampling (p=0.03); cells exposed to low glucose concentration demonstrated significantly higher GnRH secretory amplitude at 30 minutes. Although the overall magnitude of GnRH secretion over the 60 minutes course of the experiment, as assessed by AUC, was lower in cultures maintained in high glucose medium (2786 versus 3366pg/ml/million cells for high and low glucose respectively), this difference was not of statistical significance (p=0.82). The cellular content of GnRH, normalized per million cells, was significantly higher in cells maintained in low glucose medium (Figure 2, p=0.04).
Figure 1. GT1 cells maintained in high glucose concentrations demonstrate lower amplitude of GnRH secretion during the initial 45 minutes of sampling.
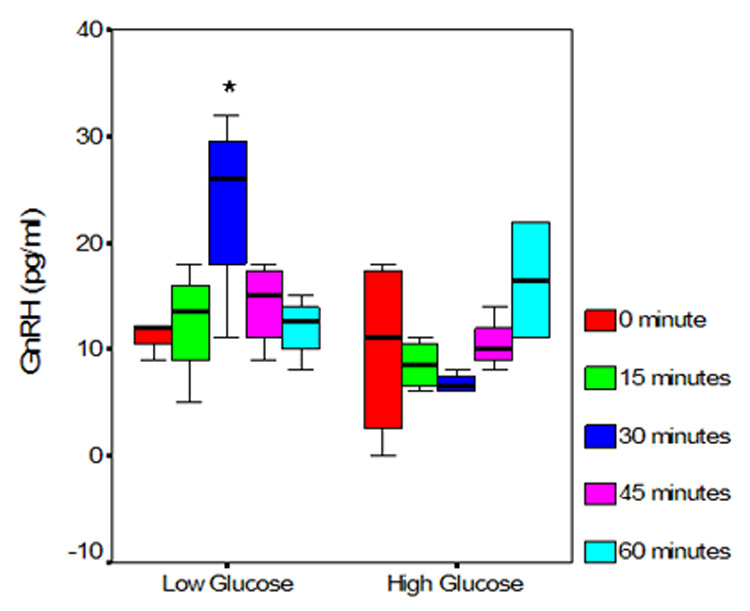
Presented data represent a single experiment (six replicates per condition) following 72 hour exposure of cells to respective glucose concentrations GnRH secretion by cells maintained in high and low glucose media, presented as box-whiskers plots. The box represents the inter-quartile range, the horizontal line represents the median and whiskers the range. Note the higher amplitude of GnRH secretion at 0, 15, 30 and 45 minutes in cells maintained at low glucose concentrations. The differences are of statistical significance at 30 minute sampling interval (*).
Figure 2. GT1 cells exposed to high glucose concentrations demonstrate significantly lower cellular GnRH content.
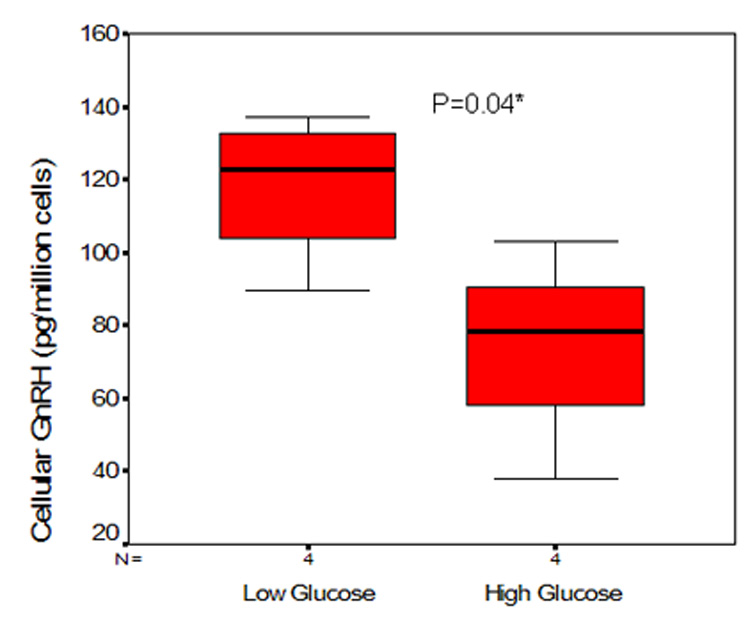
Presented data represent a single experiment (six replicates per condition) following a 72 hour exposure of cells to respective glucose concentrations. Cellular GnRH (normalized to per million cells) content in cultures maintained in high and low glucose media, presented as box-whiskers plots. The box represents the inter-quartile range, the dark line represents the median and the whiskers the 95% confidence intervals.
In a separate experiment (performed in 4 replicates per condition), similar findings of decreased pulse amplitude of GnRH secretion were noted following 48 hours of exposure to culture media with respective glucose concentrations (data not shown).
Exposure of GT1-1 cells to 2.5 mM 8 Bromo cAMP (Sigma, Aldrich) enhanced the secretion of GnRH. Peak effect was seen at 20 minutes (data not shown), confirming intact cellular functioning for subsequent interpretations.
Cell Viability
A higher percentage of non-viable cells were noted in cells cultured at high glucose concentrations, at all time periods of exposure (24, 48, 72 hours). These differences were of statistical significance at 48 and 72 hours of exposure (P<0.05). Figure 3 presents cumulative data from five experiments (following 48 and 72 hours culture in media with respective glucose concentrations), a significantly higher percentage of non-viable cells in cultures maintained in high glucose concentrations (11% versus 6%, p = 0.03).
Figure 3. Significantly higher prevalence of non-viable cells is noted in cultures maintained in high glucose concentrations.
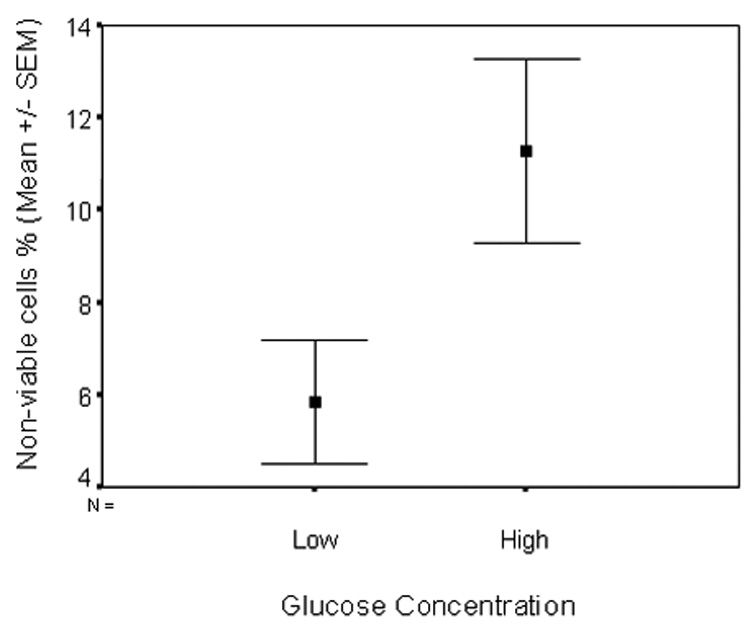
Cumulative data from 5 different experiments (three replicates per condition) following 43 (3 experiments) and 72 (2 experiments) hour exposure of cells to respective glucose concentrations. Data are presented as mean ± SEM.
Apoptosis
A significantly increased percentage of apoptotic cells, i.e. Annexin V positive and PI negative cells were observed by FACS in GT1 cultures following 72 hours of exposure to high glucose concentrations compared to cells maintained in low glucose medium, 18.75 ± 11.6% versus 7.37 ± 5.5% (mean ± SD), p=0.037. Figure 4 presents the percentage of apoptotic cells in the respective media as median ± inter-quartile interval and range. Linear regression analysis demonstrated that high glucose concentration was associated with a 13% increase in cellular apoptosis (p=0.037) and this exposure accounted for 26 % of the variability in prevalence of cell apoptosis.
Figure 4. Cultures maintained in high glucose concentrations demonstrate significantly increased prevalence of apoptotic cells (by FACS).
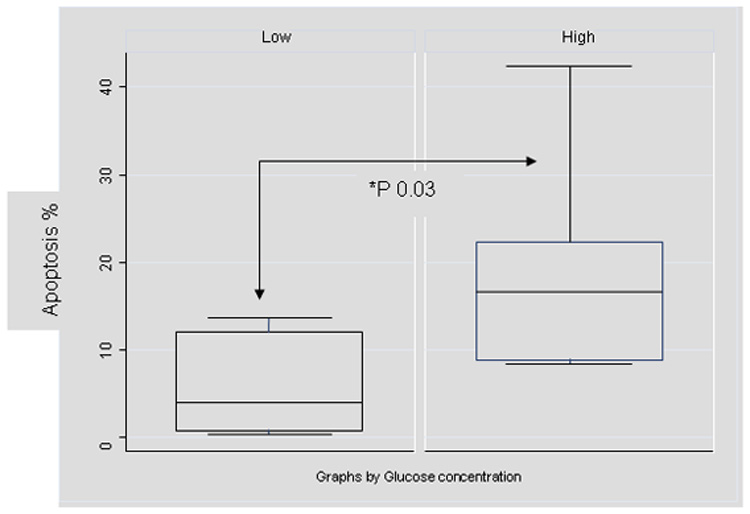
Presented data represent a single experiment (six replicates per condition) following 72 hour exposure of cells to respective glucose concentrations. Data are presented as box-whiskers plots. The box represents the inter-quartile range, the horizontal line represents the median and whiskers the range.
c. Cell cycle events
Although the cells cultured in low glucose concentrations exhibited a higher percentage of cells in G2/S phases of the cell cycle, the differences between two groups were not significant (p=0.50, data not shown).
Discussion
Evidence of glucose induced cellular toxicity is available in a variety of cells including neuronal cells, both in-vivo, as well as in in-vitro experimental models (11–19, 23–25). We herein, utilizing an in vitro culture system comprising of immortalized GnRH secreting neurons, provide evidence of direct adverse influences of high glucose concentration on cell viability, on cellular apotosis as well as synthetic and secretory capacity of GnRH by these cells Although our findings may not be directly extrapolated to neurons constituting the hypothalamic pulse generator in vivo, these findings merit further evaluation in additional experimental models so as to enhance our understanding of physiolological and pathological influences modulating the HPO axis.
Glucose induced cellular apoptosis has been demonstrated in a variety of cell types, including the neurons of dorsal root ganglia as well as in the Schwann cells (12, 14–19). Despite the evidence of glucose induced neuronal toxicity and an appreciation of the mechanisms at play, direct toxic effects of poorly controlled diabetes on the GnRH secreting neurons, specifically evidence of cellular apoptosis as a mechanism of cell death in the GnRH secreting neurons are thus far lacking. Although evaluation of hypothalamic LHRH neurons in experimental diabetes has failed to demonstrate quantitative deficiencies in the neuronal density (8), such studies however have not tested the chronicity of exposure to a high glucose milieu. Elevations in cerebral and CSF glucose levels are well described in animal models of experimental diabetes (26,27). Encouraged by our findings of apoptogenic influences of high glucose concentrations in GnRH secreting neurons, we propose that in vivo, the physical proximity of the hypothalamic nuclei to the cerebero-ventricular system theoretically facilitates neuronal exposure to a glucose replete CSF in the setting of chronic hyperglycemia and conjecture that direct glucotoxicity to the hypothalamic neurons is a plausible mechanism contributing to defects in the HPO axis as noted in poorly controlled diabetics. These findings in an in-vitro setting encourage us to propose that cellular apoptosis in the neurons constituting the GnRH pulse generator may account for the functional disturbances in the HPO axis that occur in almost a third of patients with a poorly controlled glycemic profile of long standing, despite subsequent attainment of normoglycemia (28–32). Indeed, the preponderance of available data targets the hypothalamus, and more specifically the hypothalamic GnRH secreting neurons that constitute the "pulse generator" as the site of dysfunction accounting for the reproductive deficits in the setting of poor metabolic control in diabetics as illustrated by the evidence of normal pituitary responses to exogenously administered GnRH (33,34) that reflect an intact pituitary gonadotrophe function. Similarly, ovarian responses to attempts at ovulation induction using exogenous gonadotropins are unaffected in longstanding diabetics (35), suggesting a normal ovarian complement of the HPO axis. We herein provide in vitro evidence of induction of cellular apoptosis in the GnRH secreting neurons exposed to high glucose concentrations, supporting our hypothesis that hyperglycemia may indeed inflict an irreversible insult to the GnRH secreting neurons that constitute the GnRH pulse generator, a hypothesis that merits exploration in vivo.
Glucose sensing mechanisms are demonstrated to reside within the brain stem (area postrema of medulla) in rats; glucoprivic signals have been shown to impair the LH surge (36) and an inverse relationship between LH response and serum glucose has been described, providing indications of a link between serum glucose levels and the functioning of central (hypothalamo-pituitary) components of the HPO axis. Our data utilizing an in vitro model supports an independent role for hyperglycemia in altering the physiology of the GnRH pulse generator. Unlike the rodent model, where the neurons are concentrated in specific nuclei, in the primates the GNRH secreting neurons are sparse and scattered within the ventro-medial hypothalamus, constituting the hypothalamic GnRH pulse generator. The synchronous discharge of GnRH is in turn under the influence of an extensive input from an array of inter-neuronal communication, thus rendering study of the individual neurons, in an in-vivo setting, virtually impossible. The in vitro systems provide the advantage of studying functioning of individual cells. The GT1-1 as well as other in vitro GnRH producing cellular systems (37) are ideally suited to tease out the individual contributions of a disturbed metabolic milieu on cellular function. In the data presented, we are able to successfully demonstrate detrimental influences of a "high glucose" environment on functioning and viability of the GT1-1 neurons.
We acknowledge the many limitations of our experimental model and realize that while we may propose that our data may partly explain pathophysiological mechanisms in an in-vivo setting, the presented findings need to be substantiated in-vivo. The perifusion methodology is preferable to a static culture model for evaluation of dynamic events as the cellular milieu is relatively well preserved. Nevertheless, our data utilizing the static culture model clearly suggests that the amplitude of GnRH secretion is affected by chronic exposure to high glucose concentrations. Our findings are in agreement with prior report of decreased amplitude of LH pulses in streptozocin induced diabetic male rats (38) Earlier in vitro attempts at evaluating the influences of glucose concentrations on GnRH failed to demonstrate any significant effects of acute alterations in glucose concentrations on GnRH secretion (39). We take this opportunity to highlight that these prior experiments utilized environmental manipulations over short periods (less than a few hours of exposure to high glucose concentrations) and hence may not have allowed time for the glucotoxic influences to manifest. We have attempted to recapitulate the environment of "chronic exposure" by maintaining the cells in "high glucose" concentration from the time of harvesting until the experiment (periods ranging from 24–72 hours). We demonstrate evidence of both gross cellular toxicity (poor viability, higher necrosis and evidence of cellular apoptosis) as well as provide evidence of functional (alteration in GnRH secretion) alterations (secretion of GnRH) following exposure of the GT1-1 neurons to high glucose (450mg/dl) concentrations.
Mitochondrial insult and generation of free oxygen radicals are recognized mechanisms mediating glucotoxicity induced apoptosis (4–19}. Additional data, accrued in a variety of cell types, both in vitro and in vivo, demonstrate involvement of PI3 kinase and cyclo-oxygenase pathways (23), and alterations in the ratio of pro-and anti apoptotic influences, i,e, Bax/Bcl-x(L) (25). An increased activity of Caspase 3 as well as down regulation of cellular uncoupling proteins have also been demonstrated as eventual mediators of glucotoxicity induced cellular apoptosis in various cell systems (24–25). The role of the various mechanisms at play in mediating glucotoxic insults in GnRH secreting neurons merit further evaluation.
The in vitro system utilized allows us to unravel the intracellular and molecular events within the GnRH secreting cells, modulated by chronic exposure to high glucose concentrations. Based on our observations of glucose mediated toxicity in an immortalized cell line of GnRH secreting neurons, we propose that cellular toxicity and especially apoptosis induced in the GnRH secreting neurons chronically exposed to high glucose concentrations may be a plausible mechanism that could explain the lasting disturbances in the HPO axis that are seen in up to a third of patients with poorly glycemic control. Our findings may have implications regarding the importance of stringent control of hyperglycemic state for the functioning of the reproductive axis in patients with long standing diabetes. These mechanisms need to be evaluated and substantiated in vivo.
Conclusions
Using an in vitro- cell culture model, we provide evidence for "high glucose" as an independent pathogenic mechanism contributing to irreversible cell damage in the GnRH secreting neurons. Based on this in-vitro evidence of glucose induced cellular apoptosis, we propose that this particular insult may be a mechanism underlying the functional disturbances in the hypothalamic GnRH pulse generator, that are seen in a significant proportion of patients with poorly controlled type I diabetes mellitus.
Acknowledgement
Authors would like to thank Dr. Jeffrey Pollard, Ph.D. (Department of Obstetrics & Gynecology) for the provision of GT1-1 cells and the support provided by the Reproductive Biology Center at Albert Einstein College of Medicine in the conduct of GnRH RIA. The authors acknowledge and extend their appreciation to Goli Adel, MS. and Gohar Zeitlian, MD. for their expertise and diligence in conducting the GnRH radio-immunoassays.
This work was supported by (RO1 DK064376) to GBK from the National Institute of Health – National Institute of Diabetes and Digestive and Kidney Diseases. This work was presented at the annual meetings of The Endocrine Society, 2003 and 2005.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bergqvist N. The gonadal function in female diabetics. Acta Endocrinol[Copenh] 1954;19 Suppl:3–19. [PubMed] [Google Scholar]
- 2.Djursing H. Hypothalamic-pituitary-gonadal function in insulin treated diabetic women with and without amenorrhea. Dan Med Bull. 1987;34(3):139–147. [PubMed] [Google Scholar]
- 3.Kjaer K, Hagen C, Sando SH, Eshoj O. Infertility and pregnancy outcome in an unselect group of women with insulin dependent diabetes mellitus. Am J Obstet Gynecol. 1992;166:1412–1418. doi: 10.1016/0002-9378(92)91613-f. [DOI] [PubMed] [Google Scholar]
- 4.Dorman JS, Steenkiste AR, Foley TP, Strotmeyer ES, Burke JP, Kuller LH, et al. Menopause in type 1 diabetic women: is it premature? Diabetes. 2001;50:1857–1862. doi: 10.2337/diabetes.50.8.1857. [DOI] [PubMed] [Google Scholar]
- 5.Adcock CJ, Perry LA, Lindsell DRM, Taylor AM, Holly JMP, Jones J, et al. Menstrual irregularities are more common in adolescents with type 1 diabetes: association with poor glycemic control and weight gain. Diabet Med. 1994;11:465–470. doi: 10.1111/j.1464-5491.1994.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 6.Bell RH, Jr, Hye RJ. Animal models of diabetes mellitus: physiology and pathology. J Surg Res. 1983;35(5):433–460. doi: 10.1016/0022-4804(83)90034-3. [DOI] [PubMed] [Google Scholar]
- 7.Clough RW, Kienast SG, Steger RW. Reproductive endocrinopathy in acute streptozotocin-induced diabetic male rats. Studies on LHRH. Endocrine. 1998;8(1):37–43. doi: 10.1385/ENDO:8:1:37. [DOI] [PubMed] [Google Scholar]
- 8.Wurzburger MI, Prelevic GM, Sonksen PH, Peric LA, Till S, Morris RW. Effects of improved blood glucose on insulin-induced hypoglycemia, TRH, GnRH and exercise tests in insulin-dependent diabetes. Clin Endocrinol. 1990;32(6):799–807. doi: 10.1111/j.1365-2265.1990.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 9.Kovacs P, Parlow AF, Karkanias GB. Effect of centrally administered insulin on gonadotropin-releasing hormone neuron activity and luteinizing hormone surge in the diabetic female rat. Neuroendocrinology. 2002;76(6):357–365. doi: 10.1159/000067585. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka T, Nagatani S, Bucholtz DC, Ohkura S, Tsukamura H, Maeda K, et al. Central action of insulin regulates pulsatile luteinizing hormone secretion in the diabetic sheep model. Biol Reprod. 2000;62(5):1256–1261. doi: 10.1095/biolreprod62.5.1256. [DOI] [PubMed] [Google Scholar]
- 11.Kirchick HJ, Keyes PL, Frye BE. Restoration of the LH surge and ovulation by insulin in alloxan-diabetic immature rats treated with pregnant mare's serum gonadotrophin. Acta Endocrinol (Copenh) 1982;100(2):266–273. doi: 10.1530/acta.0.1000266. [DOI] [PubMed] [Google Scholar]
- 12.Russell JW, Sullivan KA, Windebank AJ, Herrmann DN, Feldman EL. Neurons undergo apoptosis in animal and cell models of diabetes. Neurobiol Dis. 1999;6:347–363. doi: 10.1006/nbdi.1999.0254. [DOI] [PubMed] [Google Scholar]
- 13.Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. Am J Med. 1999;30(1072B):2S–8S. doi: 10.1016/s0002-9343(99)00007-8. [DOI] [PubMed] [Google Scholar]
- 14.Greene DA, Stevens MJ, Obrosova I, Feldman EL. Glucose-induced oxidative stress and programmed cell death in diabetic neuropathy. Eur J Pharmacol. 1999;30(3751–3):217–223. doi: 10.1016/s0014-2999(99)00356-8. [DOI] [PubMed] [Google Scholar]
- 15.Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, et al. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes. 2001;50(10):2363–2375. doi: 10.2337/diabetes.50.10.2363. [DOI] [PubMed] [Google Scholar]
- 16.Khera T, Martin J, Riley S, Steadman R, Phillips AO. Glucose enhances mesangial cell apoptosis. Lab Invest. 2006 Jun;86(6):566–577. doi: 10.1038/labinvest.3700418. [DOI] [PubMed] [Google Scholar]
- 17.Piconi L, Quagliaro L, Assaloni R, Da Ros R, Maier A, Zuodar G, et al. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes Metab Res Rev. 2006 May-Jun;22(3):198–203. doi: 10.1002/dmrr.613. [DOI] [PubMed] [Google Scholar]
- 18.Vincent AM, McLean LL, Backus C, Feldman EL. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005;19(6):638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- 19.Russell JW. Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci. 2002;959:368–383. doi: 10.1111/j.1749-6632.2002.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 20.Bertho AL, Santiago MA, Coutinho SG. Flow cytometry in the study of cell death. Mem Inst Oswaldo Cruz, Rio de Janeiro. 2000;95:429–433. doi: 10.1590/s0074-02762000000300020. [DOI] [PubMed] [Google Scholar]
- 21.Pozarowski P, Darzynkiewicz Z. Analysis of cell cycle by flow cytometry. Methods Mol Biol. 2004;281:301–311. doi: 10.1385/1-59259-811-0:301. [DOI] [PubMed] [Google Scholar]
- 22.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled Annexin V. J Immunol Methods. 1995;184(1):39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 23.Sheu ML, Ho FM, Yang RS, Chao KF, Lin WW, Lin-Shiau SY, et al. High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler Thromb Vasc Biol. 2005 Mar;25(3):539–545. doi: 10.1161/01.ATV.0000155462.24263.e4. [DOI] [PubMed] [Google Scholar]
- 24.Vincent AM, Olzmann JA, Brownlee M, Sivitz WI, Russell JW. Uncoupling proteins prevent glucose-induced neuronal oxidative stress and programmed cell death. Diabetes. 2004 Mar;53(3):726–734. doi: 10.2337/diabetes.53.3.726. [DOI] [PubMed] [Google Scholar]
- 25.Li ZG, Zhang W, Grunberger G, Sima AA. Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res. 2002 Aug 16;946(2):221–231. doi: 10.1016/s0006-8993(02)02887-1. [DOI] [PubMed] [Google Scholar]
- 26.Shram NF, Netchiporouk LI, Martelet C, Jaffrezic-Renault N, Cespuglio R. Brain glucose: voltammetric determination in normal and hyperglycaemic rats using a glucose microsensor. Neuroreport. 1997;8(5):1109–1112. doi: 10.1097/00001756-199703240-00009. [DOI] [PubMed] [Google Scholar]
- 27.Steffens AB, Scheurink AJ, Porte D, Jr, Woods SC. Penetration of peripheral glucose and insulin into cereberospinal fluid in rats. Am J Physiol. 1988;255(2 Pt 2):R200–R204. doi: 10.1152/ajpregu.1988.255.2.R200. [DOI] [PubMed] [Google Scholar]
- 28.Griffin ML, South SA, Yankov VI, Booth RA, Jr, Asplin CM, Veldhuis JD, et al. Insulin-dependent diabetes mellitus and menstrual dysfunction. Ann Med. 1994;26:331–340. doi: 10.3109/07853899409148347. [DOI] [PubMed] [Google Scholar]
- 29.Gens E, Michaelis D. The frequency of disturbances of somatic development in young people with type 1 diabetes is dependent on duration and age at onset of disease. Exp Clin Endocrinol. 1990;95:97–104. doi: 10.1055/s-0029-1210940. [DOI] [PubMed] [Google Scholar]
- 30.Strotmeyer ES, Steenkiste AR, Foley TP, Berga SL, Dorman JS. Menstrual Cycle Differences Between Women With Type 1 Diabetes and Women Without Diabetes. Diabetes Care. 2003;26:2003. doi: 10.2337/diacare.26.4.1016. [DOI] [PubMed] [Google Scholar]
- 31.Yeshaya A, Orvieto R, Dicker D, Karp M, Ben-Rafael Z. Menstrual characteristics of women suffering from insulin-dependent diabetes mellitus. Int J Fertil Menopausal Stud. 1995;40(5):269–273. [PubMed] [Google Scholar]
- 32.South SA, Asplin CM, Carlsen EC, Booth RA, Jr, Weltman JY, Johnson ML, et al. Alterations in luteinizing hormone secretory activity in women with insulin-dependant diabetes mellitus and secondary amenorrhea. J Clin Endocrinol Metab. 1993;76:1048–1053. doi: 10.1210/jcem.76.4.8473380. [DOI] [PubMed] [Google Scholar]
- 33.Distiller LA, Sagel J, Morley JE, Seftel HC. Pituitary responsiveness to luteinizing hormone-releasing hormone in insulin-dependent diabetes mellitus. Diabetes. 1975;24(4):378–380. doi: 10.2337/diab.24.4.378. [DOI] [PubMed] [Google Scholar]
- 34.la Marca A, Morgante G, De Leo V. Evaluation of hypothalamic-pituitary-adrenal axis in amenorrheic women with insulin-dependent diabetes. Hum Reprod. 1999;14(2):298–302. doi: 10.1093/humrep/14.2.298. [DOI] [PubMed] [Google Scholar]
- 35.Oehninger S, Hofmann GE, Kreiner D, Acosta AA, Muasher SJ. Gonadotropin stimulation for in vitro fertilization and embryo transfer in insulin-dependent diabetics: follicular response, oocyte quality, embryo development, and follicular environment. Fertil Steril. 1990;53(4):741–743. doi: 10.1016/s0015-0282(16)53476-1. [DOI] [PubMed] [Google Scholar]
- 36.Murahashi K, Bucholtz DC, Nagatani S, Tsukahara S, Tsukamura H, Foster DL, et al. Suppression of luteinizing hormone pulses by restriction of glucose availability is mediated by sensors in the brain stem. Endocrinology. 1996;137(4):1171–1176. doi: 10.1210/endo.137.4.8625886. [DOI] [PubMed] [Google Scholar]
- 37.Romanelli RG, Barni T, Maggi M, Luconi M, Failli P, Pezzatini A, et al. Role of endothelin-1 in the migration of human olfactory gonadotropin-releasing hormone-secreting neuroblasts. Endocrinology. 2005;146(10):4321–4330. doi: 10.1210/en.2005-0060. Epub 2005 Jun 30. [DOI] [PubMed] [Google Scholar]
- 38.Dong Q, Lazarus RM, Wong LS, Vellios M, Handelsman DJ. Pulsatile LH secretion in streptozotocin-induced diabetes in the rat. J Endocrinol. 1991;131(1):49–55. doi: 10.1677/joe.0.1310049. [DOI] [PubMed] [Google Scholar]
- 39.Burcelin R, Thorens B, Glauser M, Gaillard RC, Pralong FP. Gonadotropin-Releasing Hormone Secretion from Hypothalamic Neurons: Stimulation by Insulin and Potentiation by Leptin Endocrinology. 2003;144:4484–4491. doi: 10.1210/en.2003-0457. [DOI] [PubMed] [Google Scholar]


