Abstract
We have established the gene for IGF binding protein 3 (IGFBP-3) as a target for FSH action. FSH effects on this gene require the PKA pathway as well as the PI-3 kinase and MAPK pathways. At the IGFBP-3 promoter, FSH effects depend on a site for TATA box binding protein (TBP) and formation of a high molecular weight transcription complex. To further elucidate FSH effects on the downstream events involving the TBP site, we cloned a pig TAF4b cDNA into a P-Flag expression vector. By co-transfecting granulosa cells with the IGFBP-3 promoter, we found that TAF4b mimics and enhances FSH induction of IGFBP-3 reporter activity. Using RT-PCR we showed that FSH stimulates expression of TAF4b. This would suggest that the role of TAF4b in follicular development is regulated by FSH. TAF4b may thus be the TFIID component that binds to the TBP site on the IGFBP-3 promoter and is essential for FSH induction of IGFBP-3.
Keywords: granulosa cells, Follicle stimulating hormone, TAF4b
Introduction
IGF binding protein 3 (IGFBP-3) is a target for FSH action (Ongeri et al., 2004) and it may modulate FSH effects by binding IGF or through other mechanisms. FSH effects on this gene require the protein kinase A (PKA) pathway as well as the phosphatidylinositol-3 (PI-3) kinase and MAPK pathways. The FSH responsive sequence mapped to a site for TATA box binding protein (TBP) in our studies with site directed mutagenesis and gel shift assays (Ongeri et al., 2005). FSH action involved formation of a protein/DNA complex. However, we could not show an FSH-dependent increase in TBP binding to this site. Additionally, competition with antibodies directed against transcription factor IID (TFIID) did not block formation of this complex, leading us to hypothesize that a TBP-associated factor (TAF) could be the FSH target. Our current studies support that hypothesis, showing that TAF4b mimics and enhances FSH effects on the IGFBP-3 promoter. TAF4b (also known as TAFII 105), a tissue-specific TAF has previously been implicated in follicle development in vivo (Freiman et al., 2001). TAF4b null mice have defects in oocyte development (Falender et al., 2005) and maintenance of spermatogenesis (Falender et al., 2005). In our system FSH clearly induces TAF4b mRNA and activates TAF4b promoter constructs. TFII associates with ERK and is phosphorylated leading to enhanced transcription from the c-fos promoter (Kim and Cochran, 2000). In cultured human granulosa cells, TAF4b is phosphorylated by cAMP in a PKA-dependent manner (Wu et al., 2005). In pig granulosa cells, we have shown that FSH leads to the phosphorylation of ERK1/2 in a PKA-dependent manner (Ongeri et al., 2005). However, the current studies are the first to report induction of TAF4b by FSH. The mechanism of induction involves the PKA, PI-3 kinase and MAPK pathways.
MATERIALS AND METHODS
Reagents
We purchased highly purified, super-potent ovine pituitary FSH from Dr. Albert Parlow at the National Hormone and Peptide Program (Harbor-University of California-Los Angeles Medical Center, Torrence, CA). H89, LY294002, U0126 were purchased from EMD Biosciences (San Diego, CA). 32P-labeled deoxy-ATP was purchased from NEN Life Science Products (Boston, MA). A luciferase assay kit and reporter lysis buffer were purchased from Promega Corp (Madison, WI) and a beta galactosidase assay kit from BD Biosciences (Palo Alto, CA). We purchased the following from Invitrogen Technologies Inc (Carlsbad, CA); a TOPO-TA cloning kit, 3′ RACE kit, 5′ RACE kit, Lipofectin, gentamicin, OPT-MEM1 medium. Rabbit polyclonal antibodies against B-Raf, Raf1 and phospho-Raf1 ser 338 were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA) while rabbit polyclonal antibodies against Phospho-Raf1 ser 259 were purchased from Cell Signaling Technology (Danvers, MA). DNA oligonucleotides were purchased from Integrated DNA Technologies, Inc (Coralville, IA). The pLUC vector used for sub-cloning was a gift from Dr. Rich Day (University of Virginia, Charlottesville, VA). Cell culture medium was supplied by the Microbiology Department of Pennsylvania State University, Milton Hershey Medical Center (Hershey, PA).
Granulosa Cells Culture
We obtained pig ovaries from a local slaughterhouse and recovered primary granulosa cells using the dissection method as previously described (Grimes and Hammond, 1992, Grimes et al., 1992, Wandji et al., 2000, Ongeri et al., 2004). The cells were cultured in 10% fetal bovine serum to confluence, trypsinized and frozen in liquid nitrogen until used.
Cloning of the pig TAF4b coding sequence and creating of a TAF4b expression vector
Sequence for pig TAF4b is not available in the GenBank database. To evaluate the role of TAF4b in activation of IGFBP-3, we first cloned the pig TAF4b protein. The first step utilized primers designed from the human TAF4b sequence [(Dikstein et al., 1996); Genebank accession number Y09321] and amplified using pig granulosa cDNA as template. These initial primers (Forward; 5′TAA TTT GCA GCT TCC TCC AGG 3′ and Reverse; 5′CTG AAG TTT CTC CAA AAG TTA 3′) gave a 1103 bp product that was cloned into a TA vector and confirmed by sequence analysis. Using the pig TAF4b sequence we designed primers for 5′ RACE PCR and 3′ RACE PCR respectively. Both 5′ RACE and 3′ RACE were performed using the Invitrogen’s 5′ RACE and 3′RACE kits respectively (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions, with polyA+ RNA extracted from granulosa cells using Qiagen’s Oligotex kit. The sequence for cloned products was analyzed at the Pennsylvania State University, Milton Hershey Medical Center Core facility using an ABI PRISM 377 DNA sequencer (PE Applied Biosystems, Foster City CA). Based on the sequence obtained, new primers were designed for cloning the full length coding sequence for TAF4b using PCR. Using the open reading frame finder (www.ncbi.nlm.gov/gorf/orfig.cgi) we designed primers for cloning a 2520 bp product. This product was subcloned into a FLAG-CMV 5a vector (Sigma Aldrich, St Louis, MO) expression vector using KpnI and PstI restriction enzyme sites. Product sequence was confirmed by sequence analysis using Sigma’s P9350 and P5475 primers.
To evaluate the role of TAF4b in FSH induction of IGFBP-3, granulosa cells were co-transfected with a TAF4b expression vector (TAF4b in FLAG-CMV 5A vector) and a −181/+9 IGFBP-3 promoter construct in pLUC vector. The −181/+9 IGFBP-3 promoter construct was previously shown to have optimal FSH response (Ongeri et al., 2004). At 95% confluence, cells were exposed to FSH for 3h and the IGFBP-3 reporter activity assayed. Control cells were transfected with the IGFBP-3 promoter alone.
Evaluation of TAF4b expression in granulosa cells
Expression of TAF4b in granulosa cells was evaluated using RT-PCR. Granulosa cells were cultured to confluence as previously described (Ongeri et al., 2004, Ongeri et al., 2005) and treated with FSH for 0, 30, 60, 120, 180, 240 or 360 minutes. Total RNA was extracted using the Qiagen RNAeasy kit according to the manufacturer’s instructions. RT-PCR was performed as previously described using primers designed from the human TAF4b sequence in the data base [(Dikstein et al., 1996); GenBank accession # Y09321). The primers (F; 5′GG A ATG GGG CAA AAT GTG AAG3′ and R; 5′TTA AGA AAA GGA ACC AGG TGA G 3′) generated a 109 bp DNA product confirmed to be TAF4b by sequence analysis. Primers for GAPDH were used as internal controls to normalize the expression levels of TAF4b. The conditions for PCR were determined experimentally to be linear for time and mRNA.
Generation of TAF4b promoter construct and luciferase assays
Human TAF4b promoter constructs were generated by PCR using the Genomewalk approach using Invitrogen’s 3′RACE kit (Invitrogen, Carlsbad, CA). Initially human genomic DNA was digested with PvuII and used as template in combination with primers designed from the human TAF4b coding sequence [(Dikstein et al., 1996); GenBank accession # Y09321). This gave a 412 bp product from +36 to −376 that was cloned into a TA vector (Invitrogen, Carlsbad, CA). BamH I and Sal I cloning sites were incorporated into the primers to facilitate subcloning into the pLUC vector. We used software tools in Ensembl vs 34 (http://www.ensembl.org/index.html) to match the sequence obtained to human chromosome 18. From there we were able to etsbalish the position of the sequence generated with regard to human chromosome 18. Subsequently longer promoter constructs were generated using primers designed from the TAF4b gene on chromosome 18 (GenBank # AC121320). A total of 4 human promoter constructs were tested. A promoter-less pLUC vector was used as a negative control. An IGFBP-3 promoter construct known to be FSH responsive in our cell system (Ongeri et al., 2004) was included as a positive control. Granulosa cells were cultured and transfected with the TAF4b promoter constructs in a pLUC vector using the Lipofectin method as previously described (Ongeri et al., 2004, Ongeri et al., 2005). Plasmid for the housekeeping gene, beta galactosidase was used an internal control. Reporter activity for both IGFBP-3 and TAF4b promoter constructs were assayed following 3 h of FSH exposure.
Western Blot Analysis
Frozen primary granulosa cells were thawed and seeded in 10% FBS at a density of 1×105 in 100-mm Falcon brand dishes and cultured at 37°C in a CO2 incubator. At 95% confluence, the cells were serum starved overnight and exposed to 100 ng/ml FSH for 0, 30, 60 or 180 minutes. Total protein lysates were recovered as previously described (Ongeri et al., 2005). Protein concentration was determined using Bio Rad’s protein assay reagent (Bio-Rad Laboratories, Hercules, CA). Electrophoresis was carried out on 4–12% Bis-Tris gels (Invitrogen Life Technologies, Inc, Carlsbad, CA) and protein transferred to nitrocellulose membranes and blocked in 5% fat-free milk at room temperature for 1 h followed by incubation in primary antibody (1:5000 dilution) overnight at 4°C (or RT for 1 h). Following three 15 minute washes in TBS-T, the membranes were incubated in the second antibody (1:3000 dilution) for 1.5 h at room temperature. They were then washed three times in TBS-T. Antigen-antibody complexes were visualized by chemilumisence (Amersham Pharmacia, Piscataway, NJ). Where necessary the membrane was stripped by incubating in stripping buffer (0.0624M TrisHCl, pH 6.8, 2% SDS, 0.00693% b-ME) for 30 min on a shaker at 37°C, and re-probed with a second set of antibodies.
Kinase assays
Antibodies against Raf 1 and B Raf were used to immunoprecipitate protein from total lysates. Cells cultured to 95% confluence were lysed in buffer A (10 mM potassium phosphate, pH 7.0, 1 mM EDTA, 5 mM EGTA, 10 mM MgCl2, 50 mM β-glycerol phosphate, 1 mM sodium orthovanadate, 1Mm sodium pyrophosphate, 2 Mm dithiothreitol, 0.23 mM phenylmethanesulfonyl fluoride (PMSF), 0.5% Nonidet p-40 and 0.1% deoxychlorate). 2 μg antibody directed against either B-Raf or Raf1 were added to 100–500 μg protein lysate and incubated on a shaker at 4°C overnight. The immunoprecipitated protein were washed four times in PBS for 5 min at 4°C, then incubated at 37°C for 20 in a 50 μl reaction containing 0.5 μg His6-MEK1, 0.1 mM [γ-32P]ATP (10 μCi per reaction), 15 mM MgCl2, 1 mM MnCl2, 25mM Tris HCl, pH 7.4. SDS was then added to the reactions, the proteins heat denatured and then resolved by SDS-PAGE. The gels were exposed to X-ray film and activity levels visualized.
Statistical Analysis
All experiments were repeated at least 3 times. Transfections were done in triplicate with different batches of granulosa cells. Luciferase activity was normalized by the beta galactosidase activity. Data were analyzed using ANOVA (GraphPad, Inc, San Diego, CA) with p≤0.05 being considered significant. Values reported are mean±SEM.
RESULTS
Cloned Porcine TAF4b coding sequence is highly similar to human TAF4b
The pig cDNA sequence we cloned is highly similar to that of the mouse, human and rat (Figure 1). It has 79% homology to the mouse (Accession # XM_128905), 76% to rat (Accession # XM_226146), 89% to the predicted human (accession # XM_290809), and 87% to the cloned human partial sequence (Accession # Y09321). The complete pig cDNA sequence has been submitted to GenBank (Accession # EF133513).
Figure 1.
A box shade comparison of the pig peptide sequence translated from the pig TAF4b cDNA sequence determined by the open reading frame finder with that of sequence from the mouse (Mu, Accession # XM_128905), rat (Accession # XM_226146), predicted human (Hu-1, accession # XM_290809), and cloned human partial sequence (Hu-2, Accession # Y09321). The pig peptide sequence has 79% homology to the mouse, 89% to the predicted human, 87% to the cloned human, and 76% homology to the rat.
TAF4b enhances IGFBP-3 transcription activity
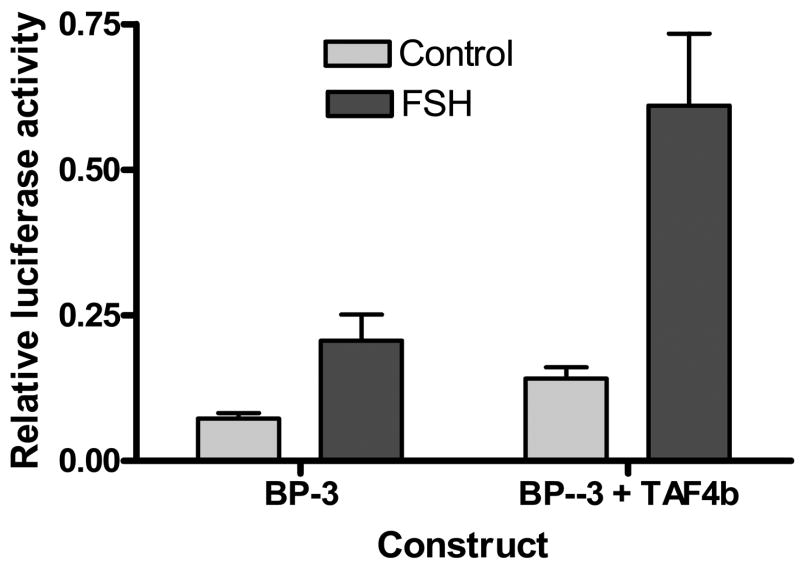
To determine if TAF4b could activate IGFBP-3 transcription in pig ovarian granulosa cells, we cloned the TAF4b coding sequence into an expression vector. Granulosa cells were then co-transfected with a −191/+9 IGFBP-3 reporter construct and the TAF4b expression vector. Granulosa cells co-transfected with TAF4b had a significantly higher level of luciferase activity (p≤ 0.05) compared to controls transfected with the IGFBP-3 reporter construct alone. The level of luciferase activity induced by TAF4b co-transfection was comparable to that induced by FSH treatment of IGFBP-3 transfected cells (Figure 2).
Figure 2.
Luciferase activity of granulosa cells cotransfected with a −191/+9 IGFBP-3 promoter construct and a TAFb expression vector. Cells were cultured in 10% FBS for 18h and transfected with either a −191/+9 IGFBP-3 promoter construct of a combination of the IGFBP-3 construct and a TAF4b expression vector in PFLAG, using the Lipofectin method. Cells were maintained in 10% FBS until 95% confluent. They were then incubated in serum-free medium overnight before being exposed to 100 ng/ml FSH for 3h. Control cells were treated with an equal volume of PBS. Beta galactosidase was used as an internal control. All transfections were done in triplicate and repeated 3 times with different batches of granulose cells. Results are mean±SEM.
When cells that were cotransfected with the TAF4b expression construct and the IGFBP-3 promoter were treated with FSH there was a further increase in luciferase activity. These results suggested that TAF4b is a plausible effector in the FSH-IGFBP-3 pathway.
FSH stimulates expression of TAF4b mRNA and TAF4b reporter activity in PKA and PI-3 Kinase and MAPK pathway-dependent manner
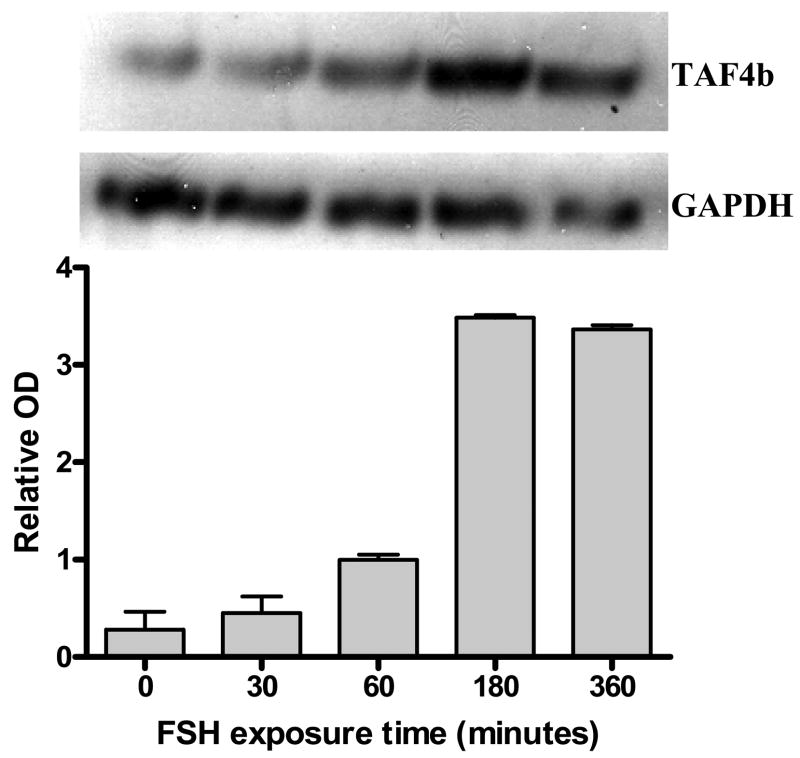
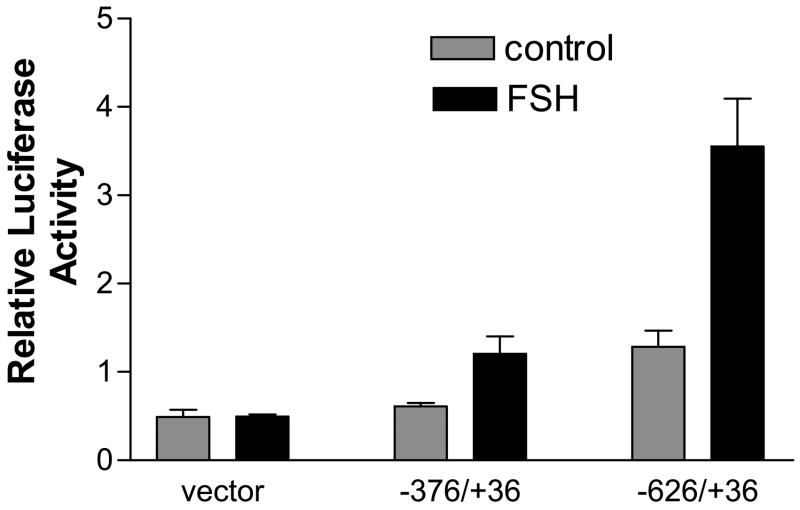
To provide additional support for TAF4b in FSH action, we examined FSH effects on TAF4b mRNA. We used RT-PCR to show that FSH stimulates expression of TAF4b mRNA, seen at 1h and sustained through 6h (Figure 3). FSH-dependent transcription of TAF4b on mRNA was confirmed in studies with granulosa cells transfected with a TAF4b-luciferase reporter construct. Cells treated with FSH had significantly higher luciferase activity (p<0.05) than control cells without FSH (Figure 4).
Figure 3.
Expression of TAF4b mRNA by granulosa cells following FSH treatment. Granulosa cells were cultured to 95% confluence, serum starved overnight and treated with 100 ng/ml FSH for 0, 30, 60, 180 or 360 minutes. Total RNA was extracted and subjected to RT-PCR. Primers for the housekeeping gene GAPDH were used as internal controls. Densitometry was used to quantify the TAF4b mRNA levels relative to the GAPDH mRNA at each time point. The blot is representative of 3 different blots, with different batches of granulose cells.
Figure 4.
Relative luciferase activity of granulosa cells transiently transfected with TAFb reporter constructs. TAF4b promoter constructs were generated by PCR using human genomic DNA as template and primers designed from the human TAF4b promoter sequence (Genbank accession # AC121320). Seeded granulosa cells were cultured in 10% FBS for 18 h and transfected with two TAF4b human promoter constructs using the Lipofectin method. A promoterless vector was used as a negative control. At 95% confluence, transfected cells were serum starved overnight and exposed to FSH for 3 h. Control cells were treated with an equal volume of PBS. All granulosa cells were co-transfected with plasmid for the housekeeping gene beta galactosidase as an internal control. All assays were done at least three times in triplicate. Results are reported as mean±SEM.
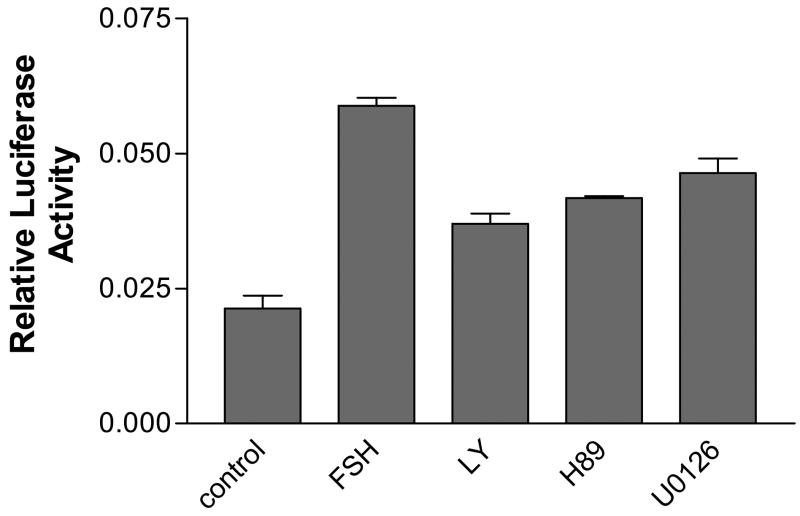
To examine the mechanism of FSH induction of TAF4b mRNA, we pre-incubated transfected granulosa cells with the PKA inhibitor H89 (10 μM), the PI-3 kinase inhibitor LY294002 (10 μM) or the MEK1/2 inhibitor U0126 (5 μM) for 1 h before adding FSH to the cell culture medium. Cells pre-exposed to these inhibitors had a significant reduction in level of FSH stimulation of luciferase activity (Figure 5). However, none of the inhibitors could completely block FSH stimulation of luciferase activity suggesting that none of the three pathways by themselves is sufficient for FSH induction.
Figure 5.
Luciferase activity of granulosa cells transfected with a −626/+36 TAF4b promoter construct exposed to FSH following a one h pre-incubation in 10 μM of the PKA inhibitor H89, 10 μM of the PI-3 kinase inhibitor LY294002 or 5 μM of the ERK1/2 inhibitor U0126. Cells were seeded in 10% FBS for 18 h, transfected in serum-free medium using the Lipofectin method for 6h, and further cultured in 10% FBS. All cells were co-transfected with beta galactosidase as an internal control. At 95% confluency, the cells were serum-starved overnight and pre-incubated in the inhibitors for 1h before being exposed to 100 ng/ml FSH for 3h. A negative control group without FSH was included. Transfection were done in triplicate and repeated at least 3 times.
FSH stimulates the kinase activity of Raf 1
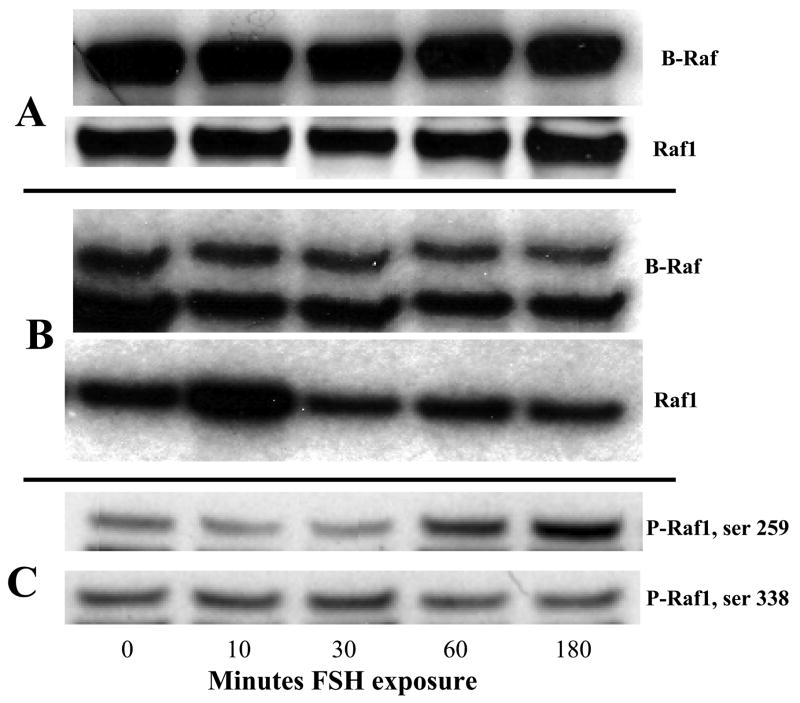
To evaluate the role of Raf in FSH signaling we first used RT-PCR to demonstrate expression of B-Raf mRNA. However, there was no significant difference in the levels of B-Raf expression in FSH treated cells compared to control cells without FSH (data not shown ). We then performed Western blot analysis with protein extracts from granulosa cells exposed to FSH for 3 h and controls without FSH, probing with antibodies directed against Raf 1 and B Raf (Figure 6, panel A). Because levels of total Raf1 and B-Raf were not changed by FSH, we evaluated the kinase activity for both Raf1 and B-Raf using recombinant His6-MEK as substrate. Our data show that FSH stimulates the kinase activity of Raf1 but not B-Raf, which is also expressed in granulosa cells (Figure 6, panel B). To determine the origin of the increased Raf1 kinase activity, we performed Western blot analysis with antibodies directed against phospho-Raf1 (P-Raf1; ser 259 and ser 338). Our data show that FSH stimulates phosphorylation of Raf1 on ser 259, one of the sites required for 14-3-3 binding and critical for Raf1 activity, but not on ser 338, another site critical for Raf1 activity (Figure 6, panel C).
Figure 6.
Western blot analysis and kinase assays of total protein lysate from granulosa cells treated with FSH. Granulosa cells were cultured to 95% confluence, serum starved overnight and treated with 100 ng/ml FSH for 0, 10, 30, 60, or 180 minutes in serum-free medium. In panel A, total protein lysate was probed with antibodies directed against B-Raf and Raf 1. In panel B total protein was immunoprecipitated against B-Raf or Raf1 antibodies. A kinase assay was performed using His6-MEK1 as substrate. In panel C, total protein was probed with antibodies directed against P-Raf1 antibodies (ser 259 and ser 338 respectively). Membranes were stripped and reprobed with antibodies against total Raf1 to validate loading intensity.
Discussion
In the present study, we evaluated the role of TAF4b in FSH signaling in porcine ovarian granulosa cells. These studies were undertaken because of our previous observations suggesting that some factor targeted to a critical TBP site could mediate FSH effects on the IGFBP-3 gene. Earlier studies in our lab had demonstrated local expression of IGFBP-3 in porcine ovarian granulosa cells in vivo (Zhou et al., 1996, Wandji et al., 2000) and in vitro (Mondschein et al., 1990, Grimes et al., 1992, Grimes and Hammond, 1992), a finding that suggested an autocrine role of IGFBP-3 in the ovary. In this system, exogenous IGFBP-3 has been shown to have inhibitory effects on granulosa cell function, although it increases IGFI binding to these cells (Samaras and Hammond, 1995). In other cells, endogenous IGFBP-3 has effects different from the exogenous protein. It has been shown to induce Akt phosphorylation and increase Akt enzyme activity in a bovine mammary epithelial cell culture system (Grill et al., 2002). In other cells, endogenous IGFBP-3 has been assigned a variety of stimulatory and inhibitory actions [Reviewed in Burger et al., (Burger et al., 2005) and (Renehan et al., 2006)]. These issues have not been resolved by the current studies. However, a better understanding of regulation of ovarian IGFBP-3 may open up new avenues to understand its significance.
We showed that a TBP site on the IGFBP-3 promoter is required for FSH induction of the IGFBP-3 gene and formation of a key protein/DNA complex involving a TBP, Sp1, Sp3, and p300. However, we could not show an FSH-dependent increase in TBP binding to this site nor could we block formation of this complex by competition with antibodies directed against TFIID (Ongeri et al., 2005). This led us to hypothesize that rather than TBP per se, a TBP-associated factor could be the FSH effector. To test this hypothesis directly, we synthesized a TAF4b expression vector, transfected granulosa cells and measured the expression of IGFBP-3 reporter constructs. TAF4b was a strong inducer of IGFBP-3 transcription, mimicked the effects of FSH on this gene as well as amplifying the effects of FSH when the two stimulators were used together. Interestingly, Geles et al., (Geles et al., 2006) using expression arrays found that TAF4b induced IGFBP-3 mRNA as well as many others in an ovarian cell line.
Next, we determined that TAF4b was expressed in our cultured granulosa cell system. Although this peptide is found in granulosa cells in vivo (Dikstein et al., 1996), studies in cultured granulosa cells are limited (Geles et al., 2006, Wu et al., 2005). Using RT-PCR we showed that this mRNA was abundantly expressed in cultured pig granulosa cells. Moreover, FSH induced a 2-fold increase in mRNA levels. To date, we have been unable to confirm this result at the protein level since antibodies which recognize porcine TAF4b are apparently unavailable. This induction was confirmed using reporter genes which we constructed with −636/+36 bases of the human TAF4b promoter.
To our knowledge, this represents the first direct demonstration of hormonal regulation of TAF4b in the ovary. The studies of Gele et al., (Geles et al., 2006) and Wu et al., (Wu et al., 2005) were consistent with FSH regulation but did not directly examine this issue. In mouse oocytes, PMSG treatment did not change the expression levels of TAF4b mRNA (Falender et al., 2005). To date we have not studied possible post translational FSH effects on this protein. The additive effects of FSH with transfected TAF4b are consistent with post translational activation of the protein by FSH dependent pathways. In other cells such post translational effects are clearly important.
In a previous study, we showed that FSH induction of IGFBP-3 required the PKA pathway as well as the PI3K pathways (Ongeri et al., 2004). Subsequently, we found that the MAPK pathway was also required. If the induction of TAF4b was critical for this FSH action, it should be mediated by similar upstream pathways. Using the PKA inhibitor H89, the PI-3 kinase inhibitor LY294002 and the ERK inhibitor U0126, we’ve shown involvement of these pathways in FSH induction of TAF4b. However, the fact that none of the inhibitors completely blocked FSH stimulation suggests that none of the pathways is sufficient for TAF4b stimulation by FSH. Information about FSH effects on MAPK pathway in porcine granulosa cells is relatively sparse. However, we and others, have previously examined the PKA and PI-3 kinase pathways in detail (Westfall et al., 2000, Cunningham et al., 2003). In different systems, others have demonstrated that the MAPK pathway impacts on TFIID (Biggs et al., 1998). Accordingly we have further established this pathway in the current studies.
We previously showed that FSH did stimulate ERK phosphorylation (Ongeri et al., 2005). In the present study we’ve demonstrated that upstream of ERK, FSH stimulates the kinase activity of Raf1 but not B-Raf. Western blot analysis showed that FSH stimulates phosphorylation of Raf1 on ser259, one of the sites required for 14-3-3 binding and critical for Raf1 activity, but not on ser 338, another site critical for Raf1 activity. These data are consistent with enhancing phosphorylation of ERK/MEK directly through activation of Raf1. However, these results are similar to FSH effects on rat granulosa cells where activation of Raf1 is apparently caused by dissociation of an inhibitory phosphotyrosine phosphatase from ERK (Cottom et al., 2003). Other members of the TAFII family have previously been shown to be targets of the MAPK pathway and ERK induced phosphorylation apparently increases their transcriptional activity (Biggs et al., 1998). However, it is not yet obvious how these pathways impinge on the TAF4b gene per se. The promoter segments we have shown to be FSH responsive do not contain canononical response element for CREB which can mediate the transcriptional activity of these pathways. Further cloning and mutational analysis of the promoter segments in hand should be informative and accessible. Such studies could add additional insights into the increasingly complex pathways mediating FSH action in ovarian cells.
As illustrated in Figure 7, interactions of TBP components with p300 provide possible mechanisms of FSH action. We have shown that FSH induction of IGFBP-3 recruited the transcription factors p300 and Sp1/Sp3 (Ongeri et al., 2004). In embryonic carcinoma cells, p300 is involved in the formation of TBP-TFIIA-containing basal transcription complex, suggesting that p300 may serve as a molecular bridge between upstream transcription activators and the basal machinery (Mitsiou and Stunnenberg, 2003). The presence of tissue-selective basal transcription factors has been suggested to be a means of diversifying transcription initiation complexes that expand promoter selectivity and tissue-specific gene expression [see review by Hochheimer and Tjian (Hochheimer and Tjian, 2003)]. Our data support and refine these concepts. A role for TAF4b in FSH action has not been previously demonstrated. However, several recent papers have established the importance of the TAF4b gene in the gonad. In male mice, TAF4b is most highly expressed in the testis. Although male homozygous KO mice are fertile (Freiman et al., 2001), more recent data have demonstrated involvement of TAF4b in the maintenance of spermatogenesis (Falender et al., 2005). Female KO mice were infertile and had defects in oocyte maturation (Freiman et al., 2001, Falender et al., 2005) suggesting that TAF4b is required for ovarian development. In the mouse ovary, TAF4b is most highly expressed in granulosa cells (Freiman et al., 2001), but is also expressed in the oocyte (Falender et al., 2005) and is thought to play a role in somatic cell – oocyte interactions. TAF4b ovaries contain fewer oocytes at postnatal day 3 compared to Wt mice ovaries (Falender et al., 2005). TAF4 b also appears to be required for release of a polar body and development beyond the two cell stage (Falender et al., 2005).
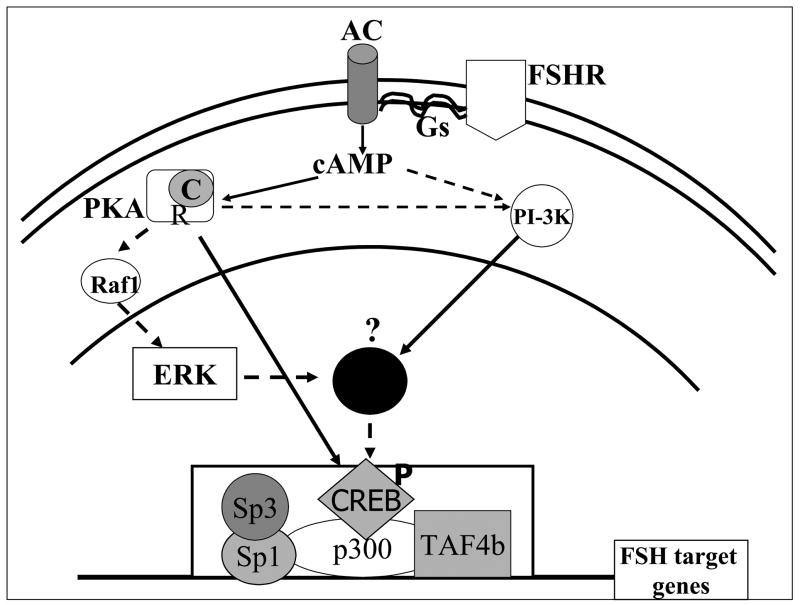
Figure 7.
Summary of FSH signaling pathways employed by FSH pig granulosa cells in the regulation of IGFBP-3 FSH target genes. Binding of FSH to its receptor, FSHR activates adenylyl cyclase via coupling with the heterodimeric G proteins. The resulting increase in cAMP activates both PKA and the PI-3 kinase. Activation of PKA causes dissociation of the catalytic subunit, which enters the nucleus and phosphorylates transcription factors such as CREB. The phosphorylated CREB then recruits the CREB binding protein homolog p300. Phospho-CREB-p300 associates with Sp1, Sp3 and TAF4b to form a complex that stimulates IGFBP-3 transcription. Solid lines represent pathways that have been documented in granulosa cells, while dashed lines represent pathways suggested by our data. A similar figure has been previously published (Ongeri et al., 2005).
Using oligonucleotide-based microarrays demonstrated down-regulation of important granulosa cell genes in TAF4b knockout animals. These include components of the inhibin-activin-follistatin pathway, aromatase p450 and cyclin D2 (Freiman et al., 2001). Levels of Foxl2, a protein that is important for transition from primordial to primary follicles is decreased in heterozygous and TAF4b null mice compared to WT controls (Falender et al., 2005). TAF4b was localized in the nucleus suggesting that it plays a role in regulating granulosa cell gene expression. However, overexpression of TAF4b did not induce a global increase in general transcription activity, suggesting TAF4b functions as a specific-transcriptional coactivator for only a limited subset of genes in the ovary (Geles et al., 2006). TAF4b has been shown to control the expression of the proto-oncogene c-jun, inhibin-α, inhibin-βA, cyclin D2 and follistatin in an immortalized rat granulosa cell line (Geles et al., 2006).
TFIID components have previously been considered as only basal transcription factors. There is now increasing evidence that they are not so basal after all (Verrijzer, 2001). Involvement of TAF4b in follicular development and apparent induction by FSH adds a new dimension to our understanding of FSH signaling mechanisms and activation of FSH target genes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of references
- BIGGS JR, AHN NG, KRAFT AS. Activation of the mitogen-activated protein kinase pathway in U937 leukemic cells induces phosphorylation of the amino terminus of the TATA-binding protein. Cell Growth Differ. 1998;9:667–76. [PubMed] [Google Scholar]
- BURGER AM, LEYLAND-JONES B, BANERJEE K, SPYROPOULOS DD, SETH AK. Essential roles of IGFBP-3 and IGFBP-rP1 in breast cancer. Eur J Cancer. 2005;41:1515–27. doi: 10.1016/j.ejca.2005.04.023. [DOI] [PubMed] [Google Scholar]
- COTTOM J, SALVADOR LM, MAIZELS ET, REIERSTAD S, PARK Y, CARR DW, DAVARE MA, HELL JW, PALMER SS, DENT P, KAWAKATSU H, OGATA M, HUNZICKER-DUNN M. Follicle-stimulating hormone activates extracellular signal-regulated kinase but not extracellular signal-regulated kinase kinase through a 100-kDa phosphotyrosine phosphatase. J Biol Chem. 2003;278:7167–79. doi: 10.1074/jbc.M203901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNNINGHAM MA, ZHU Q, UNTERMAN TG, HAMMOND JM. Follicle-stimulating hormone promotes nuclear exclusion of the forkhead transcription factor FoxO1a via phosphatidylinositol 3-kinase in porcine granulosa cells. Endocrinology. 2003;144:5585–94. doi: 10.1210/en.2003-0678. [DOI] [PubMed] [Google Scholar]
- DIKSTEIN R, ZHOU S, TJIAN R. Human TAFII 105 is a cell type-specific TFIID subunit related to hTAFII130. Cell. 1996;87:137–46. doi: 10.1016/s0092-8674(00)81330-6. [DOI] [PubMed] [Google Scholar]
- FALENDER AE, FREIMAN RN, GELES KG, LO KC, HWANG K, LAMB DJ, MORRIS PL, TJIAN R, RICHARDS JS. Maintenance of spermatogenesis requires TAF4b, a gonad-specific subunit of TFIID. Genes Dev. 2005;19:794–803. doi: 10.1101/gad.1290105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALENDER AE, SHIMADA M, LO YK, RICHARDS JS. TAF4b, a TBP associated factor, is required for oocyte development and function. Dev Biol. 2005;288:405–19. doi: 10.1016/j.ydbio.2005.09.038. [DOI] [PubMed] [Google Scholar]
- FREIMAN RN, ALBRIGHT SR, ZHENG S, SHA WC, HAMMER RE, TJIAN R. Requirement of tissue-selective TBP-associated factor TAFII105 in ovarian development. Science. 2001;293:2084–7. doi: 10.1126/science.1061935. [DOI] [PubMed] [Google Scholar]
- GELES KG, FREIMAN RN, LIU WL, ZHENG S, VORONINA E, TJIAN R. Cell-type-selective induction of c-jun by TAF4b directs ovarian-specific transcription networks. Proc Natl Acad Sci U S A. 2006;103:2594–9. doi: 10.1073/pnas.0510764103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRILL CJ, SIVAPRASAD U, COHICK WS. Constitutive expression of IGF-binding protein-3 by mammary epithelial cells alters signaling through Akt and p70S6 kinase. J Mol Endocrinol. 2002;29:153–62. doi: 10.1677/jme.0.0290153. [DOI] [PubMed] [Google Scholar]
- GRIMES RW, HAMMOND JM. Insulin and insulin-like growth factors (IGFs) stimulate production of IGF-binding proteins by ovarian granulosa cells. Endocrinology. 1992;131:553–8. doi: 10.1210/endo.131.2.1379161. [DOI] [PubMed] [Google Scholar]
- GRIMES RW, SAMARAS SE, BARBER JA, SHIMASAKI S, LING N, HAMMOND JM. Gonadotropin and cAMP modulation of IGE binding protein production in ovarian granulosa cells. Am J Physiol. 1992;262:E497–503. doi: 10.1152/ajpendo.1992.262.4.E497. [DOI] [PubMed] [Google Scholar]
- HOCHHEIMER A, TJIAN R. Diversified transcription initiation complexes expand promoter selectivity and tissue-specific gene expression. Genes Dev. 2003;17:1309–20. doi: 10.1101/gad.1099903. [DOI] [PubMed] [Google Scholar]
- KIM DW, COCHRAN BH. Extracellular signal-regulated kinase binds to TFII-I and regulates its activation of the c-fos promoter. Mol Cell Biol. 2000;20:1140–8. doi: 10.1128/mcb.20.4.1140-1148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSIOU DJ, STUNNENBERG HG. p300 is involved in formation of the TBP-TFIIA-containing basal transcription complex, TAC. Embo J. 2003;22:4501–11. doi: 10.1093/emboj/cdg419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONDSCHEIN JS, SMITH SA, HAMMOND JM. Production of insulin-like growth factor binding proteins (IGFBPs) by porcine granulosa cells: identification of IGFBP-2 and -3 and regulation by hormones and growth factors. Endocrinology. 1990;127:2298–306. doi: 10.1210/endo-127-5-2298. [DOI] [PubMed] [Google Scholar]
- ONGERI EM, VERDERAME MF, HAMMOND JM. Follicle-stimulating hormone induction of ovarian insulin-like growth factor-binding protein-3 transcription requires a TATA box-binding protein and the protein kinase A and phosphatidylinositol-3 kinase pathways. Mol Endocrinol. 2005;19:1837–48. doi: 10.1210/me.2004-0487. [DOI] [PubMed] [Google Scholar]
- ONGERI EM, ZHU Q, VERDERAME MF, HAMMOND JM. Insulin-like growth factor-binding protein-3 in porcine ovarian granulosa cells: gene cloning, promoter mapping, and follicle-stimulating hormone regulation. Endocrinology. 2004;145:1776–85. doi: 10.1210/en.2003-1552. [DOI] [PubMed] [Google Scholar]
- RENEHAN AG, HARVIE M, HOWELL A. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and breast cancer risk: eight years on. Endocr Relat Cancer. 2006;13:273–8. doi: 10.1677/erc.1.01219. [DOI] [PubMed] [Google Scholar]
- SAMARAS SE, HAMMOND JM. Insulin-like growth factor binding protein-3 inhibits porcine granulosa cell function in vitro. Am J Physiol. 1995;268:E1057–64. doi: 10.1152/ajpendo.1995.268.6.E1057. [DOI] [PubMed] [Google Scholar]
- VERRIJZER CP. Transcription factor IID--not so basal after all. Science. 2001;293:2010–1. doi: 10.1126/science.1064980. [DOI] [PubMed] [Google Scholar]
- WANDJI SA, GADSBY JE, SIMMEN FA, BARBER JA, HAMMOND JM. Porcine ovarian cells express messenger ribonucleic acids for the acid-labile subunit and insulin-like growth factor binding protein-3 during follicular and luteal phases of the estrous cycle. Endocrinology. 2000;141:2638–47. doi: 10.1210/endo.141.7.7563. [DOI] [PubMed] [Google Scholar]
- WESTFALL SD, HENDRY IR, OBHOLZ KL, RUEDA BR, DAVIS JS. Putative role of the phosphatidylinositol 3-kinase-Akt signaling pathway in the survival of granulosa cells. Endocrine. 2000;12:315–21. doi: 10.1385/ENDO:12:3:315. [DOI] [PubMed] [Google Scholar]
- WU Y, LU Y, HU Y, LI R. Cyclic AMP-dependent modification of gonad-selective TAF(II)105 in a human ovarian granulosa cell line. J Cell Biochem. 2005;96:751–9. doi: 10.1002/jcb.20577. [DOI] [PubMed] [Google Scholar]
- ZHOU J, ADESANYA OO, VATZIAS G, HAMMOND JM, BONDY CA. Selective expression of insulin-like growth factor system components during porcine ovary follicular selection. Endocrinology. 1996;137:4893–901. doi: 10.1210/endo.137.11.8895362. [DOI] [PubMed] [Google Scholar]