Abstract
The author discusses the implications of a new phase I trial investigating the role of rapamycin in patients with glioblastoma.
During the past several decades, and with an accelerating pace in the past several years, a primary focus of cancer research and treatment has been the development and refinement of specific, biologically directed therapies [1,2]. A number of attractive targets have been identified, dissected, and validated molecularly and biochemically, including multiple members of the family of receptor tyrosine kinases [1,2]. These potent enzymes, frequently concentrated or overexpressed on the surface of cancer cells, phosphorylate target proteins, with varied and manifold effects on numerous downstream, intracellular signaling pathways, leading to profound alterations in transcription and translation, cell growth, differentiation, apoptosis, angiogenesis, and invasion and metastatic potential [1,2]. A number of small molecular inhibitors of these tyrosine kinases (TKs) have been developed in recent years. Imatinib, for example, has shown impressive activity in many patients with chronic myelogenous leukemia [3,4].
The success of imatinib in human trials, and subsequent work in the laboratory and the clinic in several other cancers in which TKs appear causative and where TK inhibitors (TKIs) appeared likely to be efficacious, spurred a great deal of interest and enthusiasm throughout the oncologic community [1,2]. This was equally true in neurooncology, where progress in treating patients with malignant gliomas, especially glioblastoma (GBM), has been slow and incremental [4–7].
Treating Glioblastomas
GBM is an aggressive, primary tumor of the central nervous system [8]. Because of their intrinsic, infiltrative nature, GBMs follow a malignant clinical course. Classified as World Health Organization grade IV astrocytic tumors, GBMs have a pronounced mitotic activity, substantial tendency toward neoangiogenesis (microvascular proliferation), necrosis, and proliferative rates three to five times higher than grade III tumors, the anaplastic astrocytomas. The clinical behavior of GBMs is often mimicked by unusual pathological presentations, which gave rise to the old moniker of “glioblastoma multiforme” (Figure 1). Even with the survival advantage provided by the recently developed protocol of concurrent chemoradiation followed by adjuvant alkylating chemotherapy with temozolomide (the Stupp regimen), the prognosis of patients with GBM remains poor, with median overall survival in the range of 9–15 months and two-year survival rates of 26% in the most favorable subgroup [9].
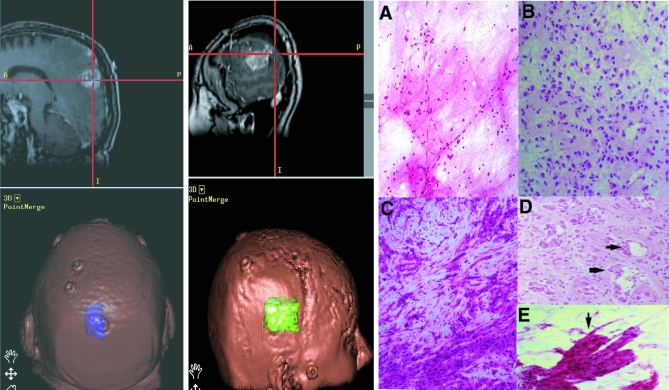
Figure 1. Clinicopathological Features of Glioblastoma.
Left, a sagittal (top), contrast-enhanced, T-1 weighted magnetic resonance (MR) image from a patient shows a left posterior parietal GBM, centered within the red cross during intra-operative navigation. The tumor is overlaid in purple on the skull (left, bottom); the several small discs seen on the surface of the scalp are used for intra-operative localization. Middle, a sagittal (top), contrast-enhanced, T-1 weighted MR image from a different patient shows a GBM within the right anterior parietal and posterior temporal lobes, represented in green on the bottom image. Right, histological variability of GBMs. A, normal paucicellular temporal lobe. B, typical, hypercellular GBM from one patient 50 years of age. C, excessive stromal proliferation within a separate portion of the same patient seen in B. D and E, areas of pronounced vascular proliferation (arrows) found throughout the specimen from a second patient, also 50 years of age, whose clinical presentation (headache and seizure) and tumor on MR imaging was nearly identical to that of the patient in B. The patient in B had little vascular proliferation compared to the patient depicted in D; conversely, patient D had no areas of stromal proliferation. Magnification in A–D, 200×; 400× in E. Hematoxylin and eosin staining.
Several common genetic alterations, such as EGFR (epidermal growth factor receptor) amplifications on chromosome 7p, as well as losses on 9p (p16), 10q (PTEN, or phosphatase and tensin homolog deleted on chromosome 10), and 17p (p53) have been identified in a significant proportion of patients with malignant gliomas (reviewed thoroughly in [8]). Two clinically recognized forms of GBM, de novo or primary and secondary or progression, have been identified clinically and recapitulated at the molecular genetic level [8]. In de novo or primary GBMs, EGFR gene amplifications, often combined with gene rearrangements that lead to a constitutively active, truncated receptor (the most common is EGFRvIII), occur in GBMs that generally express wild-type p53 [8,10–16]. In secondary tumors, progression from a low-grade glioma to a GBM involves the serial accumulation of genetic alterations that inactivate tumor suppressor genes such as p53, p16, Rb, and PTEN, or activate oncogenes such as MDM2 and CDKs 4 and 6; alterations in EGFR are less common or absent [8]. Frequently, loss of PTEN function is a common feature in both types of GBMs [8]. Response to chemotherapy may be modified by the level of expression of methyl guanine methyl transferase (MGMT) [9]. MGMT hypermethylation decreases production of MGMT, which leads to a diminished ability to repair DNA damage caused by an alkylating agent; presence of hypermethylated MGMT correlated with an approximately two-month improved median survival in patients treated with the Stupp regimen compared with those without hypermethylation [9]. However, promoter methylation analysis of MGMT is highly dependent on the tumor, collection method, specimen quality, and operator, and there is no standard alternative to the Stupp regimen in patients with intact MGMT [9].
Linked Research Article.
This Research in Translation discusses the following new study published in PLoS Medicine:
Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, et al. (2008) Antitumor activity of rapamycin in patients with recurrent PTEN-deficient glioblastoma. PLoS Med 5: e8. doi:10.1371/journal.pmed.0050008
In a phase I trial Charles Sawyers and colleagues investigated the role of rapamycin in patients with PTEN-deficient glioblastoma.
The high incidence of EGFR overexpression, amplification, or coexpression of the truncated, constitutively active EGFRVIII in GBMs raised expectations that TKIs of the EGFR, such as gefitinib or erlotinib, would have significant positive treatment effects, while minimizing toxicity compared to other therapies [1,2,8]. EGFR activates an intracellular TK that leads to a signal transduction cascade that enhances survival and infiltration of GBM cells in vitro [10–14]. Overexpression of EGFR correlates with increased cellular proliferation, tumorigenesis, decreased apoptosis, and a poorer prognosis and may be associated, as well, with radioresistance [12,14–16]. In GBM cell lines, TKIs suppress anchorage-independent growth, prevent proliferation, and enhance apoptosis [5,7,8].
While the inhibition of EGFR with TKIs showed promise preclinically, these inhibitors have subsequently shown only moderate activity as single agents in patients with GBM and other cancers. In one trial, 10 of 24 (42%) patients with recurrent or progressive GBM receiving erlotinib had a partial response or stable disease with a median time to progression of about 4.5 months—but the results of others have not been as favorable [5,6]. Analogous results were observed with EGFR antagonists in patients with non-small cell lung and pancreatic cancers [17,18]. Furthermore, Vogelbaum et al., for example, have observed that response to erlotinib is not determined by EGFR amplification status or EGFR overexpression [5]; patients with normal and elevated levels of EGFR were equally likely to have a clinical response.
Meanwhile, Haas-Kogan et al. and Mellinghoff et al. suggested that EGFR status and the activation status of some direct and indirect EGFR pathway components together play a role in the response to therapy in that fraction of patients (9%–18%) who respond favorably to erlotinib [19,20]. For example, coexpression of EGFRVIII and PTEN was the most favorable molecular marker of response (six of seven patients who responded and were tested, from the nine patients out of 49 who had an objective treatment response) in the study by Mellinghoff et al. at the University of California, Los Angeles (UCLA) [19]. By contrast, none of the responders expressed EGFRVIII in the study by Haas-Kogan et al., although overall elevated levels of EGFR and low or absent phosphorylated Akt levels were favorable predictors of response [20]. This has been confirmed by several groups who have returned to the laboratory to dissect the molecular mechanisms in vitro and animal preclinical models: in GBM cells with low PTEN expression levels, inhibition of the mammalian target of rapamycin, a downstream target of the phosphatidylinositol 3-kinase (PI3K) pathway through Akt (Figure 2), showed substantial efficacy [21–31].
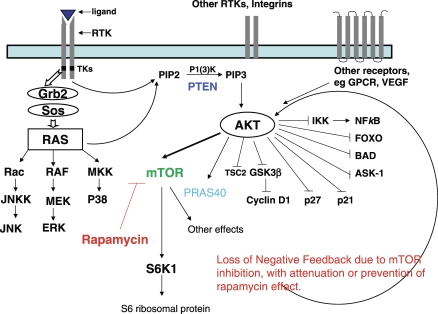
Figure 2. Cartoon Representation of Receptor Tyrosine Kinase and Phosphatidylinositol 3-Kinase (PI3K)/Akt/mTOR Pathways.
The cell surface is represented as a light blue rectangle and contains a variety of receptor tyrosine kinases, such as EGFR, insulin-like growth factor 1 (IGF-1R), and a variety or other receptors such as integrins, G-protein-coupled receptors (GPCRs), and the receptor for vascular endothelial growth factor (VEGF). Activation of the RTK by ligand (dark blue triangle) on the cell surface leads to dimerization of two receptors and phosphorylation at the tyrosine kinases, with intracellular activation of Grb2 and then Sos. Canonical activation of Ras leads to downstream activation of Rad, Raf, and MKK (mitogen-activated protein kinase kinase). It also leads, directly and indirectly through Ras, to generation of 3′-phosphoinositides, with activation of Akt; PTEN opposes the function of PI3K by removing its 3′-phosphate groups. Akt acts on a number of molecules and processes, both by activation (arrowheads) and by inhibition (lines with cross hatches), as indicated to the right of the figure.
For our purposes, Akt directly activates mTOR, which is present in two complexes, not depicted here: TORC1 (mTOR bound to Raptor, whose substrates include S6K1 and PRAS40 and which is inhibited by rapamycin and its analogues) and TORC2 (mTOR bound to Rictor). mTOR activates S6K1, as shown, an effect inhibited by rapamycin (in red). As Cloughesy et al. demonstrate, however, this effect may be more complex than previously appreciated, since loss of mTOR activity by rapamycin blockade initiates a loss of negative feedback control on Akt, which may enhance its other growth-promoting effects.
Definitions: ASK-1, apoptosis signal-regulating kinase, involved in regulating progression to apoptosis; BAD, the Bcl2 antagonist of cell death, involved in regulating progression to apoptosis; FoxO, forkhead box, involved in transcription and proliferation; GSK3β, glycogen synthase kinase 3-beta, involved in cell metabolism and growth; IKK, IκB kinase; NFκB, nuclear factor κB; PIP2, phosphatidylinositol-3,4-biphosphate; PIP3, phosphatidylinositol-3,4,5-triphosphate; TSC2, tuberous sclerosis complex 2.
A New Study of Rapamycin for Recurrent GBM
These findings spurred the UCLA group to design an important, molecularly focused clinical study, published in this issue of PLoS Medicine [32], to analyze the effect of rapamycin in a subset of patients with recurrent GBM in whom activity of the tumor suppressor PTEN was absent. The study design, which is outlined in their Figure 1 [32], is a “treat-biopsy-treat” paradigm, in which only patients with the appropriate molecular features are selected to receive a targeted biological agent. In this case, patients who were known to have PTEN loss at the time of initial resection were chosen, after recurrence of tumor following standard treatment (surgery, radiation, and temozolomide), for inclusion in the study. Patients (n = 15) were treated for approximately one week with single-agent rapamycin, underwent resection, then resumed therapy and continued it until it was determined that tumor had recurred (time-to-progression).
A variety of well-designed molecular studies were conducted, including determination of serum and intratumoral concentration of rapamycin; markers of proliferation (Ki-67 labeling); assessment of the impact of mammalian target of rapamycin (mTOR) inhibition as measured by activation status of downstream targets of mTOR, including phospho-S6; and feedback loop inhibition of AKT (see Figure 4A in [32]). In seven of 14 patients (50%), suppression of mTOR correlated directly with inhibition of tumor cell proliferation, although in several other cases (non-responders), adequate intratumoral concentrations of rapamycin did not translate unequivocally into mTOR inhibition. In other words, the probability of response was greatest in patients with the greatest degree of mTOR inhibition ([32], Figure 3).
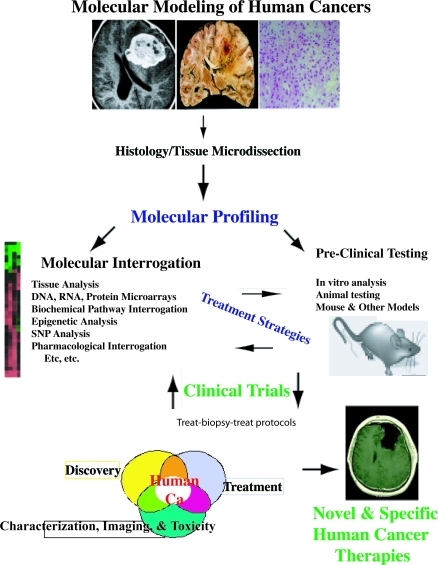
Figure 3. A Schematic Representation of the Potential for Novel Molecular Modeling of Human Cancer Therapy.
One potential paradigm is illustrated. Other methods and paradigms are possible.
This illustrates the importance of performing these studies directly in humans, since preclinical data had failed to suggest that the levels of rapamycin used would be a problem. This failure is likely due to several features such as (1) the use in preclinical settings of higher doses (for greater saturation) than may be tolerable in humans; (2) factors such as the blood–brain and blood–tumor barriers, which influence pharmacodynamic bioavailability; (3) variability in vascularization and necrosis within the tumor; and (4) other host factors (see Figure 1). Thus, even in a carefully chosen cohort of patients with the molecular features that predict response, only 50% responded. Why?
An interesting and unexpected molecular feature appears to be responsible, at least in part: inhibition of mTOR led to feedback loop activation of Akt (depicted in Figure 2). This disinhibition appears to explain the diminished response rate, especially through activation of the downstream Akt target PRAS40. Genetic investigation of the factors associated with PRAS40 induction during mTOR inhibition identified amplification of EGFR, MDM2, and PDGFRA as more common in the non-responder subgroup, a finding not predicted from preclinical work.
Unpredictable results such as this have recently been echoed in three important studies, in which it has been shown in advanced solid epithelial malignancies, such as lung cancer and gliomas, that activation of multiple signaling pathways, as well as alteration of their feedback mechanisms, are common features and that successful treatment strategies must account for these novel characteristics of the neoplastic state [2,33–35]. Thus, use of combination therapy (for example, rapamycin plus a TKI or a TKI plus an inhibitor of Akt), as Cloughesy et al. suggest, or more permissive and less specific TKIs that work on several activation pathways (see Figure 2), as Arbiser advocates, is more likely to be successful when applied to specific subgroups of patients identified carefully along the lines described in the study published here [32]; see also the review of Arbiser [2].
Five Key Papers in the Field.
Engelman et al., 2007 [33] This paper shows how amplification of the MET oncogenes leads to enhancement of signaling through a receptor not generally activated in lung cancer.
Furnari et al., 2007 [8] A comprehensive review of the molecular biology of the malignant gliomas.
Kummar et al., 2007 [37] A cogent review of the issues surrounding careful interrogation of novel therapeutics. Essential reading.
Stommel et al., 2007 [34] This paper, along with [33], illustrates the synergy between multiple receptor kinases and the potential need for more promiscuous TKIs, perhaps in combination with other signaling inhibitors such as rapamycin.
Mellinghoff et al., 2005 [19] An elegant study that illustrates the dilemma of PTEN and Akt activation with the successes and failures associated with EGFR blockade in patients with GBM.
Towards Individualized Therapy
The ultimate goal of most oncologists is to tailor therapy that takes into account—and exploits—the individual tumor's unique biological features. While individualized therapy may be some years in the future, the work of Cloughesy et al. [32], and that of many others, is pointing a way toward rational design of therapy for stratified groups of patients who share common molecular features [2,35–37]. Therefore, one strategy in designing the next generation of clinical trials in oncology must be to address both the known interactions, and, as Cloughesy et al. have done here, interrogate clinical studies and tissues at an early stage to identify genetic and biochemical features that distinguish responders and non-responders so that both types of patients receive optimal therapy (Figure 3).
This is likely to be an iterative process, which, as technology advances and neural network/machine-learning processes become integrated into clinical care, is likely to allow cancer researchers and clinicians to reach toward the holy grail of individualized therapy in the not-so-distant future [36]. It is also a method that has recently gained attention from both the United States Food and Drug Administration and the National Cancer Institute, with respect to rapid drug development timelines in cancer therapeutics and for tissue bio-repositories, with emphasis on what are being called “Phase 0 trials” [37]. The work of Cloughesy and his colleagues helps point the way.
Acknowledgments
I wish to thank the Melvin Burkhardt chair in neurosurgical oncology and the Karen Colina Wilson research endowment fund within the Brain Tumor and Neuro-Oncology Center at the Cleveland Clinic Foundation for research and administrative support.
Glossary
Abbreviations
- EGFR
epidermal growth factor receptor
- GBM
glioblastoma
- MGMT
methyl guanine methyl transferase
- MR
magnetic resonance
- mTOR
mammalian target of rapamycin
- PI3K
phosphatidylinositol 3-kinase
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- TK
tyrosine kinase
- TKI
tyrosine kinase inhibitor
- UCLA
University of California, Los Angeles
Footnotes
Robert J. Weil is at the Brain Tumor and Neuro-Oncology Center, Department of Neurosurgery and the Neurological Institute, Cleveland Clinic, Cleveland, Ohio, United States of America. E-mail: weilr@ccf.org
Funding: The author received no specific funding for this article.
Competing Interests: The author has declared that no competing interests exist.
References
- Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23:2445–2459. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]
- Arbiser JL. Why targeted therapy hasn't worked in advanced cancer. J Clin Invest. 2007;117:2762–2765. doi: 10.1172/JCI33190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- Vogelbaum MA, Peereboom D, Stevens G, Barnett GH, Brewer C. Response rate to single agent therapy with the EGFR tyrosine kinase inhibitor erlotinib in recurrent glioblastoma multiforme: results of a phase II study [abstract TA–359] 2004. Proceedings of the Ninth Meeting of the Society for Neuro-Oncology; 18–21 November 2004; Toronto, Ontario, Canada.
- Prados MD, Lamborn KR, Chang S, Burton E, Butowski N, et al. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro Oncol. 2006;8:67–78. doi: 10.1215/S1522851705000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halatsch ME, Gehrke EE, Vougioukas VI, Botefur IC, A-Borhani F, et al. Inverse correlation of epidermal growth factor receptor messenger RNA induction and suppression of anchorage-independent growth by OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in glioblastoma multiforme cell lines. J Neurosurg. 2004;100:523–533. doi: 10.3171/jns.2004.100.3.0523. [DOI] [PubMed] [Google Scholar]
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–7270. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Schlegel J, Merdes A, Stumm G, Albert FK, Forsting M, et al. Amplification of the epidermal-growth-factor-receptor gene correlates with different growth behaviour in human glioblastoma. Int J Cancer. 1994;56:72–77. doi: 10.1002/ijc.2910560114. [DOI] [PubMed] [Google Scholar]
- Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- Halatsch ME, Gehrke E, Borhani FA, Efferth T, Werner C, et al. EGFR but not PDGFR-beta expression correlates to the antiproliferative effect of growth factor withdrawal in glioblastoma multiforme cell lines. Anticancer Res. 2003;23:2315–2320. [PubMed] [Google Scholar]
- Lund-Johansen M, Bjerkvig R, Humphrey PA, Bigner SH, Bigner DD, et al. Effect of epidermal growth factor on glioma cell growth, migration, and invasion in vitro. Cancer Res. 1990;50:6039–6044. [PubMed] [Google Scholar]
- Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63:6962–6970. [PubMed] [Google Scholar]
- Barker FG, 2nd, Simmons ML, Chang SM, Prados MD, Larson DA, et al. EGFR overexpression and radiation response in glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;51:410–418. doi: 10.1016/s0360-3016(01)01609-1. [DOI] [PubMed] [Google Scholar]
- Chakravarti A, Chakladar A, Delaney MA, Latham DE, Loeffler JS. The epidermal growth factor receptor pathway mediates resistance to sequential administration of radiation and chemotherapy in primary human glioblastoma cells in a RAS-dependent manner. Cancer Res. 2002;62:4307–4315. [PubMed] [Google Scholar]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Goldstein D, Hamm J, Kotecha J, Gallinger S, et al. Erlotinib improves survival when added to gemcitabine in patients with advanced pancreatic cancer. 2005. A phase III trial of the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) [abstract 77]. 2005 Gastrointestinal Cancers Symposium; 27–29 January 2005; Hollywood, Florida, United States of America. Available: http://www.asco.org/portal/site/ASCO/menuitem.34d60f5624ba07fd506fe310ee37a01d/?vgnextoid=76f8201eb61a7010VgnVCM100000ed730ad1RCRD&vmview=abst_detail_view&confID=36&abstractID=10376. Accessed 17 December 2007.
- Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- Haas-Kogan DA, Prados MD, Tihan T, Eberhard DA, Jelluma N, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- Mellinghoff IK, Cloughesy TF, Mischel PS. PTEN-mediated resistance to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. 2007;13:378–381. doi: 10.1158/1078-0432.CCR-06-1992. [DOI] [PubMed] [Google Scholar]
- Sarkaria JN, Yang L, Grogan PT, Kitange GJ, Carlson BL, et al. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther. 2007;6:1167–1174. doi: 10.1158/1535-7163.MCT-06-0691. [DOI] [PubMed] [Google Scholar]
- Omuro AM, Faivre S, Raymond A. Lessons learned in the development of targeted therapy for malignant gliomas. Mol Cancer Ther. 2007;6:1909–1919. doi: 10.1158/1535-7163.MCT-07-0047. [DOI] [PubMed] [Google Scholar]
- Wang MY, Lu KV, Zhu S, Dia EQ, Vivanco I, et al. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006;66:7864–7869. doi: 10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]
- McLendon RE, Turner K, Perkinson K, Rich J. Second messenger systems in human gliomas. Arch Pathol Lab Med. 2007;131:1585–1590. doi: 10.5858/2007-131-1585-SMSIHG. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- LoPiccolo J, Granville CA, Gills JJ, Dennis PA. Targeting Akt in cancer therapy. Anticancer Drugs. 2007;18:861–874. doi: 10.1097/CAD.0b013e3280cc2c6f. [DOI] [PubMed] [Google Scholar]
- Zhang HH, Lipovsky AI, Dibble CC, Sahin M, Manning BD. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Mol Cell. 2006;24:185–197. doi: 10.1016/j.molcel.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MJ, Crowder R. “PIKing” the winner for phosphatidylinositol 3-kinase inhibitors in ErbB2-positive breast cancer: let's not “PTENed” it's easy! Clin Cancer Res. 2007;13:5661–5662. doi: 10.1158/1078-0432.CCR-07-1698. [DOI] [PubMed] [Google Scholar]
- Huang PH, Cavenee WK, Furnari FB, White FM. Uncovering therapeutic targets for glioblastoma: a systems biology approach. Cell Cycle. 2007;6:2750–2754. doi: 10.4161/cc.6.22.4922. [DOI] [PubMed] [Google Scholar]
- Huang PH, Mukasa A, Bonavia R, Flynn RA, Brewer ZE, Cavenee WK, et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci U S A. 2007;104:12867–12872. doi: 10.1073/pnas.0705158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, et al. Antitumor activity of rapamycin in patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- Nissen LJ, Cao R, Hedlund EM, Wang Z, Zhao X, et al. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007;117:2766–2777. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordon-Cardo C, Kotsianti A, Verbel DA, Teverovskiy M, Capodieci P, et al. Improved prediction of prostate cancer recurrence through systems pathology. J Clin Invest. 2007;117:1876–1883. doi: 10.1172/JCI31399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummar S, Kinders R, Rubinstein L, Parchment RE, Murgo AJ, et al. Compressing drug development timelines in oncology using phase ‘0’ trials. Nat Rev Cancer. 2007;7:131–139. doi: 10.1038/nrc2066. [DOI] [PubMed] [Google Scholar]