Abstract
OBJECTIVE
To determine if the mature peptide product of the Vcsa1 gene, sialorphin, could restore erectile function in ageing rats, and whether these effects are mediated through relaxation of corporal smooth muscle tissue, as we recently reported that Vcsa1 is one of the most down-regulated genes in the corpora of rats in three distinct models of erectile dysfunction, and gene transfer of plasmids expressing Vcsa1 into the corpora of ageing rats restored erectile function.
MATERIALS AND METHODS
Sialorphin was injected intracorporeally into retired breeder rats, and the effect on the physiology of corporal tissue was analysed by intracorporal/blood pressure (ICP/BP) measurement at different times after injection. In organ-bath studies, the ability of sialorphin (1 μg/mL) to enhance C-type natriuretic peptide (CNP) relaxation of corporal smooth muscle tissue strips was investigated after pre-contraction with 1 μM phenylephrine.
RESULTS
Intracorporal injection of 100 μg sialorphin into retired breeder rats resulted in a time-dependent increase in the ICP/BP response to electrostimulation of the cavernosal nerve. After 55–65 min the ICP/BP ratio increased to ≈ 0.6, a value associated with normal erectile function. In organ-bath studies after pre-contraction with 1 μM phenylephrine, 1 μM CNP significantly (67%) increased the relaxation rate of corporal tissue. This rate of relaxation was increased by 2.5-fold after incubation with sialorphin (1 μg/mL) compared with carrier alone.
CONCLUSION
These results show that sialorphin has a role in erectile function, probably through a mechanism that involves relaxation of corporal smooth muscle tissue.
Keywords: Vcsa1, sialorphin, ageing, erectile dysfunction
INTRODUCTION
Recently it was reported that the variable coding sequence a1 gene (Vcsa1; also known as a submandibular rat 1 gene) transcript is one of the most down-regulated genes in the corpora of rats in three distinct models of erectile dysfunction (ED): diabetic, age-related, and neurogenic (bilaterally ligated cavernosal nerves) models of ED [1,2]. These reports led to the suggestion that Vcsa1 is a potential marker for ED. Furthermore, when we performed gene transfer of plasmids expressing Vcsa1 (5 μg and 25 μg pVax-Vcsa1) into the corpora of ageing rats, their erectile function was restored. Transfer of even more of the gene (100 μg pVax-Vcsa1) caused priapism, implicating that the protein products of Vcsa1 have a direct role in erectile physiology. Vcsa1 encodes a precursor protein that gives rise to three peptide products [3]; an undecapeptide, a hexapeptide and a pentapeptide. The final mature peptide is the pentapeptide, named sialorphin. There is considerable sexual dimorphism in the regulation of Vcsa1 gene expression, essentially through transcriptional regulation by androgens. Androgen induction of Vcsa1 expression results in 1000 times more Vcsa1 mRNA in acinar cells of rat submandibular gland and in 100–500 times greater circulating sialorphin peptide levels in adult males than in females [4–7]. In addition, 0.3, 1 and 3 μg/kg sialorphin administered through dorsal tail vein injections was shown to modulate male sexual behaviour in the rat [8]. Also, sialorphin was reported to show analgesic activity, and this has been related to its ability to act as an inhibitor of rat membrane-bound neutral endopeptidase (NEP) [4].
The synthetic NEP inhibitors, phosphoramidon and thiorphan, have been shown to enhance C-type natriuretic peptide (CNP)-induced relaxation of porcine isolated coronary artery smooth muscle [9]. Interestingly, CNP has also been suggested to have a role in erectile function [10]. CNP binds to corporal smooth muscle guanylyl cyclase B (GC-B) receptor present in both rabbits and rats, and it was shown that CNP could cause relaxation of isolated rabbit corporal smooth muscle tissue [11,12].
Our previously published results showed that gene transfer of plasmids expressing Vcsa1 into the rat penis improved erectile function, as measured by the intracorporal pressure/systemic blood pressure (ICP/BP) ratio after electrostimulation of the cavernosal nerve. Because penile erection is the end result of smooth muscle relaxation in the penis [13,14], and ageing results in increased corporal smooth muscle tone [15], we hypothesized that intracorporal gene transfer of Vcsa1 into the ageing rat, resulting in enhanced expression of sialorphin, which acts as a NEP inhibitor, might cause relaxation of the corporal smooth muscle tissue, leading to the restoration of erectile function. In the present experiments, we investigated the ability of the mature peptide product of Vcsa1, sialorphin, to restore erectile function in the ageing rat. In addition, we determined in organ-bath studies if sialorphin could mediate relaxation of corporal smooth muscle tissue.
MATERIALS AND METHODS
Experiments were done on 9–10-month-old retired breeder male Sprague-Dawley rats weighing >500 g (as described previously [16]). When measurements were complete, the rats were killed in a CO2 gas chamber. All protocols were approved by the Animal Use Committee and Internal Review Board at the Albert Einstein College of Medicine.
The sialorphin peptide (2 mg) was dissolved in 0.5 mL of 0.01 M acetic acid and then was vortexed, and the solution was centrifuged for a few seconds at ≈ 1000 g at 4 °C (to gather all the liquid at the bottom of the tube). The stock solution was stored in 25-μL aliquots (100 μg) at −70 °C. Before use, the stock was thawed on ice and PBS (pH 7.4) was added to bring the volume to 150 μL.
Intracorporal micro-injection of sialorphin into rat corporal tissue was modified from previously described procedures, where plasmids were injected intracorporeally to investigate their effect on the ICP/BP ratio [16–18]. The modifications from the previously described protocols were used in anticipation that as a mature peptide product, there would be a direct effect by sialorphin (compared with the gene transfer studies where a plasmid gene would have to be transcribed, translated, and be processed before exerting an effect). Also, as a hormonal peptide mediator, the sialorphin would be very susceptible to proteases or peptidases, reducing the time for it to exert its physiological effect. Therefore, the time from administration to cavernosal nerve stimulation was reduced (to <1.5 h after administration) and the rats were used as their own control (i.e. measurements were taken before and after treatment).
Briefly, rats were anaesthetized with an i.p. injection of pentobarbital sodium (35 mg/kg), the crus was exposed and a 100 μg sialorphin in 150 μL of carrier solution (PBS, pH 7.4) was micro-injected. The other crus was isolated, and a 25 G needle was inserted to record ICP. The cavernosal nerves were identified adjacent to the prostate gland and for electrostimulation, currents of 0.75 and 4 mA at 20 Hz and duration of 0.2 ms were applied.
The changes in ICP and systemic BP were recorded at both levels of neurostimulation. After two duplicate measurements, rats were given an intracorporal injection with 150 μL of 100 μg of sialorphin in carrier solution. After 20 min, ICP/BP measurements were initiated with applied currents of 0.75 and 4 mA. The mean (SD) ICP/BP (seven rats) was calculated before and after treatment. A significant change in the ICP/BP value from that of the pretreatment group was determined by Student’s t-test. Studies of contractility of isolated smooth muscle tissue were carried out as described previously [19,20].
Four longitudinal strips of cavernosal tissue from the crura of old rats (four) were dissected free from the tunica albuginea and were suspended between small surgical hooks in a tension-measuring device (Multimyograph Model 610M, Copenhagen, Denmark) that allows simultaneous monitoring of four muscle strips. Tissue was equilibrated for 90 min in 6 mL Krebs-Henseleit buffer (in mM; 110 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 11 glucose, and dextrose in glass-distilled water). Organ chambers were maintained at 37 °C and continuously bubbled with 95% O2 and 5% CO2 to maintain a mean pH of 7.4 ± 0.1. Tension developed by the muscle strips was continuously recorded using Powerlab software (Chart version 4.2.4, AD Instruments, CO, USA) on a dedicated computer. The strips of corpus cavernosum were first pre-contracted with phenylephrine (10−6 M). Then, relaxation was induced using CNP dissolved in the carrier dimethyl sulphoxide (DMSO; final concentration in organ bath, CNP 10−6 M, DMSO 0.1%). The change in the rate of relaxation after the addition of 1 μg/mL sialorphin was derived from the slope of the recording, as the change in tension (g) over time (min). The results from four separate strips from four rats were averaged and the significance determined using Student’s t-test.
RESULTS
The ICP/BP ratio of the rats was measured before and immediately after injection with 10 and 100 μg sialorphin, and then at 15–25, 35–45, and 55–65 min after injection. There was no effect of sialorphin on the systemic BP. The results of a representative experiment are shown in Fig. 1A, where ICP (upper panel) and BP (lower panel) were measured after electrostimulation at 0.75 and 4 mA before treatment with sialorphin and at various times after treatment. There was an apparent time-dependent significant increase in the effect of sialorphin on erectile function. This effect was noted at 35–45 and 55–65 min after intracorporal injection with sialorphin for the lower level of stimulation (0.75 mA). At 4 mA stimulation at the longest time studied after administration of sialorphin (55–65 min), there was a significant improvement of erectile function. The ICP/BP ratio approached 0.6, which is considered a value at which an erection can be achieved. Carrier alone (three rats) and the lower dose of sialorphin (10 μg, three rats) had no significant effect on erectile function.
FIG. 1.
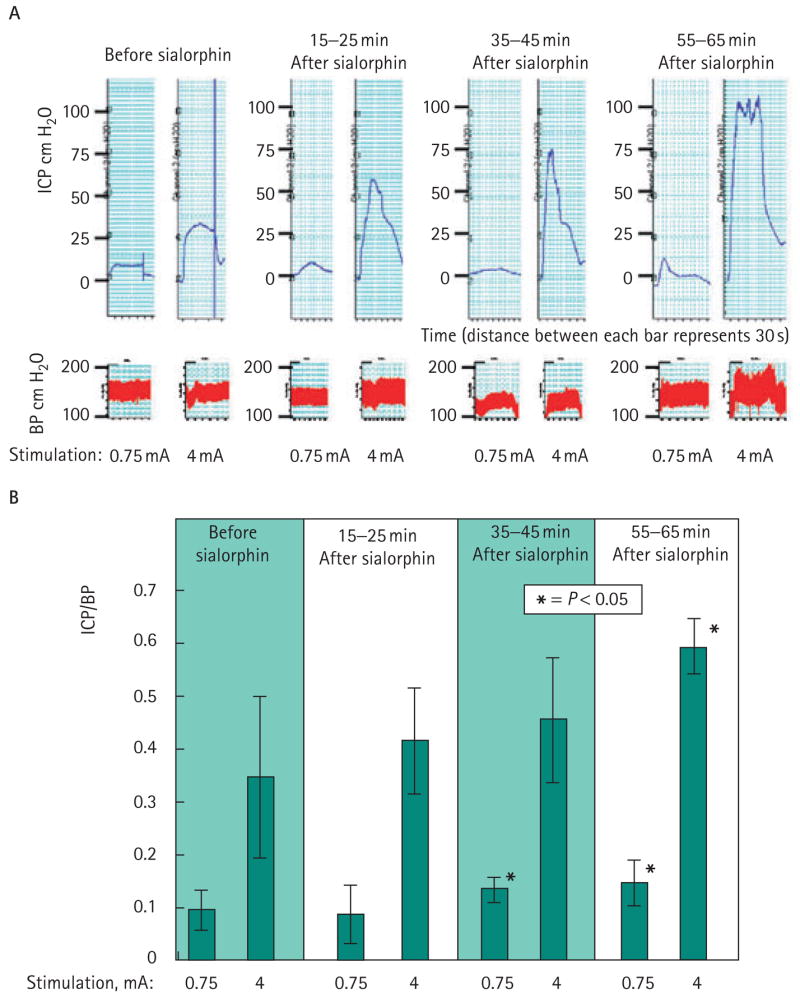
A, Results of a typical experiment in which (upper panel) ICP and (lower panel) BP was measured after electrostimulation at 0.75 and 4 mA before treatment with sialorphin and at various times after treatment. B, the mean of ICP/BP measurements for seven rats. There is an apparent time-dependent increase in the effect of sialorphin on erection increasing with time. There was a significant effect (P < 0.05, Student’s t-test) on erectile function at 35–45 min and 55–65 min after intracorporal injection with sialorphin for the lower level of stimulation (0.75 mA) and after 1 h for the higher level of stimulation (4 mA) as compared to baseline.
The studies of contractility of isolated smooth muscle tissue were as previously described [19,20]. Typical experiment results are shown in Fig. 2 and in Table 1. Tissue strips rapidly contracted on adding 1 μM phenylephrine to the organ-bath media. Under the experimental conditions used in these experiments, the corpora strips slowly relaxed, taking >60 min to return to the baseline, at a rate of tension reduction of 0.052 g/min. However, on adding CNP (1 μM) there was a significant (67%) increase in the relaxation rate of the tissue. This rate of relaxation was increased further by adding sialorphin (10 μg/mL), such that there was a 2.5-fold increase compared with the corporal strips treated with carrier alone. If sialorphin was incubated with the corporal strips with no CNP, there was no significant effect on the relaxation rate, suggesting that sialorphin enhances the effect of CNP rather than the relaxation rate being affected independently.
FIG. 2.
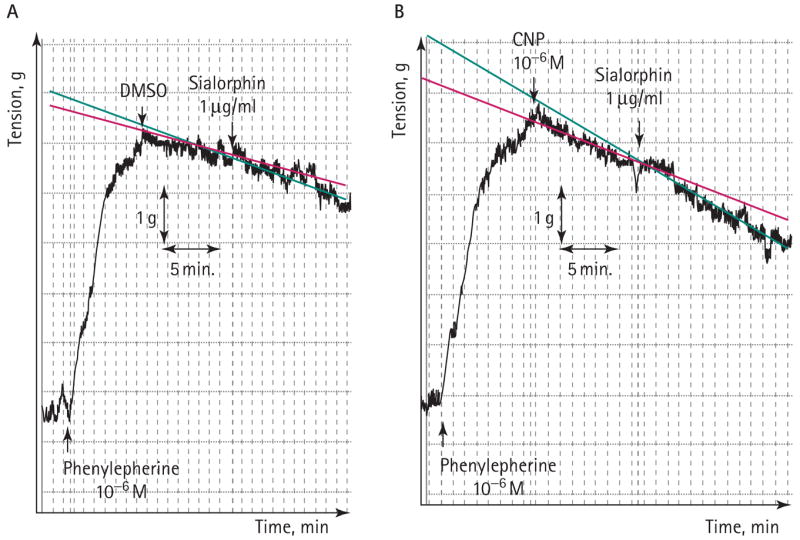
A, A myograph of corporal strips contracted with 1 μM phenylephrine. Relaxation was induced by adding CNP (1 μM). Adding sialorphin (1 μg/mL) further increased the rate of relaxation. The change in the rate of relaxation after adding 1 μg/mL sialorphin was derived from the slope of the recording as the change in tension (g) over time (min) (as shown).
TABLE 1.
Sialorphin can increase the relaxation of isolated corporal tissue caused by CNP
| Variable | Rate of tension loss, g/min |
|---|---|
| Mean (SD): | |
| Carrier (DMSO) | 0.052 (0.017) |
| 1 μm CNP | 0.087 (0.012)* |
| 1 μg/mL sialorphin | 0.072 (0.018) |
| 1 μm CNP + 1 μg/mL sialorphin | 0.13 (0.014)* |
The mean (SD) rate of tension loss was measured from four corporal smooth muscle strips from four different rats.
A significant increase in rate of tension loss (P < 0.05, Student’s t-test) compared with strips treated with carrier alone.
DISCUSSION
Vcsa1 was shown to be one of the most down-regulated genes in the corpora of rats in a neurogenic model of ED (bilaterally ligated cavernosal nerves), using microarrays [2]. Our work using microarrays (data not shown) showed that Vcsa1 also is the most down-regulated gene in the corpora of rats with diabetes-induced ED, and we recently showed by quantitative PCR that the gene is down-regulated in the corpora of diabetic and retired breeder rats with ED [1]. We therefore suggest that the expression of Vcsa1 is a marker for organic ED. When we reintroduced the gene by intracorporal injection into ageing rats, there was improved erectile function at lower doses, and priapism occurred at higher doses [1], suggesting a direct involvement of the Vcsa1 product in erectile function.
Penile erection depends on the relaxation of smooth muscle in the corpora cavernosum [13,14]. It was shown that endogenous selective μ-opioid receptor peptide agonists (endomorphins 1 and 2) can relax aortic vascular smooth muscle from the rat aorta by an endothelium-dependent mechanism [21]. In addition, synthetic inhibitors of NEP, such as thiorphan or phosphoramidon, will enhance CNP-induced relaxation of porcine coronary artery [9]. Vasopeptidase inhibitors are used to treat hypertension because of their ability to reduce vasocontraction and to enhance vasodilatation [22]. As described above, the mature peptide product of Vcsa1, sialorphin, has potent analgesic activity in rats because of its ability to act as a NEP inhibitor, thereby activating μ- and δ-opioid receptor-dependent enkephalin pathways [4].
Combining these observations, a reasonable hypothesis for the mechanism of action of gene transfer of Vcsa1 in restoring erectile function is that the gene product, sialorphin, acts locally as an inhibitor of NEP, thereby enhancing the activity of agonist-to-opioid receptors that stimulate smooth muscle relaxation. It is possible that NEP inhibitors at higher levels result in sustained smooth muscle relaxation, so that there is activation of the pathways involved in priapism [23]. We reported that priapism results at higher levels of Vcsa1 transfer [1]. In addition to vasorelaxant effects, CNP also mediates hyperpolarizing effects. The hyperpolarizing events for CNP were significantly diminished by iberiotoxin, a selective maxi-K-channel blocker [24]. Further evidence for the involvement of potassium channels in natriuretic peptide-induced relaxation of smooth muscle cells is that high KCl potently suppressed relaxation [24]. We recently reported that gene transfer of plasmids expressing the maxi-K channel (pVAX-hSlo) can be used to treat ED in ageing rats [25], and we are investigating the potential of this therapy to treat human ED in clinical trials [16]. Activation or overexpression of the maxi-K channels results in hyperpolarization of cells, which inhibits L-type calcium channels, lowering intracellular calcium. Lowering intracellular calcium inactivates myosin light-chain kinase, and then the myosin is dephosphorylated by smooth muscle myosin phosphatase, leading to smooth muscle relaxation [26–28] (Fig. 3).
FIG. 3.
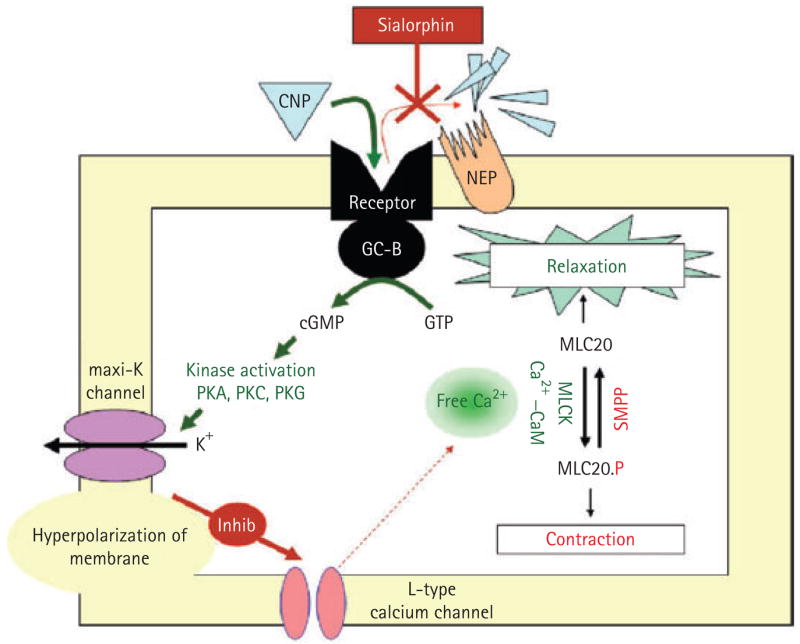
The proposed pathway by which sialorphin causes smooth muscle relaxation and thereby improves erectile function. CNP binds to its membrane receptor on corporal smooth muscle cells and activates GC-B. The CNP is degraded by NEP. However, in the presence of sialorphin (acting as a NEP inhibitor), the CNP has a prolonged effect, activating downstream mechanisms resulting in smooth muscle relaxation, mediated by the secondary messenger cGMP. Among these downstream activators are maxi-K channels. Efflux of potassium from the cells causes hyperpolarization of the smooth muscle cell membrane, inhibiting influx of Ca2+ through calcium channels. Lowered intracellular calcium causes inactivation myosin light-chain kinase (MLCK), thereby promoting smooth muscle relaxation. GTP, guanosine triphosphate, PKA, protein kinase A, PKC, protein kinase C; PKG, protein kinase G, MLC20, myosin light chain.
As described above, CNP binds to the corporal smooth muscle GC-B receptor present in both rats and rabbits, and CNP can cause relaxation of isolated rabbit corporal smooth muscle tissue [11,12]. Therefore, the potential downstream mediators of sialorphin-induced relaxation of corporal smooth muscle cells might be through its action as a NEP inhibitor causing prolonged binding of CNP to corporal smooth muscle GC-B receptor present on the rat’s smooth muscle tissue membrane, with consequent raising of the intracellular cGMP, causing activation of the maxi-K channel. A proposed pathway by which sialorphin causes smooth muscle relaxation is shown in Fig. 3. These reports suggest that gene transfer protocols using the maxi-K channel might have improved efficacy when combined with NEP inhibitors such as sialorphin.
Overall, these results suggest that gene transfer of a plasmid expressing Vcsa1 results in improved erectile function because of expression of its gene product, sialorphin. Furthermore, a component of ED observed with diabetes, ageing and neuronal injury is probably associated with decreased production of sialorphin, and that we have now shown that erectile function can be restored with either the administration of the gene (Vscsa1) or its protein product (sialorphin). The results show that the role of sialorphin in erectile function is complex and indirect, being mediated through the inhibition of NEP, an enzyme that functions as a control mechanism to prevent sustained peptide-GC-B-receptor-cGMP activity. The limiting action of NEP is similar to that of phosphodiesterase activity in the smooth muscle cells; it is an enzyme that when blocked by phosphodiesterase inhibitors also results in prolonged erection. The results of the present experiments show that injection of the sialorphin (the mature peptide product of Vcsa1) into the penile corpora significantly improves erectile function and suggests that inhibitors of NEP might provide novel targets for treating ED or might be useful for increasing the activity of existing treatments.
Abbreviations
- Vcsa1
variable coding sequence a1 gene (also known as a submandibular rat 1 gene)
- ED
erectile dysfunction
- NEP
neutral endopeptidase
- CNP
C-type natriuretic peptide
- ICP/BP
intracorporal pressure/systemic blood pressure ratio
- GC-B
guanylyl cyclase B
- DMSO
dimethyl sulphoxide
Footnotes
CONFLICT OF INTEREST
Source of funding: grants P01-DK060037 and K01-DK67270 (awarded to K.P. Davies) from the NIH, NIDDK.
References
- 1.Tong Y, Tar M, Davelman F, Christ G, Melman A, Davies KP. Variable coding sequence protein A1 as a marker for erectile dysfunction. BJU Int. 2006;98:396–401. doi: 10.1111/j.1464-410X.2006.06247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.User HM, Zelner DJ, McKenna KE, McVary KT. Microarray analysis and description of SMR1 gene in rat penis in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;170:298–301. doi: 10.1097/01.ju.0000060882.75475.5a. [DOI] [PubMed] [Google Scholar]
- 3.Rougeot C, Rosinski-Chupin I, Njamkepo E, Rougeon F. Selective processing of submandibular rat 1 protein at dibasic cleavage sites. Salivary and bloodstream secretion products. Eur J Biochem. 1994;219:765–73. doi: 10.1111/j.1432-1033.1994.tb18556.x. [DOI] [PubMed] [Google Scholar]
- 4.Rougeot C, Messaoudi M, Hermitte V, et al. Sialorphin, a natural inhibitor of rat membrane-bound neutral endopeptidase that displays analgesic activity. Proc Natl Acad Sci USA. 2003;100:8549–54. doi: 10.1073/pnas.1431850100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosinski-Chupin I, Rougeot C, Courty Y, Rougeon F. Localization of mRNAs of two androgen-dependent proteins, SMR1 and SMR2, by in situ hybridization reveals sexual differences in acinar cells of rat submandibular gland. J Histochem Cytochem. 1993;41:1645–9. doi: 10.1177/41.11.8409372. [DOI] [PubMed] [Google Scholar]
- 6.Rosinski-Chupin I, Tronik D, Rougeon F. High level of accumulation of a mRNA coding for a precursor-like protein in the submaxillary gland of male rats. Proc Natl Acad Sci USA. 1988;85:8553–7. doi: 10.1073/pnas.85.22.8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosinski-Chupin I, Huaulme JF, Rougeot C, Rougeon F. The transcriptional response to androgens of the rat VCSA1 gene is amplified by both binary and graded mechanisms. Endocrinology. 2001;142:4550–9. doi: 10.1210/endo.142.10.8428. [DOI] [PubMed] [Google Scholar]
- 8.Messaoudi M, Desor D, Nejdi A, Rougeot C. The endogenous androgen-regulated sialorphin modulates male rat sexual behavior. Horm Behav. 2004;46:684–91. doi: 10.1016/j.yhbeh.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Marton Z, Pataricza J, Krassoi I, Varro A, Papp JG. NEP inhibitors enhance C-type natriuretic peptide-induced relaxation in porcine isolated coronary artery. Vascul Pharmacol. 2005;43:207–12. doi: 10.1016/j.vph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Walther T, Stepan H. C-type natriuretic peptide in reproduction, pregnancy and fetal development. J Endocrinol. 2004;180:17–22. doi: 10.1677/joe.0.1800017. [DOI] [PubMed] [Google Scholar]
- 11.Kuthe A, Reinecke M, Uckert S, et al. Expression of guanylyl cyclase B in the human corpus cavernosum penis and the possible involvement of its ligand C-type natriuretic polypeptide in the induction of penile erection. J Urol. 2003;169:1918–22. doi: 10.1097/01.ju.0000055602.35858.8f. [DOI] [PubMed] [Google Scholar]
- 12.Kim SZ, Kim SH, Park JK, Koh GY, Cho KW. Presence and biological activity of C-type natriuretic peptide-dependent guanylate cyclase-coupled receptor in the penile corpus cavernosum. J Urol. 1998;159:1741–6. doi: 10.1097/00005392-199805000-00104. [DOI] [PubMed] [Google Scholar]
- 13.Andersson KE. Pharmacology of penile erection. Pharmacol Rev. 2001;53:417–50. [PubMed] [Google Scholar]
- 14.Andersson KE. Erectile physiological and pathophysiological pathways involved in erectile dysfunction. J Urol. 2003;170:S6–14. doi: 10.1097/01.ju.0000075362.08363.a4. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Cadavid NF, Rajfer J. Molecular pathophysiology and gene therapy of aging-related erectile dysfunction. Exp Gerontol. 2004;39:1705–12. doi: 10.1016/j.exger.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. The first human trial for gene transfer therapy for the treatment of erectile dysfunction: preliminary results. Eur Urol. 2005;48:314–8. doi: 10.1016/j.eururo.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Christ GJ, Day N, Santizo C, et al. Intracorporal injection of hSlo cDNA restores erectile capacity in STZ-diabetic F-344 rats in vivo. Am J Physiol Heart Circ Physiol. 2004;287:H1544–53. doi: 10.1152/ajpheart.00792.2003. [DOI] [PubMed] [Google Scholar]
- 18.Christ GJ, Rehman J, Day N, et al. Intracorporal injection of hSlo cDNA in rats produces physiologically relevant alterations in penile function. Am J Physiol. 1998;275:H600–8. doi: 10.1152/ajpheart.1998.275.2.H600. [DOI] [PubMed] [Google Scholar]
- 19.Chang S, Hypolite JA, Changolkar A, Wein AJ, Chacko S, DiSanto ME. Increased contractility of diabetic rabbit corpora smooth muscle in response to endothelin is mediated via Rho-kinase beta. Int J Impot Res. 2003;15:53–62. doi: 10.1038/sj.ijir.3900947. [DOI] [PubMed] [Google Scholar]
- 20.Spektor M, Rodriguez R, Rosenbaum RS, Wang HZ, Melman A, Christ GJ. Potassium channels and human corporeal smooth muscle cell tone: further evidence of the physiological relevance of the Maxi-K channel subtype to the regulation of human corporeal smooth muscle tone in vitro. J Urol. 2002;167:2628–35. [PubMed] [Google Scholar]
- 21.Hugghins SY, Champion HC, Cheng G, Kadowitz PJ, Jeter JR., Jr Vasorelaxant responses to endomorphins, nociceptin, albuterol, and adrenomedullin in isolated rat aorta. Life Sci. 2000;67:471–6. doi: 10.1016/s0024-3205(00)00631-7. [DOI] [PubMed] [Google Scholar]
- 22.Lapointe N, Rouleau JL. Cardioprotective effects of vasopeptidase inhibitors. Can J Cardiol. 2002;18:415–20. [PubMed] [Google Scholar]
- 23.Champion HC, Bivalacqua TJ, Takimoto E, Kass DA, Burnett AL. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci USA. 2005;102:1661–6. doi: 10.1073/pnas.0407183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otsuka K, Tanaka H, Horinouchi T, Koike K, Shigenobu K, Tanaka Y. Functional contribution of voltage-dependent and Ca2+ activated K+ (BK (Ca)) channels to the relaxation of guinea-pig aorta in response to natriuretic peptides. J Smooth Muscle Res. 2002;38:117–29. doi: 10.1540/jsmr.38.117. [DOI] [PubMed] [Google Scholar]
- 25.Melman A, Zhao W, Davies KP, Bakal R, Christ GJ. The successful long-term treatment of age related erectile dysfunction with hSlo cDNA in rats in vivo. J Urol. 2003;170:285–90. doi: 10.1097/01.ju.0000063375.12512.6e. [DOI] [PubMed] [Google Scholar]
- 26.Hartshorne DJ, Ito M, Erdodi F. Myosin light chain phosphatase: subunit composition, interactions and regulation. J Muscle Res Cell Motil. 1998;19:325–41. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- 27.Leblanc N, Ledoux J, Saleh S, et al. Regulation of calcium-activated chloride channels in smooth muscle cells: a complex picture is emerging. Can J Physiol Pharmacol. 2005;83:541–56. doi: 10.1139/y05-040. [DOI] [PubMed] [Google Scholar]
- 28.Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol. 2005;83:215–42. doi: 10.1139/y05-016. [DOI] [PubMed] [Google Scholar]


