Abstract
v7−3 (SLC6A15) is the prototype for a gene subfamily whose members have sequence homologies to classical Na+- and Cl−-dependent neurotransmitter transporters but display unusual features that include characteristic large fourth extracellular loops. Interest in v7−3 has been increased by elucidation of its expression in neurons located in cerebral cortex, hippocampus, cerebellum, midbrain, and olfactory bulb. To help clarify the role of v7−3 in brain functions, we have created and characterized v7−3 knockout mice. These mice lack functional v7−3 protein, but are viable and fertile. While our studies were in progress, v7−3 expression was reported to confer transport of proline and branched-chain amino acids in in vitro expression systems (Takanaga et al. 2005; Broer et al. 2006). Assessment of amino acid uptake into cortical synaptosomes of v 7−3 knockouts identifies 15% and 40% reductions in sodium-dependent proline and leucine transport, respectively, compared to wild type controls. Despite these biochemical changes, v7−3 knockout mice demonstrate only modest alterations in rotarod performance with aging and lack reproducible alterations in other motor, memory, anxiety or olfactory tests. Compensation for the lack of v7−3 via other amino acid carriers is likely to leave v7−3 knockouts without gross behavioral manifestations. The current results place v7−3 in the context of other brain transporters that accumulate proline and branched-chain amino acids.
Keywords: v7−3 knockout, orphan neurotransmitter transporter, B0AT2, SBAT1
1. Introduction
Work in the 1990's in our laboratory and others elucidated genes that encoded sodium- and chloride-dependent neurotransmitter transporters including those for dopamine (Giros et al. 1991; Shimada et al. 1991) and GABA (Guastella et al. 1990). These studies also identified a family of genes that encode proteins with structures that were similar to Na+/Cl−-dependent neurotransmitter transporters but whose substrates were not identified. The first member of this family was termed v7−3, since it derived from the ventral midbrain cDNA library used to clone the dopamine transporter (Uhl et al. 1992). Elucidation of the v7−3 gene, now also termed SLC6A15, was followed by identification of NTT4/Rxt1 (SLC6A17), a product of efforts to clone GABA transporters (Liu et al. 1993; el Mestikawy et al. 1994). More peripherally-expressed members of this family included ROSIT/XT2 (SLC6A18) and rB21a/SIT1 (SLC6A20) (Wasserman et al. 1994; Smith et al. 1995). All these apparent transporters shared sequence homologies with the prototypical Na+/Cl−-dependent neurotransmitter transporters; each also encoded an unusually large glycosylated domain that was tentatively identified as a fourth extracellular loop (Uhl et al. 1992). All these genes also resisted initial attempts to identify their physiological substrates; they were thus described as an “orphan” transporter subfamily.
V7−3 and NTT4 are the members of this orphan transporter subfamily that are expressed almost exclusively in neurons. Proteins encoded by these two genes display 66% amino acid identity with other subfamily members, though they manifest longer C- and N-terminal cytoplasmic tails. v 7−3 is expressed at higher levels than NTT4 (Hoglund et al. 2005). Nevertheless, in situ hybridization studies reveal largely overlapping expression patterns, with highest levels identified in olfactory bulb, cerebral cortex, hippocampus and cerebellum (Inoue et al. 1996; Luque et al. 1996; Masson et al. 1996). Whereas most epitope-tagged v7−3 is expressed at the cell surface (Farmer et al. 2000), transfection, fractionation and immunocytochemical results suggest that much NTT4 immunoreactivity may reside on synaptic vesicles (Masson et al. 1999).
While the studies described below were in progress, v7−3 expression was reported to confer transport of proline and branched-chain amino acids in Xenopus oocytes (Takanaga et al. 2005; Broer et al. 2006). However, these interesting observations have still left broad uncertainties about the physiological importance of the amino acid transport mediated by the v7−3 gene's products. Brain proline transport by a number of systems has been described: 1) the brain-specific high affinity proline transporter PROT (SLC6A7) is expressed in a subset of glutamatergic nerve terminals where it is likely to help regulate excitatory transmission (Fremeau et al. 1992; Crump et al. 1999; Renick et al. 1999); 2) members of the amino acid transport system A, SNAT1 (SLC38A1) and SNAT2 (SLC38A2), are expressed by both glutamatergic neurons and non-neuronal cells (Yao et al. 2000; Gonzalez-Gonzalez et al. 2005); 3) proton-coupled amino acid transporters PAT1 (SLC36A1), which is implicated in the transport of lysosomal proteolysis products into the cytosol, and PAT2 (SLC36A2) contribute to neuronal transport and sequestration of amino acids such as proline, glycine and alanine (Agulhon et al. 2003; Rubio-Aliaga et al. 2004; Kennedy et al. 2005); and 4) system IMINO/rB21a/SIT1 (SLC6A20) is expressed in pia mater and choroid plexus, suggesting potential involvement in the transport of proline into and/or out of the brain (Kowalczuk et al. 2005; Takanaga et al. 2005). The existence of such a variety of brain systems for proline compartmentalization suggests a strong biological requirement for regulation of local concentrations of this amino acid, perhaps befitting proline's abilities to both modulate neurotransmission and mediate excitotoxicity (Nadler et al. 1988). In this context, the role of v7−3 in physiological proline compartmentalization in brain, and in still-less-well understood mechanisms involving leucine uptake, has remained poorly defined.
We now report results of attempts to help elucidate the roles of v7−3 in brain function and the contributions of v7−3 to proline and leucine transport by creating and characterizing v7−3 knockout mice. Studies of these animals support a modest-to-moderate quantitative role for this transporter in synaptosomal accumulation of proline and leucine. Data from these mice also support the idea that other systems active in proline transport seem to be able to compensate for the lack of v7−3. Mice lacking functional v7−3 protein are viable, fertile, and fail to demonstrate any striking abnormality in performance in almost all of a battery of tests of physiological, motor and sensory functions.
2. Results
2.1. Generation of v7−3 knockout mice
To investigate v7−3 function in vivo, we disrupted exon 3 of the v7−3 gene in embryonic stem cells using homologous recombination (Fig. 1a). Exon 3 contains the initiation codon and the first transmembrane domain involved in substrate binding (Yamashita et al. 2005); its disruption should thus inactivate the v7−3 gene product. Correctly-argeted ES cell clones were identified by PCR (Fig. 1b) and confirmed by Southern blotting (Fig. 1c). Recombinant ES cells were injected into blastocysts, which were implanted into pseudopregnant females and resulted in four chimeric mice. Mating of the heterozygous progeny of the chimeric mice produced wild type, heterozygous and knockout pups at expected Mendelian frequencies without sexual bias. Pups were genotyped by PCR with a set of 3 primers that simultaneously amplified wild type (527 bp) and knockout (694 bp) alleles (Fig. 1d). The disruption of the v7−3 locus was further confirmed by RT-PCR. Amplification using the oligonucleotides J52 (homologous to the deleted part of the gene) and J53 produced an amplicon of the expected size in DNA extracted from wild type and heterozygous samples, but not from homozygous knockouts (Fig. 1e).
Figure 1. Generation and analysis of v7−3 knockout mice.
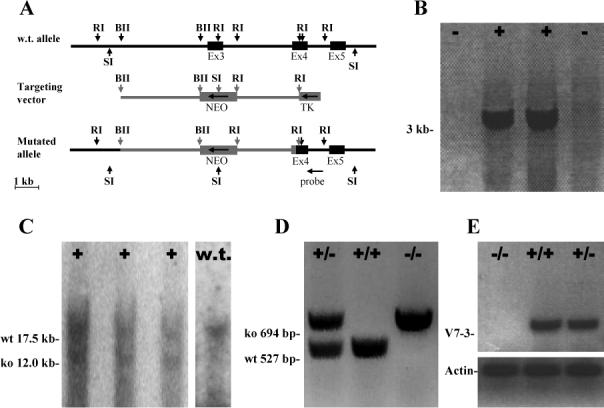
(A) Genomic organization of the v7−3 gene (black) and the gene targeting construct (gray). Full boxes represent exons and arrow heads important restriction sites: RI, EcoRI; BII, Bgl II, SI, Sac I. Direction of transcription for Neo is opposite to that of v7−3 as indicated by the arrow. (B) Two positive and two negative ES cell clones screened by PCR with primers J5 and J8. (C) Southern blot analysis of the clones positive by PCR. SacI digestion produced a 17.5 kb fragment from the wild type allele, and a 12.0 kb fragment from the mutated allele because the Neo cassette introduced a new SacI site. (D) PCR genotyping of v7−3 mice. (E) RT-PCR analysis of brain expression of the v7−3 (primers J52, J53) and actin (ACTf, ACTr) in wild type and mutant mice.
2.2. Proline transport
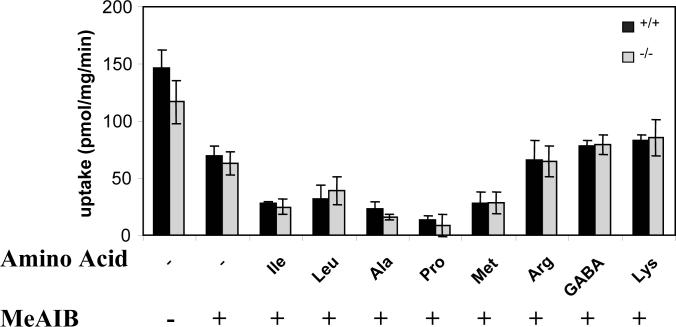
L-[14C]proline was efficiently transported by crude cortical synaptosomes prepared from wild type mice. This transport was inhibited by YGGFL (0.1 mM) or MeAIB (10 mM) to about 60 % of control values (data not shown and Figure 2, respectively). Residual L-[14C] proline uptake was further inhibited by unlabeled 10 mM isoleucine, leucine, alanine, methionine or proline. Neither arginine, GABA nor lysine were effective (Fig. 2).
Figure 2. Inhibition of proline transport by different amino acids.
Proline transport into 40 μg of crude synaptosomal fraction (P3 pellet) was measured in the presence of 10 mM MeAIB and 10 mM L-amino acid as indicated. Data are expressed as means ± SEM obtained from triplicate samples and confirm previous observations (Broer et al. 2006).
Synaptosomes prepared from brains of v7−3 KO mice displayed transport activities that were modestly lower than those found in synaptosomes prepared from wild type mice. Sensitivity of this uptake to competition by other amino acids was indistinguishable from the sensitivity found in synaptosomes prepared from wild type mice. MeAIB-resistant proline transport activity thus cannot be ascribed solely to v7−3.
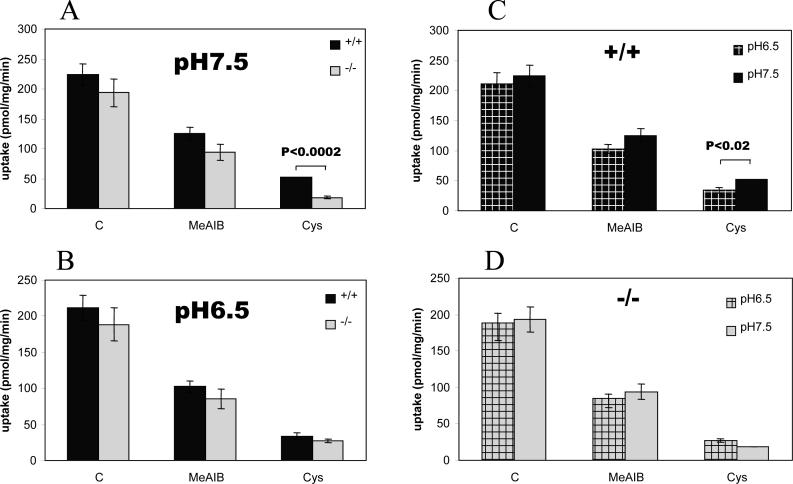
To better estimate the quantitative contribution of v7−3 to synaptosomal proline accumulation, we sought a competitor that would selectively inhibit much of the proline uptake that was not mediated through v7−3. Cysteine inhibits the high affinity proline transporter PROT (SLC6A7) with Ki = 91 μM (Fremeau et al. 1992), but is reported to be ineffective at blocking v7−3 (Broer et al. 2006). In our experiments with synaptosomal preparations from wild type mice cerebral cortex, cysteine inhibited [14C]proline accumulation by ca 80 %. In v7−3 knockout mice, less cysteine-resistant [14C]proline transport activity was found in cortical synaptosomes (p < 0.0005, Fig. 3a). This difference between wild type and v7−3 knockouts is ca. 15 % of the total proline accumulated by synaptosomes from wild type mice.
Figure 3. Effect of pH and Cysteine on proline transport.
Proline transport into 40 μg of crude synaptosomal fraction (P3 pellet) was measured in the presence of 10 mM MeAIB or 10 mM L-cysteine. (A) At pH 7.5 cysteine resistant transport activity of synaptosomes from v7−3 KO was significantly lower than the activity of wild type synaptosomes. (B) At pH 6.5 there was no difference in cysteine resistant transport activity between wild type and KO synaptosomes. At pH 6.5, cysteine resistant transport activity is significantly lower in the wild type synaptosomes (C), but not in the KO synaptosomes(D). Data are expressed as means ± SEM obtained from 3 independent experiments using triplicate samples.
v7−3 uptake activity has been reported to demonstrate strong pH dependence (Takanaga et al. 2005; Broer et al. 2006). We thus compared cysteine-resistant proline uptake at lower pH in synaptosomes from wild type vs v7−3 knockouts. At pH 6.5, the difference between wild type and knockout synaptosomes disappeared (Fig. 3b) due to a 48 % reduction of proline uptake in the wild type mice (Fig. 3c). The residual proline transport in v7−3 KO synaptosomes assayed in the presence of 10 mM cysteine was unaffected (Fig. 3d). In the absence of more specific pharmacological tools, these data each support the idea that a significant portion of the cysteine-resistant proline uptake into cortical synaptosomes can be attributed to v7−3.
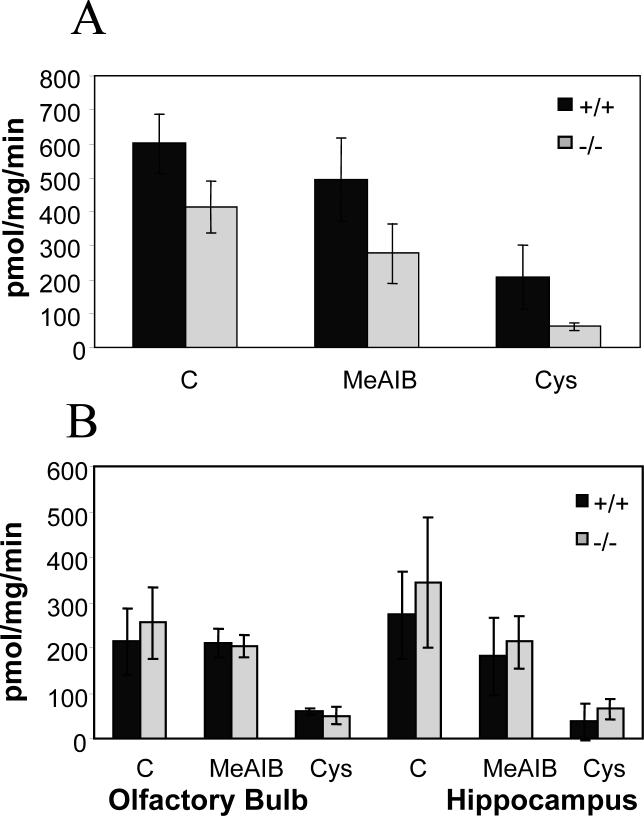
To provide further support for these observations, we repeated uptake experiments in synaptosomal preparations enriched by phase partitioning. Purified synaptosomes prepared from v7−3 KOs retained a substantial amount (ca 65 %) of the MeAIB-resistant proline accumulation found in wild type synaptosomes. Conversely, cysteine-resistant proline accumulation was almost abolished in the knockouts (Fig. 4a). This result supports the likelihood that observations made in crude synaptosomal preparations are due to their content of synaptosomes that are assessed in enriched preparations.
Figure 4. Proline transport.
(A) Proline transport into 20 μg of synaptosomes purified by phase partitioning was measured in the presence of 10 mM MeAIB or 10 mM L-cysteine. (B) Proline transport into 40 μg of crude synaptosomal fraction (P3 pellet) from hippocampus and olfactory bulb was measured in the presence of 10 mM MeAIB or 10 mM L-cysteine. Data are expressed as means ± SEM obtained from 3 independent experiments using triplicate samples.
The results with cerebral cortical synaptosomes were not replicated in crude synaptosomal fractions from the olfactory bulb and hippocampus. v7−3 deletion exerted no significant effect on proline accumulation in either area (Figure 4b), consistent with more robust involvement of other proline transport systems in these regions of the nervous system.
2.3. Leucine transport
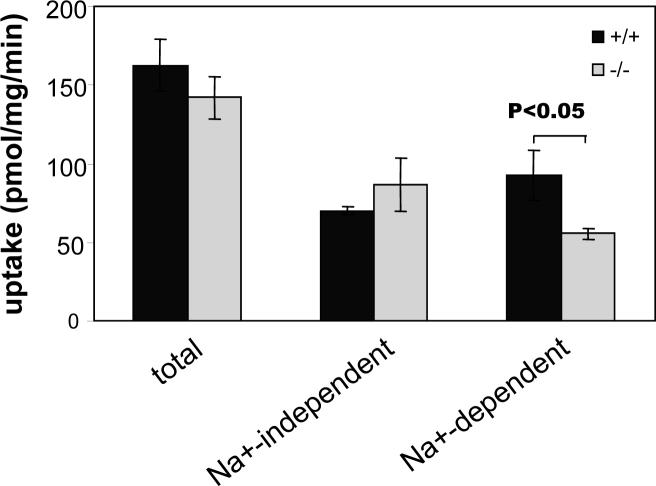
v7−3 has been reported to transport branched chain amino acids, such as leucine, with twice its affinity for proline (Takanaga et al. 2005; Broer et al. 2006). Total and Na+-independent leucine transport into crude cortical synaptosomes prepared from mutant mice did not differ significantly from transport into wild type synaptosomes. However, the Na+-dependent fraction of leucine transport was lower in knockout synaptosomes than in the control preparations (p < 0.05, Figure 5). The difference between wild type and v7−3 knockout transport represented 10 − 20 % of the total leucine accumulation in wild type synaptosomes. Similar results were obtained with cortical synaptosomes purified by phase partitioning and with crude synaptosomes from olfactory bulb and hippocampus (data not shown), suggesting a significant role for v7−3 in leucine uptake in these regions as well.
Figure 5. Leucine transport into cortical synaptosomes.
Leucine transport into 40 μg of crude synaptosomal fraction (P3 pellet) was assayed in NaCl containing buffer (total transport) or in the same buffer where NaCl was replaced with ChoCl (Na+-independent transport). Na+-dependent transport was calculated as the difference between total and Na+-independent values. Data are expressed as means ± SEM obtained from 3 independent experiments using triplicate samples.
2.4. Fertility and behavioral phenotype
In assessments of breeding, v7−3 KO females were mated with C57Bl/6J males and v7−3 KO males were mated with C57Bl/6J females. There was no difference between the two sorts of pairings in: 1) latency to produce offspring, 2) litter sizes, and 3) maternal behavior in retrieving 3−5 day old pups. v7−3 females displayed estrous cycles of the same duration as their wild type littermates.
No neurological or behavioral abnormalities were observed in the v7−3 KO mice during a number of tests (Table 2). Knockouts gained weight at the same rate as their wild type siblings during their first year of life. The animals displayed posture, gait and reflexes that were indistinguishable from those of their wild type littermates. Eye blink, ear twitch or whisker reflexes elicited by light touches with cotton swabs were all similar to responses in wild type mice. v7−3 knockouts failed to display wild running, freezing, circling, or stereotypy.
Table 2.
Behavioral phenotyping of the V73KO mice. Number of subjects is in brackets.
| Age | Sex | +/+ | +/− | −/− | P | |
|---|---|---|---|---|---|---|
| Observational Batery | ||||||
| Piloerection, bald Patches | 8 w | M, F | No (7) | No (11) | No (6) | |
| Ear/foot color | 8 w | M, F | pink | pink | pink | |
| Posture, gait, grooming, righting | 8 w | M, F | normal | normal | normal | |
| Reflexes (Eye blink, ear twitch, wisker orienting) | 8 w | M, F | normal | normal | normal | |
| Abnormal behavior (cicrling, stereotypy, freezing) | 8 w | M, F | No | No | No | |
| Weight | 8 w | M | 35 g (10) | 38 g (10) | 31 g (6) | 0.25 |
| Locomotion (60min, total distance traveled in m) | ||||||
| Saline | 2−3 M | M, F | 2.58 (10) | 1.88 (7) | 3.44 (12) | 0.31 |
| Cocaine (10 mg/kg, s.c.) | 2−3 M | M, F | 3.84 (10) | 6.81 (7) | 5.50 (12) | 0.45 |
| Ethanol (2 mg/kg, i.p.) | 2−3 M | M, F | 6.43 (10) | 7.05 (10) | 9.18 (10) | 0.52 |
| Morphine (3 mg/kg, s.c.) | 2−3 M | M, F | 2.46 (10) | 2.77 (10) | 2.28 (10) | 0.95 |
| Conditioned Place Preference (Cocaine, 10 mg/kg, s.c.) | ||||||
| Time change in drug-paired environment (s) | 2−3 M | M, F | 234 (9) | 193 (10) | 0.70 | |
| Motor functions | ||||||
| latency to fall off of an accelerating rotarod (s) | 8 w | M, F | 253 (10) | 206 (13) | 252 (12) | 0.19 |
| 6 M | M | 203 (14) | 192 (13) | 228 (11) | 0.47 | |
| 6 M | F | 233 (8) | 265 (11) | 281 (10) | 0.15 | |
| 12 M | M | 159 (10) | 132 (6) | 172 (7) | 0.59 | |
| 12 M | F | 188 (17) | 219 (20) | 258 (14) | 0.07 | |
| Learning in Morris Water Maze | ||||||
| Latency to platform quadrant (s) | 2−3 M | M, F | 2.3 (10) | 2.9 (10) | 1.9 (13) | 0.32 |
| Latency to new location (s) | 2−3 M | M, F | 1.7 (10) | 2.8 (10) | 2.9 (13) | 0.35 |
| Time in new/time in old | 2−3 M | M, F | 2.4 (10) | 2.2 (10) | 2.2 (13) | 0.92 |
| Breeding behavior | ||||||
| Estrous cycle -cycle duration (days) | 2−3 M | F | 6.6 (12) | 8.6 (12) | 0.25 | |
| Latency to produce a litter (days) | 2−3 M | F | 32 (13) | 33 (10) | 0.87 | |
| Litter size (# of pups) | 2−3 M | F | 6.9 (13) | 6.2 (10) | 0.61 | |
| Latency to collect 4 days old pups (s) | 2−3 M | F | 23 (6) | 31 (5) | 0.66 | |
| Olfaction | ||||||
| Latency to approach opposite sex's urine (s) | 2−3 M | M | 60 (12) | 43 (5) | 41 (10) | 0.64 |
| 2−3 M | F | 15 (10) | 52 (12) | 101 (11) | 0.0007* | |
| Latency to approach peanut butter odor (s) | 2−3 M | M | 33 (15) | 36 (13) | 33 (11) | 0.41 |
| 2−3 M | F | 53 (12) | 97 (18) | 92 (13) | 0.13 | |
| Olfactory discrimination (%correct) | ||||||
| 100 % odorant A | 2−3 M | M, F | 82 (11) | 92 (12) | ||
| 60 % odorant A, 40 % odorant B | 2−3 M | M, F | 79 (11) | 72 (12) | ||
| 58 % odorant A, 42 % odorant B | 2−3 M | M, F | 67 (11) | 69 (12) | ||
| Avoidance of aversive stimulus (TMT) | ||||||
| Time spent in TMT environment (s) | 2−3 M | M, F | 259 (11) | 338 (19) | 0.045* | |
| Analgesia | ||||||
| Latency to tail flick (53 °C water bath) | 2−3 M | M, F | 5.9 s (10) | 5.6 s (11) | 5.8 s (11) | 0.90 |
| Latency to jump/lick a paw (55 °C hot plate) | 2−3 M | M, F | 10.9 s (10) | 15.3 s (11) | 14.7 s (11) | 0.55 |
| Seizures: latency to tonic/clonic (s) | ||||||
| Pentylene tetrazole 80 mg/kg | 2−3 M | M, F | 111 (4) | 96 (4) | 0.39 | |
| Pentylene tetrazole 60 mg/kg | 2−3 M | M, F | 181 (14) | 289 (14) | 0.39 | |
| Anxiety | ||||||
| Elevated + maze — Latency to open (s) | 2−3 M | M, F | 34 (10) | 69 (10) | 48 (10) | 0.65 |
| Time in open (s) | 2−3 M | M, F | 37.4 (10) | 26.7 (10) | 30.7 (10) | 0.54 |
| Entries to open | 2−3 M | M, F | 5.5 (10) | 5.3 (10) | 4.9 (10) | 0.90 |
| Dark box - Latency to emerge (s) | 2−3 M | M, F | 40 (12) | 33 (7) | 39 (12) | 0.86 |
| Time in light compartment (s) | 2−3 M | M, F | 241 (12) | 196 (7) | 139 (12) | 0.28 |
| Open field - Time in center (s) | 2−3 M | M, F | 84 (12) | 40 (7) | 92 (12) | 0.17 |
| Number of entries | 2−3 M | M, F | 129 (12) | 104 (12) | 0.52 | |
| Novel object exploration (s) | 2−3 M | M, F | 26 (11) | 22 (9) | 25 (10) | 0.9 |
| Sress-Induced Anxiety | ||||||
| Dark box - Latency to emerge (s) baseline | 2−3 M | M, F | 481 (12) | 472 (12) | 0.96 | |
| stress | 2−3 M | M, F | 140 (12) | 2178 (12) | 0.025 | |
| - Time outside (s) baseline | 2−3 M | M, F | 241 (12) | 193 (12) | 0.16 | |
| stress | 2−3 M | M, F | 240 (12) | 136 (12) | 0.057 | |
| Open field - Time in center (s) baseline | 2−3 M | M, F | 83 (12) | 92 (12) | 0.76 | |
| stress | 2−3 M | M, F | 50 (12) | 24 (12) | 0.07 | |
| Number of entries baseline | 2−3 M | M, F | 129 (12) | 104 (12) | 0.49 | |
| stress | 2−3 M | M, F | 63 (12) | 32 (12) | 0.11 |
Not replicated in the subsequent experiments
v7−3 knockouts did not differ from wild type littermates in locomotor behaviors observed after injection of saline, cocaine (10 mg/kg), ethanol (2 mg/kg), or morphine (3 mg/kg). The knockout animals showed robust preferences for the places in which they received cocaine (10 mg/kg) in a conditioned place preference paradigm; data was similar to that obtained from wild type mice. v7−3 knockouts and wild type littermates displayed equal sensitivities to induction of epileptiform activity by pentylene tetrazole (60−80 mg/kg) or cocaine (80 mg/kg).
Since v7−3 is highly expressed in the olfactory bulb and since urines from mice of the opposite gender provides a salient olfactory stimulus, knockout mice were initially tested for their responsiveness to such urine samples. Mutant females appeared less responsive to odors from male urine than their wild type littermates in an initial experiment (Table 2). This difference, however, could not be reproduced in later experiments (data not presented). Since the estrous cycle could have been a confounding factor in this assay, we tested another group of mice for their response to another odorant, TMT. v7−3 knockouts showed less avoidance of this aversive olfactory stimulus in initial experiments, but this observation could not be repeated in subsequent attempts at replication. In an olfactory discrimination task performed under food deprived conditions, v7−3 knockouts again performed as well as wild type mice. Therefore, we concluded that these mice have grossly normal olfactory acuity, although their performance may be modulated by factors that we have not controlled in the present experiments in ways that are different from modulation in wildtype animals.
Hippocampus and cerebellum are two other brain areas with the highest expression of v7−3 mRNA. To determine if v7−3 inactivation affects spatial learning, knockout mice were tested in the Morris water maze. There was no difference between KO mice and their wild type siblings in the rate at which they learned to locate the hidden platform, the time they spent searching for it in the correct quadrant during the probe trial, or reversal learning. To find out if v7−3 has a greater effect in older animals, the experiment was repeated in 1-year-old mice. Although the older mice learned more slowly, the learning curves for the wild type controls and the mutants were essentially identical (data not shown).
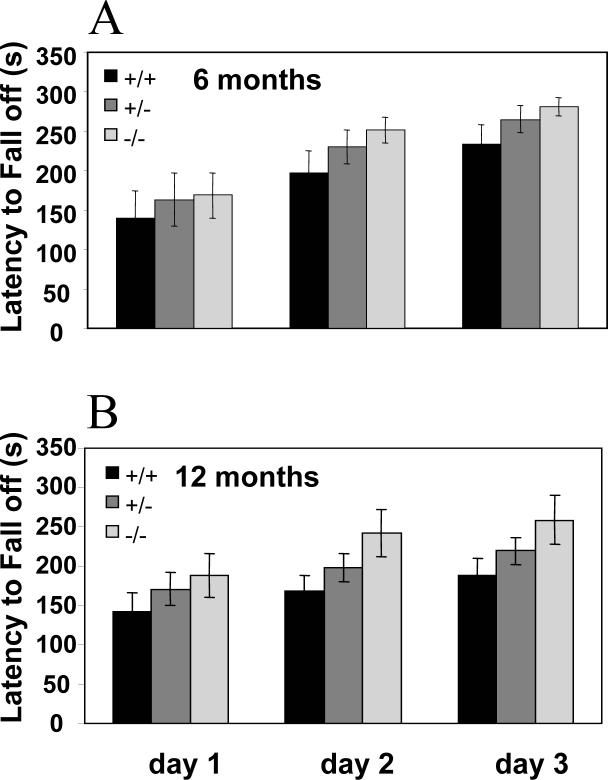
We studied locomotor coordination and learning on the accelerating rotarod in both young and mature animals. In young mice of either gender or in aged males, v7−3 mutation had no negative consequences in this behavioral task. Interestingly however, mature homozygote v7−3 KO females maintained their balance on the rotating cylinder for longer periods of time than their wild type littermates. Heterozygous mice displayed an intermediate phenotype (p < 0.05 for combined 6 and 12 month groups; Fig. 6).
Figure 6. Motor function assessment on accelerating rotarod.
(A) Latency of 6 month old female mice to fall off the rotating cylinder (data are expressed as means ±SEM for 8 − 10 animals/group). (B) Latency of 12 month old female mice to fall off the rotating cylinder. (data are expressed as means ± SEM for 14 − 20 animals/group). 6−12 months old homozygote v7−3 KO females maintain their balance on the rotating cylinder longer than their wild type littermates. Heterozygote mutants have an intermediate phenotype. (p = 0.0476, ANOVA for all groups combined)
Since v7−3 knockout produced few significant behavioral deficits in any of these circumstances, we asked if differences might be observed under more stressful circumstances. Mice were subjected to the stresses of forced swim testing were evaluated in light-dark box and open field paradigms immediately afterwards. The forced swim stress increased assessments of anxiety to similar levels in wild type and knockout mice. v7−3 thus does not appear to be necessary for normal stress effects on these behaviors.
3. Discussion
Generation and characterization of v7−3 knockout mice provides a new tool that helps us to place this transporter into appropriate biochemical and behavioral contexts in vivo. We discuss the data from these animals, and the limits of interpretation of this data, in this light.
3.1. Proline transport
Several brain systems capable of proline transport were described prior to elucidation of proline as a v7−3 substrate. SNAT1 (SLC38A1), SNAT2 (SLC38A2), system IMINO (SLC6A20), and PAT2 (SLC36A2) can be pharmacologically distinguished from v7−3 since they are all inhibited by MeAIB at concentrations that do not block v7−3 (Yao et al. 2000; Takanaga et al. 2002; Gonzalez-Gonzalez et al. 2005; Kennedy et al. 2005; Kowalczuk et al. 2005). The high affinity proline transporter PROT (SLC6A7) is inhibited by cysteine with Ki = 91 μM (Fremeau et al. 1992). At this concentration, cysteine had no effect on proline uptake by v7−3 (Broer et al. 2006). Since effects of MeAIB on PROT have not been well described, we used cysteine as a pharmacological tool to distinguish proline transport mediated by PROT from that mediated by v7−3.
MeAIB-resistant proline uptake in mouse brain synaptosomes that constitutes approximately 40% of total proline uptake with sensitivity to competition by the other v7−3 substrates isoleucine, leucine, alanine, and methionine was previously ascribed to v7−3 (Broer et al. 2006). In our present experiments with cortical synaptosomes from wild type mice, we confirmed ca 50% inhibition of proline uptake by MeAIB and competition for this MeAIB resistant transport by isoleucine, leucine, alanine, and methionine. v7−3 KO mice displayed trends toward reduced proline transport both with and without MeAIB (Fig. 2, 3 and 4a). However, the sensitivity of synaptosomes prepared from v7−3 KOs to amino acid competitors was indistinguishable from the profile of synaptosomes from wild type mice (Fig. 2). Availability of v7−3 KO mice allows us to conclude that MeAIB resistant proline uptake activity cannot be ascribed solely to v7−3. These conclusions are different from those that were previously drawn based on data from wild type animals (Broer et al. 2006), highlighting the power of knockouts when few specific pharmacologic analytic tools are available. In our experiments with crude synaptosomes prepared from wild type mice, cysteine inhibited [14C] proline accumulation by 80%. The remaining cysteine-resistant activity was almost abolished in crude synaptosomes prepared from the v7−3 KO mice. This cysteine-resistant uptake activity displayed pH dependence (Fig. 3) that paralleled the pH sensitivity previously reported for v7−3-mediated transport (Takanaga et al. 2005; Broer et al. 2006).
Based on each of these lines of evidence, we thus estimate that the contribution of v7−3 to the total proline uptake into cortical synaptosomes is ca 15 %, with smaller involvement in the olfactory bulb and hippocampus (Figure 4b).
3.2. Leucine transport
Few studies on Na+-dependent leucine uptake into rodent brain synaptosomes have been reported. Synaptosomal leucine uptake can be divided into high affinity and low affinity components (Rao et al. 1995). It has also been split into a Na+--independent component and a smaller (ca. 20 % of total), Na+-dependent component (Tan et al. 1988). In our experiments, Na+-dependent transport constituted almost 60 % of the total leucine uptake into the wild type synaptosomes. This Na+-dependent activity was reduced by 40% in the KO synaptosomes (Fig. 5), suggesting a significant contribution of v7−3 to Na+-dependent leucine transport. Based on all of these lines of evidence, we estimate that the contribution of v7−3 to total leucine transport is ca 15% but that it may provide as much as 40% of the Na+-dependent leucine uptake. This more robust contribution may be reflected in our observations that leucine uptake activity was reduced by the v7−3 deletion in olfactory bulb and hippocampus (data not shown), areas in which the knockouts did not display significant reductions in proline uptake.
3.3. Behavioral characterization of v7−3 KO mice
The biochemical results that document modest effects on v7−3 knockout on brain amino acid uptake properties are paralleled by the lack of evidence for any large effects on the health or fertility of these animals. A substantial battery of behavioral tests, with special attention to tasks sensitive to dysfunctions in the brain regions with high levels of v7−3 expression (e.g. olfactory bulb, hippocampus, thalamus, and cerebellum, (Inoue et al. 1996) failed to reveal any significant differences between WT and v7−3 KO mice (Table 2). The only exception was that mature homozygous v7−3 KO females consistently outperformed their wild type siblings on the accelerating rotarod task. The modest difference was greater in mice tested at 12 months of age (38%) than in mice tested at 6 months (20%). Conceivably, v7−3 transport activity might affect intra- or extra cellular concentrations of certain osmolytes such as proline, which might influence formation of protein aggregates (Ignatova and Gierasch 2006) that accumulate in the aging brain. v7−3 KO effects on locomotor control in aging animals thus appear worthy of further exploration.
3.4. Limitations
Knockout mice display limitations that are important to consider in evaluating the current data. We have focused on recently-elucidated v7−3 substrates in characterizing these mice. However, the present results do not at all exclude the possibility that other amino acids or analogues not tested here might represent major, still unelucidated physiological v7−3 substrates. Uptake experiments in v7−3 KO mice indicate that inactivation of the v7−3 gene does affect proline and leucine transport. The modest extent of changes in proline and leucine accumulation is consistent with expression of other transporters for these amino acids. The lack of obvious behavioral changes in the v7−3 knockouts is thus consistent with the possibility that these other transporters could help to compensate for loss of v 7−3. Proline can be excitotoxic with neuronal toxicities comparable to those exerted by glutamate (Nadler et al. 1988). The systems for proline transport that are expressed in brain might thus well display redundancies and abilities to compensate for defects in any one proline transport system, consistent with the data from the present study. Compensatory changes in other transport systems, further, might arise during development of the v7−3 knockout animals. Since the nearest v7−3 homologue, NTT4, is expressed in many of the same brain regions, we have sought altered NTT4 mRNA levels in cortex, cerebellum, hippocampus and olfactory bulb. However, NTT4 mRNA levels were identical in wild type and v7−3 KO mice (JD, unpublished observations). It appears likely that we may need to eliminate v7−3 in combination with other proline transporters in order to reveal phenotypes arising from more marked changes in the system whose redundancies and/or abilities to adapt are unable to compensate for loss of several of these transporters.
In conclusion, the results from these analyses of v7−3 knockout mice demonstrate that the v7−3 protein makes moderate contributions to proline and leucine transport into brain synaptosomes in ways that allow normal development, survival and reproduction in its absence.
4. Experimental Procedure
4.1. Generation of v7−3 knockout mice
A 129/sjl genomic library in λ fixII was screened with a 32P-labeled probe derived from a 425 bp BamH I and EcoR I fragment of a rat v7−3 cDNA (Liu et al. 1993). Three positive clones were obtained, each containing a different insert of about 30 kb. We sequenced 60 kb of v7−3 genomic sequence (AY149282), constructed a precise restriction map and found that v7−3 mRNA was alternatively spliced within its 5'UTR. Thus, exon 2 could be either included or excluded to form isoforms v7−3a (AY149280) and v7−3b (AY149281). A Bgl II genomic fragment (4.2 kb) upstream of exon 3 was inserted 3' from the Neo gene (1.8 kb) and EcoR I genomic fragment (2.9 kb) downstream of exon 3 was inserted to EcoR I site 5' of Neo gene in bluescript SKII vector. A Sal I fragment of the TK gene (2.0 kb) was then inserted downstream to the insertion site (Fig. 1a). The final construct was linearized with Not1 and electroporated into 129.3 mouse MC1 embryonic stem (ES) cells (JHU Transgenic Core Laboratory). Primers J5, in the Neo cassette and J8, outside the construct were used for PCR screen of 576 colonies resistant to G418 and Gancyclovir (Fig. 1b). All 30 ES cell clones positive by PCR were further confirmed by Southern blot after DNA digestion with Sac I (Fig. 1c). The external 270-bp probe used was generated by PCR with primers J14 and J15. Two of the correctly targeted ES cell clones were microinjected into C57BL/6J E3.5 blastocysts at the Johns Hopkins University transgenic core facility. Heterozygous v7−3 offspring of male chimeras were mated and their progeny genotyped by PCR with primers V73f, J2, and NEO1 to amplify wild type (527 bp) and knockout (694 bp) alleles simultaneously (Fig. 1d).
4.2. RNA extraction and reverse transcription-PCR
Total brain RNA was prepared with RNeasy Lipid Tissue Kit (Qiagen, Valencia, CA). cDNA was synthesized from oligo dT primers using Superscript III (Invitrogen, Carlsbad, CA). For quantitative comparison of different RNA species, RT-PCR was performed with SYBR Green PCR Master mix (Applied Biosystems, Foster City, CA) and a 7900HT Sequence detection system (Applied Biosystems). Data were collected at 80°C.
4.3. Preparation of synaptosomes
Mice were sacrificed by decapitation and brains were quickly dissected on an ice-cold glass plate. Synaptosomes were prepared by differential centrifugation and phase partitioning adapted from (Lopez-Perez 1994). Cerebral cortex from a single mouse was homogenized in 10 ml of ice-cold buffer A (0.32 M sucrose, 1 mM potassium EDTA, and 10 mM Tris-HCl, pH 7.4) in a glass homogenizer using 8 strokes of a Teflon pestle at 500 rpm. The homogenate was diluted with additional 10 ml of buffer A and centrifuged twice at 1,300 g for 3 min. The resulting supernatant was further centrifuged at 17,000 g for 10 min, the pellet resuspended in 10 ml of buffer B (0.32 M sorbitol, 0.1 mM potassium EDTA, and 5 mM potassium phosphate, pH 7.4) and centrifuged at 12,000 g for 10 min. The ensuing pellet, P3 or crude synaptosomal fraction, was resuspended to a final protein concentration of 2.0 mg/ml in buffer B and immediately used for uptake assays. When indicated, a scaled down version of the method of (Lopez-Perez 1994) was used to prepare purified cortical synaptosomes from single mice.
4.4. Proline transport
Proline uptake was assayed as described by Broer et al., (2006). 20 μl of synaptosomal suspension (50 μg of protein from P3 pellet or 10 μg of protein from purified synaptosomes) was added to 180 μl of HEPES-buffered Hank's balanced salt solution (HHBSS) (1.4 g/l CaCl2, 4 g/l KCl, 0.6 g/l KH2PO4, 1 g/l MgCl2 × 6 H2O, 1 g/l MgSO4 × 7 H2O, 80 g/l NaCl, 0.9 g/l Na2HPO4 × 7 H2O, 1 g/l glucose and 10 mM HEPES pH 7.5) containing 50 μM proline and 0.1 μCi of L-[14C]proline (Amersham, Piscataway, NJ). The mixture was incubated at 23°C for 10 min and uptake was terminated by addition of 1 ml of ice-cold 5 mM proline in HHBSS. Synaptosomes were collected on polyethylenimine pretreated glass fiber filters (GF/B, Whatman, Maidstone, UK) and washed 3 × with 2 ml of ice-cold 5 mM proline in HBSS. Accumulated radioactivity in triplicate samples was assessed by scintillation counting. Proline uptake was defined by subtracting proline binding measured at 0°C from total radioactivity. This amount of radioactivity was essentially the same as the amount accumulated during 10 min incubation at 23°C in the absence of Na+ (data not shown).
4.5. Leucine transport
20 μl of synaptosomal P3 pellet was added to 180 μl of HHBSS (see above) containing 25 μM leucine and 0.1 μCi of L-[14C]leucine (Amersham, Piscataway, NJ). The mixture was incubated at 23°C for 5 min. Uptake was terminated by addition of 1 ml of ice-cold 5 mM leucine in HHBSS. To assess the Na+-independent leucine transport, NaCl in the assay buffer was replaced by choline chloride (ChoCl) and all other Na+-containing salts with K+ salts. Na+-dependent leucine transport was calculated as the difference between the total and Na+-independent leucine transport.
4.6. Behavioral testing
v7−3 KO mice were evaluated for their general health, reflexes and gross behavioral abnormalities as suggested by J. Crawley (Crawley 1999).
4.6.1
Locomotor activity was assessed as described (Hall et al. 2003). After 1 h habituation, the mice were injected with saline and 1 hour later with cocaine (10 mg/kg, s. c), ethanol (2 mg/kg, i. p.), or morphine (3 mg/kg, s. c.). Distance traveled was monitored optically for an additional 1h.
4.6.2
Cocaine reward was assessed with cocaine hydrochloride (10 mg/kg s. c.) by conditioned place preference paradigm as described in (Hall et al. 2003).
4.6.3
Motor coordination and motor learning were measured on an accelerating rotarod in three consecutive days as described in (Lalonde et al. 1995; Crawley 1999).
4.6.4
Memory and learning were evaluated in the Morris Water Maze as described in (Morris 1984; Crawley 1999). The apparatus consisted of a black pool (90 cm diameter) filled with room temperature water made opaque with white tempera paint. A 9 cm diameter platform was located in the center of one quadrant. For the first 6 trials the platform was visible, during all subsequent trials it was hidden 0.5 cm below the water level. Each trial lasted a maximum of 60 seconds followed by a 15 second rest period on the platform. After two trials, the mice were returned to their home cages for about 2 hours and then submitted to another 2-trial session for total of 2 sessions per day. Each time, latency to reach the platform was recorded. The probe trial, when the platform was removed, was videotaped and analyzed using Ethovision software (Noldus, Netherlands). After initial acquisition, the platform was moved and reversal learning was assessed under the same conditions.
4.6.5
To determine the stage of estrous cycle, vaginal lavages were taken daily between 9 and 12 AM and examined under a light microscope as described in (Nelson et al. 1982).
4.6.6
Olfaction was assessed in three different paradigms: (1) Responses to appetitive stimuli: Each mouse was placed in the center of a Plexiglas box with two pieces of filter paper attached at each end. After 5 min, the animal was removed for 30 sec and 5 μl of oil from peanut butter or urine (male urine for female subjects, female urine for male subjects) was applied to one of the filters. Latency to sniff the loaded filter was measured with a maximum duration of the test 150 s. (2) Response to aversive stimuli: Mice were placed into 2-chambered Plexiglas boxes with free access to both sides. 0.5 μl of fox fecal odor (trimethylthiazoline, TMT from PheroTech, Delta, BC, Canada) was applied to one of the chambers. Time spent in each chamber was monitored for 10 min before and after the application of the odorant. (3) Olfactory discrimination: This task was adapted from (Pho et al. 2005). Mice deprived of food overnight were placed individually into clean cages. After 5 min habituation, 2 plastic Petri dishes filled with corncob bedding were lowered into the cages at random locations. The bedding in one of the dishes was scented with 200 μl of 0.02% amyl acetate, the other dish was scented with 0.02% citronellal. One of the odorants (CS+) always indicated presence of a 3 mm cube of honey graham cracker hidden under the bedding. The other (CS-) always indicated its absence. Digging in the dish with food was considered a correct response and was rewarded with the cracker. Digging in the dish without food or not digging within 2 min was considered an incorrect response and resulted in removal of the dishes and termination of the trial. There were 3 trials per day with 10 min inter-trial intervals. In the additional sessions, the + odorant contained increasing fractions of the – odorant.
4.6.7
Analgesia was assessed using tail-flick and hot-plate paradigms. First, the tail of a mouse was immersed into a 53°C hot water bath and the latency to flick was measured. Immediately afterwards, the mouse was placed onto a 55°C hot plate and the latency to either jump or lick one of the hind paws was taken. To prevent injury, the cut off times for the tail-flick and hot-plate tests were 15 s and 30 s respectively.
4.6.8
To measure susceptibility to seizures, mice were injected intraperitoneally with pentylenetetrazole (60−80 mg/kg) or cocaine (80 mg/kg), placed in transparent Plexiglas cages and observed for 30 minutes. Latencies to initial myoclonic twitches, repetitive tonic-clonic convulsions, and tonic seizures were recorded.
4.6.9
Anxiety was assessed in paradigms modified from (Crawley 1999). (1) Open Field. Mice were placed singly in an open field (42 × 42 cm) illuminated with red light, for twenty minutes. The time the animal spent in the central quadrant and the number of fecal pellets was recorded. (2) Novel object interaction: Immediately after the initial open field test, an object was attached to a side wall 10 cm above the floor, and the time spent in direct contact with it was recorded for 5 min. (3) Light-Dark Test: The testing cage (18 × 36 cm) consisted of two compartments: a dark chamber (18 × 18 cm) with black walls and a small opening (5 cm) leading to a Plexiglas compartment illuminated with red light. An animal was placed into the dark chamber. The latency to emerge and the time spent outside the dark chamber during the 10 min trial were measured using the Optovarimax system (Columbus Instruments, OH). (4) Elevated Plus Maze: Mice were placed in an automated elevated plus maze apparatus (Onaivi et al. 1989). The time spent in the open and closed arms of the apparatus, as well as the number of entries into each arm were recorded.
4.6.10
Depression-related behavior was assessed in the forced swim test. Mice were placed in the center of a 4 L beaker (18 cm diameter) containing 3 L of room temperature water for 15 min (Hall et al. 1998). The last 5 minutes were digitally recorded and analyzed by a visual observation using the TIMER program (NIH) for swimming, struggling and floating behaviors.
4.6.11
Stress induced anxiety was assessed in the dark box and immediately after that, in the open field 10 min after the termination of the forced swim test.
Experiments were conducted under protocols approved by the NIDA-IRP Animal Care and Use Committee.
4.7. Data analysis
Quantitative data were analyzed using t-test or ANOVA with the between subjects factors of genotype and sex where appropriate.
Table 1.
DNA primers used in the study
| Primer name | Primer sequence |
|---|---|
| J2 | 5′GTCCTTCACTGAGTCTGGCAC |
| J5 | 5′GCGCATGCTCCAGACTGCCTT |
| J8 | 5′CTTACTACACAGCTTGCGAAGCC |
| J14 | 5′GAATGCACCTCACTCCTGAACTT |
| J15 | 5′ACTGCTGCCTGGCTACCTTCC |
| J52 | 5′GGCCAGCCTGGAACAGTAAGC |
| J53 | 5′TCCACTGTCAGAGATGGAGCTG |
| J54 | 5′GACCTCACAGACATGTTAGGATTT |
| J55 | 5′TGAGGCTTGCGTCATCTCCTTC |
| ACTf | 5′GCTCTGGCTCCTAGCACCAT |
| ACTr | 5′CTGCTTGCTGATCCACATCTG |
| NEO1 | 5′CATCAGAGCAGCCGATTGTCTG |
| V73f | 5′GGCAGAATACCTAAGAGCCTTGT |
Acknowledgements
We acknowledge the valuable comments and assistance on ES cell culturing from Dr. Ichiro Sora, the substantial efforts of Shigeo Kitayama to elucidate v7−3 substrates, and financial support by the National Institutes of Health Intramural Research Program (NIDA); DHHS.
Abbreviations
- HBSS
HEPES-buffered Hank's balanced salt solution
- MeAIB
α-(Methylamino)isobutyric acid
- PTZ
pentylenetetrazole
- TMT
trimethylthiazoline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section 1. Cellular and Molecular Biology of Nervous Systems
References
- Agulhon C, Rostaing P, Ravassard P, Sagne C, Triller A, Giros B. Lysosomal amino acid transporter LYAAT-1 in the rat central nervous system: an in situ hybridization and immunohistochemical study. J Comp Neurol. 2003;462:71–89. doi: 10.1002/cne.10712. [DOI] [PubMed] [Google Scholar]
- Broer A, Tietze N, Kowalczuk S, Chubb S, Munzinger M, Bak LK, Broer S. The orphan transporter v7−3 (slc6a15) is a Na+-dependent neutral amino acid transporter (B0AT2). Biochem J. 2006;393:421–430. doi: 10.1042/BJ20051273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J. What's wrong with my mouse? behavioral phenotyping of transgenic and knockout mice. John Wiley & Sons, Inc.; New York, Chichester, Weinheim, Brisbane, Singapore, Toronto: 1999. [Google Scholar]
- Crump FT, Fremeau RT, Craig AM. Localization of the brain-specific high-affinity l-proline transporter in cultured hippocampal neurons: molecular heterogeneity of synaptic terminals. Mol Cell Neurosci. 1999;13:25–39. doi: 10.1006/mcne.1998.0727. [DOI] [PubMed] [Google Scholar]
- el Mestikawy S, Giros B, Pohl M, Hamon M, Kingsmore SF, Seldin MF, Caron MG. Characterization of an atypical member of the Na+/Cl(−)-dependent transporter family: chromosomal localization and distribution in GABAergic and glutamatergic neurons in the rat brain. J Neurochem. 1994;62:445–455. doi: 10.1046/j.1471-4159.1994.62020445.x. [DOI] [PubMed] [Google Scholar]
- Farmer MK, Robbins MJ, Medhurst AD, Campbell DA, Ellington K, Duckworth M, Brown AM, Middlemiss DN, Price GW, Pangalos MN. Cloning and characterization of human NTT5 and v7−3: two orphan transporters of the Na+/Cl− -dependent neurotransmitter transporter gene family. Genomics. 2000;70:241–252. doi: 10.1006/geno.2000.6387. [DOI] [PubMed] [Google Scholar]
- Fremeau RT,, Jr., Caron MG, Blakely RD. Molecular cloning and expression of a high affinity L-proline transporter expressed in putative glutamatergic pathways of rat brain. Neuron. 1992;8:915–926. doi: 10.1016/0896-6273(92)90206-s. [DOI] [PubMed] [Google Scholar]
- Giros B, el Mestikawy S, Bertrand L, Caron MG. Cloning and functional characterization of a cocaine-sensitive dopamine transporter. FEBS Lett. 1991;295:149–154. doi: 10.1016/0014-5793(91)81406-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gonzalez IM, Cubelos B, Gimenez C, Zafra F. Immunohistochemical localization of the amino acid transporter SNAT2 in the rat brain. Neuroscience. 2005;130:61–73. doi: 10.1016/j.neuroscience.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Guastella J, Nelson N, Nelson H, Czyzyk L, Keynan S, Miedel MC, Davidson N, Lester HA, Kanner BI. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GF, Pert A. The effects of social isolation on the forced swim test in Fawn hooded and Wistar rats. J Neurosci Methods. 1998;79:47–51. doi: 10.1016/s0165-0270(97)00155-6. [DOI] [PubMed] [Google Scholar]
- Hall FS, Drgonova J, Goeb M, Uhl GR. Reduced behavioral effects of cocaine in heterozygous brain-derived neurotrophic factor (BDNF) knockout mice. Neuropsychopharmacology. 2003;28:1485–1490. doi: 10.1038/sj.npp.1300192. [DOI] [PubMed] [Google Scholar]
- Hoglund PJ, Adzic D, Scicluna SJ, Lindblom J, Fredriksson R. The repertoire of solute carriers of family 6: identification of new human and rodent genes. Biochem Biophys Res Commun. 2005;336:175–189. doi: 10.1016/j.bbrc.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Ignatova Z, Gierasch LM. Inhibition of protein aggregation in vitro and in vivo by a natural osmoprotectant. Proc Natl Acad Sci U S A. 2006;103:13357–13361. doi: 10.1073/pnas.0603772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Sato K, Tohyama M, Shimada S, Uhl GR. Widespread brain distribution of mRNA encoding the orphan neurotransmitter transporter v7−3. Brain Res Mol Brain Res. 1996;37:217–223. doi: 10.1016/0169-328x(95)00298-7. [DOI] [PubMed] [Google Scholar]
- Kennedy DJ, Gatfield KM, Winpenny JP, Ganapathy V, Thwaites DT. Substrate specificity and functional characterisation of the H+/amino acid transporter rat PAT2 (Slc36a2). Br J Pharmacol. 2005;144:28–41. doi: 10.1038/sj.bjp.0706029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczuk S, Broer A, Munzinger M, Tietze N, Klingel K, Broer S. Molecular cloning of the mouse IMINO system: an Na+- and Cl−-dependent proline transporter. Biochem J. 2005;386:417–422. doi: 10.1042/BJ20050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R, Bensoula AN, Filali M. Rotorod sensorimotor learning in cerebellar mutant mice. Neurosci Res. 1995;22:423–426. doi: 10.1016/0168-0102(95)00916-h. [DOI] [PubMed] [Google Scholar]
- Liu QR, Mandiyan S, Lopez-Corcuera B, Nelson H, Nelson N. A rat brain cDNA encoding the neurotransmitter transporter with an unusual structure. FEBS Lett. 1993;315:114–118. doi: 10.1016/0014-5793(93)81145-p. [DOI] [PubMed] [Google Scholar]
- Lopez-Perez MJ. Preparation of synaptosomes and mitochondria from mammalian brain. Methods Enzymol. 1994;228:403–411. doi: 10.1016/0076-6879(94)28040-1. [DOI] [PubMed] [Google Scholar]
- Luque JM, Jursky F, Nelson N, Richards JG. Distribution and sites of synthesis of NTT4, an orphan member of the Na+/Cl(−)-dependent neurotransmitter transporter family, in the rat CNS. Eur J Neurosci. 1996;8:127–137. doi: 10.1111/j.1460-9568.1996.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Masson J, Pohl M, Aidouni Z, Giros B, Hamon M, el Mestikawy S. The two orphan Na+/Cl(−)-dependent transporters Rxt1 and V-7−3−2 have an overlapping expression pattern in the rat central nervous system. Receptors Channels. 1996;4:227–242. [PubMed] [Google Scholar]
- Masson J, Riad M, Chaudhry F, Darmon M, Aidouni Z, Conrath M, Giros B, Hamon M, Storm-Mathisen J, Descarries L, El Mestikawy S. Unexpected localization of the Na+/Cl−-dependent-like orphan transporter, Rxt1, on synaptic vesicles in the rat central nervous system. Eur J Neurosci. 1999;11:1349–1361. doi: 10.1046/j.1460-9568.1999.00540.x. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Nadler JV, Wang A, Hakim A. Toxicity of L-proline toward rat hippocampal neurons. Brain Res. 1988;456:168–172. doi: 10.1016/0006-8993(88)90358-7. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod. 1982;27:327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Todd S, Martin BR. Behavioral effects in the mouse during and following withdrawal from ethanol ingestion and/or nicotine administration. Drug Alcohol Depend. 1989;24:205–211. doi: 10.1016/0376-8716(89)90057-4. [DOI] [PubMed] [Google Scholar]
- Pho V, Butman ML, Cherry JA. Type 4 phosphodiesterase inhibition impairs detection of low odor concentrations in mice. Behav Brain Res. 2005;161:245–253. doi: 10.1016/j.bbr.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Rao KV, Vemuri MC, Murthy CR. Synaptosomal transport of branched chain amino acids in young, adult and aged rat brain cortex. Neurosci Lett. 1995;184:137–140. doi: 10.1016/0304-3940(94)11189-p. [DOI] [PubMed] [Google Scholar]
- Renick SE, Kleven DT, Chan J, Stenius K, Milner TA, Pickel VM, Fremeau RT,, Jr. The mammalian brain high-affinity L-proline transporter is enriched preferentially in synaptic vesicles in a subpopulation of excitatory nerve terminals in rat forebrain. J Neurosci. 1999;19:21–33. doi: 10.1523/JNEUROSCI.19-01-00021.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Aliaga I, Boll M, Vogt Weisenhorn DM, Foltz M, Kottra G, Daniel H. The proton/amino acid cotransporter PAT2 is expressed in neurons with a different subcellular localization than its paralog PAT1. J Biol Chem. 2004;279:2754–2760. doi: 10.1074/jbc.M305556200. [DOI] [PubMed] [Google Scholar]
- Shimada S, Kitayama S, Lin CL, Patel A, Nanthakumar E, Gregor P, Kuhar M, Uhl G. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science. 1991;254:576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- Smith KE, Fried SG, Durkin MM, Gustafson EL, Borden LA, Branchek TA, Weinshank RL. Molecular cloning of an orphan transporter. A new member of the neurotransmitter transporter family. FEBS Lett. 1995;357:86–92. doi: 10.1016/0014-5793(94)01328-x. [DOI] [PubMed] [Google Scholar]
- Takanaga H, Mackenzie B, Suzuki Y, Hediger MA. Identification of mammalian proline transporter SIT1 (SLC6A20) with characteristics of classical system imino. J Biol Chem. 2005;280:8974–8984. doi: 10.1074/jbc.M413027200. [DOI] [PubMed] [Google Scholar]
- Takanaga H, Mackenzie B, Peng JB, Hediger MA. Characterization of a branched-chain amino-acid transporter SBAT1 (SLC6A15) that is expressed in human brain. Biochem Biophys Res Commun. 2005;337:892–900. doi: 10.1016/j.bbrc.2005.09.128. [DOI] [PubMed] [Google Scholar]
- Takanaga H, Tokuda N, Ohtsuki S, Hosoya K, Terasaki T. ATA2 is predominantly expressed as system A at the blood-brain barrier and acts as brain-to-blood efflux transport for L-proline. Mol Pharmacol. 2002;61:1289–1296. doi: 10.1124/mol.61.6.1289. [DOI] [PubMed] [Google Scholar]
- Tan CH, Leong MK, Ng FH. A novel sodium-dependent uptake system for l-leucine in rat brain synaptosomes. Neurochem Int. 1988;12:91–95. doi: 10.1016/0197-0186(88)90153-2. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Kitayama S, Gregor P, Nanthakumar E, Persico A, Shimada S. Neurotransmitter transporter family cDNAs in a rat midbrain library: ‘orphan transporters’ suggest sizable structural variations. Brain Res Mol Brain Res. 1992;16:353–359. doi: 10.1016/0169-328x(92)90246-8. [DOI] [PubMed] [Google Scholar]
- Wasserman JC, Delpire E, Tonidandel W, Kojima R, Gullans SR. Molecular characterization of ROSIT, a renal osmotic stress-induced Na(+)-Cl(−)-organic solute cotransporter. Am J Physiol. 1994;267:F688–694. doi: 10.1152/ajprenal.1994.267.4.F688. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Yao D, Mackenzie B, Ming H, Varoqui H, Zhu H, Hediger MA, Erickson JD. A novel system A isoform mediating Na+/neutral amino acid cotransport. J Biol Chem. 2000;275:22790–22797. doi: 10.1074/jbc.M002965200. [DOI] [PubMed] [Google Scholar]