Abstract
Teleost gut associated lymphoid tissue (GALT) consists of leucocyte populations located both intraepithelially and in the lamina propria with no structural organization. The present study aims to assess different protocols for the isolation of GALT cells from an important fish species in the Mediterranean aquaculture, the gilthead seabream. Mechanical, chemical and enzymatic treatments were assayed. Nylon wool columns and continuous density gradients were used for further separation of cell subpopulations. Light microscopy and flow cytometry showed that the highest density band (HD) consisted of a homogeneous lymphocytic population, whereas the intermediate density band (ID) corresponded to epithelial and secretory cells and some lymphocytes. Respiratory burst activity of total cell suspensions revealed very low numbers of potential phagocytic cells, reflecting results from light microscopy and reports in other teleost species. The present data set up the basis for future functional characterization of GALT in seabream.
Keywords: Sea Bream, Flow Cytometry, Respiratory Burst
Introduction
The vertebrate immune system includes lymphoid organs considered primary and secondary, according to their ontogenic and functional characteristics. Secondary lymphoid organs, whose lymphoid cell populations depend on the primary lymphoid organs, include the spleen, lymph nodes and mucosa-associated lymphoid tissue (MALT), which includes the gut-associated lymphoid tissue (GALT).
Research into the immunity of mammalian GALT has led to the development of numerous techniques that have subsequently been applied to other vertebrates. Different protocols have been reported to isolate and purify GALT cells, all of them based on one or more of the following treatments: mechanical, chemical or enzymatic (1-2). Even more sophisticated techniques are available involving the use of monoclonal antibodies and immunomagnetic (3-4).
Teleost fish also possess primary and secondary lymphoid organs but there are major structural and morphological differences between fish and mammalian immune systems. Fish have a thymus but lack bone marrow, the kidney being the main lymphoid organ (5). As regards the secondary lymphoid organs the most obvious difference is the absence of lymph nodes in fish, although both, spleen and MALT are present. Fish MALT includes skin, gills and gut-associated lymphoid tissues. GALT has been described in both cartilaginous and bony fish species (6), where it does not appear to form structured aggregates such as the Peyer´s patches, which are characteristic of higher vertebrates. Histologically, the gut tissue is composed by three layers: the serosa, muscularis and mucosa. The mucosal layer is made up by an epithelium with its corresponding basal membrane and underlying connective tissue named lamina propria. The lymphoid cells found in fish gut seem to be distributed in a diffuse manner along the lining of the alimentary canal both within the epithelium and in the lamina propria (6) although available data reveals great interspecies variability in this respect (7).
Fish gut immune cells merit investigation due to their direct implication in enteric diseases, antigen uptake, immunoglobulin production and, therefore, oral vaccination or therapy (8). Protocols developed to purify fish GALT cells (8-11) have been adapted from those used routinely in mammalian species. No studies have yet applied them to the seabream, one of the most important farmed fish species in the Mediterranean. The diversity of fish ecology, biology, nutritional behaviour and physiology result in highly variable digestive system morphologies. Moreover, the alimentary canal exhibits adaptive flexibility in response to changes in dietary composition (12).
Therefore, herbivorous, detritivorous, omnivorous and carnivorous fish species differ from each other in terms of the presence or absence of a stomach, the length of the intestine (from 1 to more than 20 times the body length), number and presence of pyloric caeca, intestinal loops and valves (13). In the particular case of the gilthead seabream, a carnivorous marine species, the digestive tract consists of an oesophagus, the stomach, few pyloric caeca and the intestine. The intestine includes a loop and it can be divided into foregut and hingut since it narrows from the stomach towards the anus. In other finfish species such as the carp, the intestine length is greater. It shows two loops and can be divided in up to four segments but no stomach or pyloric caeca are found (14). Salmonids, in turn, posses a short intestine that appears as a simple tube and the stomach is surrounded by hundreds of pyloric caeca. Thus, optimisation of the procedure is necessary in terms of time and cost due to the obvious anatomical and physiological differences in the gut between mammals and fish and between different fish species.
This study was therefore undertaken to evaluate different protocols for the isolation and enrichment of immune cells from the gilthead seabream (Sparus aurata L.) digestive tract. Results are discussed according to the time and cost of the protocol, the number of cells, their viability and the different cell populations obtained as studied by light microscopy and flow cytometry.
Materials and Methods
Fifty specimens (150 g mean weight) of the hermaphrodite protandrous seawater teleost gilthead seabream (Sparus aurata L.) gilthead seabream obtained from Culmarex S.A (Murcia, Spain) were kept in running seawater tanks (flow rate 1500 l/h) at 20°C with a 12 h dark: 12 h light photoperiod and fed with a commercial pellet diet (ProAqua, Palencia, Spain). Fish were allowed to acclimatise for 15 days and, before sampling, they were starved for 48 hours. Specimens were sacrificed by an overdose of benzocaine, bled and then ventrally dissected to obtain their gut.
Protocols for the isolation of gut leucocytes
The digestive tract of each specimen from the pyloric caeca to the anus was collected. Head-kidneys was obtained as a positive control as explained elsewhere (15). After weighing, the dissected tissues were placed in a Petri dish and washed in ice cold phosphate buffer saline (PBS) to remove the connective tissue and gut contents. The whole sample was dissected into approximately 1 cm long fragments, which were then longitudinally opened by means of a scalpel. The range of protocols tested was the result of reviewing the current literature (1, 8-10, 16-18), with incubation time and enzyme concentration as the two variable parameters.
Mechanical
The isolation of gut leucocytes without the use of chemical or enzymatic treatments involved the mechanical stripping of the gut epithelium from the underlying submucosa by means of a cell scraper. Briefly, gut segments of about 1 cm long were placed in a Petri dish with 10 ml of cold sRPMI containing 10 I.U./ml heparin (Sigma). Fragments were dissected longitudinally and the epithelium was carefully separated from the underlying mucosa. Then, after passing through a sterile 100 μm mesh, they were washed twice in sRPMI as before.
Chemical
Gut fragments were placed in 50 ml sterile test-tubes containing 15 ml of cold PBS with ethylenediamintetraacetic acid (EDTA) (0.37 mg/ml) and dithiothreitol (DTT) (0.145 mg/ml, Sigma). DTT is a reducing agent frequently used to reduce the disulfide bonds of proteins and, more generally, to prevent intramolecular and intermolecular disulfide bonds from forming between cysteine residues of proteins. In the case of the gut tissue, it reduces the bonds present at the tight junctions present between the epithelial cells. The tubes were mechanically shaken (10 min, 60 rpm, 22°C) so that cells located between epithelial cells were released from the tissue into the suspension. Supernatants were collected (S1) and sieved through a 100 μm sterile mesh. Solid fragments were washed in cold washing medium (Hanks Buffer Saline Solution (HBSS), 5% foetal bovine serum (Gibco), 100 I.U./ml penicillin (Flow), 100 μg/ml streptomycin (Flow), DNAse I (0.05 mg/ml; Sigma) to prevent possible inhibition of collagenase by DTT.
Enzymatic
Enzymatic treatment was carried out by stirring the sample (same conditions as before) in 15 ml of washing medium with 0; 0.15 or 0.37 mg/ml collagenase (Sigma) for 30, 60 or 120 min at room temperature. During the collagenase treatment, the connective tissue that houses LPL’s is digested and cells are released into the media. Afterwards, the supernatants as well as the gut fragments were sieved through a 100 μm sterile mesh. Total cell suspensions (S2) were washed twice in 10 ml sRPMI [RPMI-1640 culture medium with 0.35% sodium chloride (to adjust the medium’s osmolarity to gilthead seabream plasma osmolarity, 353.33 mOs), 100 I.U./ml penicillin, 100 μg/ml streptomycin and 5% foetal bovine serum] by centrifuging (10 min, 400 g, 4°C).
Tissue fragments from intestines were taken at different stages of the isolation protocols (chemical treatment consisting of 10 min in DTT-EDTA; enzymatic treatments after 30, 60 or 120 min incubation in washing media containing collagenase (0, 0.15 or 0.37 mg/ml) in order to visualise the different degrees of digestion undergone by the gut epithelium and lamina propria. Light microscopy samples consisted of fragments fixed immediately in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2, 3 h, 4°C), washed in 0.1 M cacodylate buffer, then postfixed in OsO4 and embedded in Epon. Thin sections were stained with toluidin blue and examined by a Leica (DMRB) light microscope.
Cell counts
At all times, it was necessary to thoroughly mix cell pellets with a Pasteur pipette prior to counting in order to avoid large cell clusters. Cell aggregation was minimised by the use of nylon wool columns. The purified seabream gut cells were counted in a Neubauer chamber and adjusted to 107 cells/ml.
Cell viability and flow cytometry study
Aliquots of 50 ml cell suspensions from the different isolation procedure assays were placed in 5 ml test-tubes (Falcon, Becton Dickinson) and 30 μl of propidium iodide (PI) (400 μg/ml, Sigma) were added to each sample. The tubes were gently mixed before analysis in a FACScan (Becton Dickinson, Madrid, Spain) flow cytometer with an argon-ion laser adjusted to 488 nm. Analyses were performed on 3,000 cells, which were acquired at a rate of 300 cells/s. Instrument setting were identical throughout the whole study. Data were collected in the form of two-parameter side scatter (granularity) (SSC) and forward scatter (size) (FSC) dotplots, while red fluorescence (FL2) histograms were made on a computerised system. Dead cells were estimated as the percentage of cells with propidium iodide (red-PI fluorescent cells).
Nylon wool columns
Nylon wool columns were not used to separate a particular cell population of the total gut cell suspensions. They were made by tightly packing 0.5 g of nylon wool fibre (Kisker-Biotech) into a 5 ml syringe. The column size was chosen according to the manufacturer’s specifications. The columns were autoclaved and incubated for 1 hour at 24°C with 5 ml sRPMI. Then 5 ml (107 cells/ml) of the gut cell suspension (S2) were loaded into the column and incubated for 1 h at 24°C after which, non-adherent cells were eluted by rinsing the column with sRPMI and washed twice as before. The resulting cell suspension was called NW. Viability, cell counts and flow cytometry analyses were conducted on this suspension. Additionally, nylon wool fibres from columns incubated with seabream gut cell suspensions from three specimens were fixed after being eluted. The latter aimed to further investigate which elements of the original suspension were being retained by the fibres. Briefly, samples were fixed in 3% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2–7.4, for 2 h at 4°C and postfixed in 1% OsO4. Postfixed samples were dehydrated in acetone, critical-point dried, sputter-coated with gold and studied with a Jeol JSM 6100 scanning electron microscope.
Continuous Percoll gradients
A continuous Percoll gradient was established between 25% and 75% in a 50 ml plastic test-tube. Three millilitres of GALT leucocyte suspensions (S2 or NW) were carefully layered over the Percoll gradients. Density beads (Sigma) were used to determine the density of the bands found after centrifuging for 30 min at 400 g and 4°C. After centrifugation, each band of cells present in the Percoll gradient was collected and washed twice in sRPMI prior to observation by light microscopy and flow cytometry. Cell viability was measured as before.
Microscopy
Light microscopy (LM) samples of the different cell suspensions obtained during the protocol were prepared by cytocentrifugation. Additionally, cytocentrifugation of aliquots of 5 x 105 cells (10 min, 690 g, 22°C) of each band was also performed on glass slides. The slides were air dried, fixed for 3 min in pure methanol and stained with Giemsa (Merck; 1:10 in tap water) for 60 min. The slides were then washed to discard excess Giemsa, air-dried and mounted on DPX. Images were acquired with a Leica DC500 digital camera attached to a light microscope (Leica DMRB) with x100 oil objective.
Respiratory burst (NBT) assay
Superoxide anion (O2 -) production, by total cell suspensions obtained from seabream intestines by mechanical stripping, after 60 min in collagenase (0.15 mg/ml) or after 120 min in collagenase (0.37 mg/ml) was measured by the nitroblue tetrazolium (NBT, Sigma) method adapted from Muñoz et al. (2000) (19). These three protocols were chosen because they theoretically represent different degradation levels of the gut lamina propria housing the phagocytic cells. Briefly, 100 μl of each cell suspension were plated in 96-well plates in triplicate. 100 μl of NBT (Sigma, 2 mg/ml in HBSS) with or without phorbol myristate acetate (PMA, Sigma) (2 μg/ml) were added to each well. Positive controls consisted of suspensions of head kidney leucocytes from the same fish. Head kidney is known to contain high numbers of phagocytic cells in teleost fish that respond to PMA giving positive respiratory burst reactions. Plates were incubated for 1h at 24°C in a moisture chamber and then washed twice in PBS. PBS was carefully removed with a pipette and the plates were immediately examined under a Leica inverted light microscope coupled to a Leica DFC280 digital camera with an x40 objective. Cells containing formazan precipitates (NBT positive) were counted in each well.
Data analysis
The results are expressed as mean ± sd (standard error of the mean). The data were examined for significance by one-way analysis of variance and Tukey´s comparison of means when necessary, considering P<0.05 as statistically significant.
Results
Protocols for the isolation of seabream GALT cells, cell counts and viability
The total mean cell numbers per gram of tissue and their mean viability calculated for each of the tested protocols for the isolation of gut leucocytes are shown in Table 1.
| Total number of cells per gram of seabream intestine tissue as counted under a Neubauer chamber after different isolation protocols and their viability (assessed by PI staining). Results are expressed as mean ± sd (n= 5 fish). One-way analysis of variance and Tukey test were conducted when necessary. Different letters stand for statistically significant differences (p<0.05) between isolation protocols. | ||
| Treatment | Numbers of cells/g tissue x 107 | Viability (%) |
| DTT 10 min | 0.26 ± 0.14a | 65 ± 9.6a |
| Collagenase 0 mg/ml | ||
| 30 min | 8.15 ± 3.4c | 82.5 ± 6.3b |
| 60 min | 42.8 ± 37.9e | 83.5 ± 9.1b |
| 120 min | 48.2 ± 6.7e | 76.6 ± 6.5b |
| Collagenase 0.15 mg/ml | ||
| 30 min | 19.4 ± 0.9d | 82.6 ± 4.7b |
| 60 min | 22.6 ± 2.4d | 80.5 ± 4.5b |
| 120 min | 40.5 ± 3e | 74 ± 4.6b |
| Collagenase 0.37 mg/ml | ||
| 30 min | 18.7 ± 7.6d | 88 ± 7.1b |
| 60 min | 48.75 ± 3e | 82.75 ± 7.1b |
| 120 min | 75.6 ± 5.1f | 77 ± 2.8b |
| Mechanical stripping | 4.95 ± 1.5b | 82.2 ± 2b |
Note that chemical treatment alone for 10 min (S1) yielded significantly lower cell counts than other protocols, being the obtained cells also statistically less viable than any of the other cell collected suspensions. This lower viability could be due to the dissection procedure and consequent tissue damage during the initial stages of the protocol. However, the subsequent purification steps allowed refinement of the cell suspensions and the increase of the final cell viability.
When no collagenase was added to the washing medium, high numbers of cells were obtained, especially after gently shaking for 60 or 120 min. The viability of the cells was always higher than 75%. Intestines incubated for 30 min in washing media without collagenase released statistically fewer cells than when longer incubation times or higher enzyme concentrations were used.
Increasing the collagenase concentration from 0.15 mg/ml to 0.37 mg/ml and the digestion time from 30 to 120 min resulted in increasingly higher numbers of cells. These differences were statistically significant in all cases. Despite the absence of a significant decrease in viability, it was observed that cells obtained after longer enzymatic digestion tended to be slightly less viable than when shorter protocols were used.
Shortening collagenase digestion from 120 to 60 min yielded similar S2 both in number and composition according to flow cytometry analysis. However, further reduction of the treatment (30min) resulted in a different distribution of the dot plots obtained by flow cytometry.
The use of a purely mechanical method involving a cell scraper was the most time and cost-effective although the latter varied from fish to fish. Nonetheless, the viability of the cells obtained was high (88%) and similar to the viability using collagenase. Examination under LM revealed the presence of whole fragments of tissue where enterocytes were still attached to each other forming a simple cylindrical epithelium (data not shown). Therefore, the isolation of cells as a suspension was sometimes incomplete.
Flow cytometry studies of seabream GALT cells obtained by different protocols
Identification of GALT cell subsets according to the FSC and SSC values obtained by flow cytometry was not easy because the dot plots showed no well defined subsets. Three cell subsets of variable relative abundance could be distinguished, such variations appearing not to be associated to a particular purification protocol but to differences between specimens. In general, the majority of cells corresponded to low FSC and low SSC values (R1). A second subpopulation, R2, consisted of medium FSC values but with relatively high spread and medium SSC values. Finally, a small population (R3) of low FSC and low to high SSC values was found (Fig. 1A).
Fig. 1.
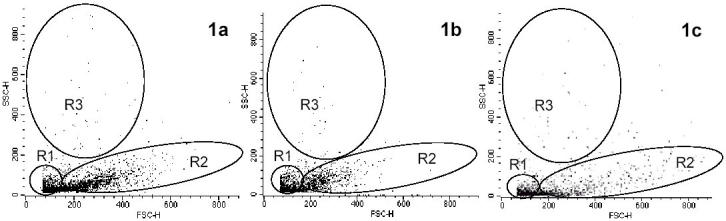
Flow cytometry SSC and FFC dot plots of seabream intestine cells showing three subpopulations (R1, R2 and R3). (a) Representative dot plot of a S2 suspension obtained after isolation of cells using both chemical (DTT, 10 min) and enzymatic (collagenase 0.15 mg/ml, 60 min) treatments. (b) Representative dot plot obtained after the same suspension in Figure 1a is passed through a nylon wool column (NW) for 60 min. (c) Representative dot plot of a total suspension obtained by mechanical stripping,
When mechanical stripping was conducted, cell suspensions showed a different picture according to their FSC and SSC values, with a R2 region that was typically more spread in the FSC axis compared to other treatments (Fig. 1C).
Use of nylon wool columns after isolation of seabream GALT
Nylon wool columns were an effective tool for eliminating mucus and yielded cleaner cell suspensions that did not form aggregates that were present prior to its use. No other methods to eliminate mucus were evaluated in the present study. The recovery of the loaded cells was over 90% and, after one hour in the nylon wool column, their viability was unaffected. Moreover, this step was necessary for the Percoll separation since the presence of mucus sometimes precluded adequate separation of cells according to their relative density. When NW cell pools were studied under flow cytometer, their viability was slightly higher that of S2 suspensions and FSC scattering was reduced. All three subpopulations were still present although R2 showed less variability in the FSC axis compared to R2 from Figure 1A (Fig. 1B).
Observation under scanning electron microscopy of nylon wool fibres from columns incubated for one hour with seabream gut cells, revealed considerable amounts of mucus attached to them (Fig. 2A). Additionally, some rounded small cells (diameter around 5μm) adhered to the fibres (Fig. 2B).
Fig. 2.
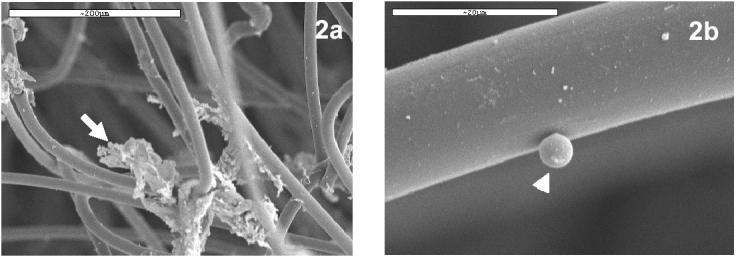
Scanning electron micrographs of nylon wool fibers from nylon wool columns incubated with seabream gut cells. (a) Mucus (arrow) from seabream gut trapped between nylon wool fibres. Bar: 200 μm (b) Small rounded cell (arrow head) adhered to a nylon wool fibre. Bar: 20 μm.
Purification by continuous Percoll gradients
Percoll gradients on cell suspensions that had not been passed through nylon wool columns sometimes yielded one sole layer at the interface between the Percoll and sRPMI medium, where almost all the cells were present due to the presence of mucous that caused cell aggregation.
When cell suspensions were previously purified in the nylon wool column and then layered over continuous Percoll gradients, three main bands were found: i) a low density band (LD) (density lower than 1.033 g/l) that remained in the supernatant; ii) a thicker intermediate band (ID) located between 1.055 and 1.060 g/l; iii) a higher density band (HD) with an approximate density of 1.075 g/l. The three bands were not consistently found in all the trials, sometimes we observed more bands and in few instances less. The LD, ID and HD pattern were nonetheless the most frequently observed.
Flow cytometry analysis of these bands revealed that the viability of ID and HD was higher than in the original cell suspension since dead cells stayed mainly in LD. Moreover, LD contained a cell subpopulation resembling those found in the S2 and NW pools. The ID band was enriched in cells of intermediate size and low-medium complexity (Fig. 3A). Finally, HD was enriched in a cell populations characterised by both very low FSC and SSC (Fig. 3B).
Fig. 3.
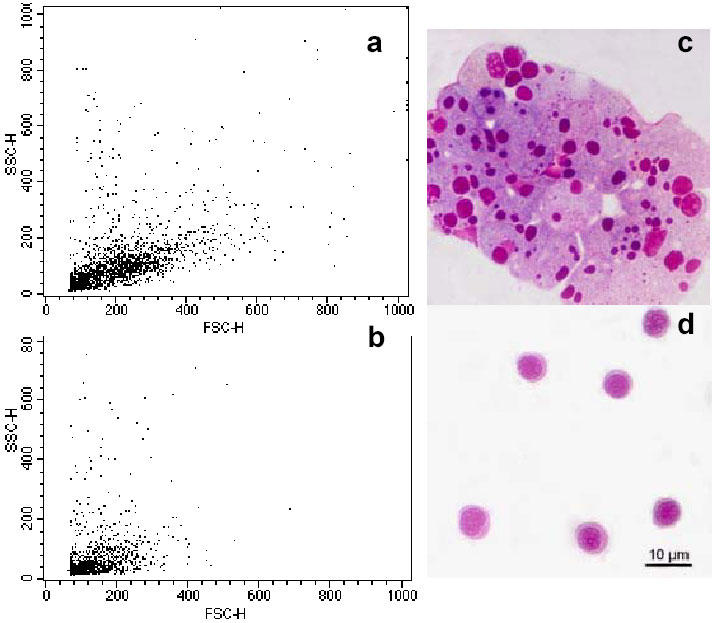
Purification of seabream GALT cells by Percoll density gradients. (a) Representative flow cytometry dot plot of intermediate density band (ID) corresponding to 1.060 g/l. (b) Representative flow cytometry dot plot of cells found at the highest density band (HD) (1.075 g/l). (c) Light micrograph of a Giemsa stained cytocentrifugation of ID band cells. (d) Light micrograph of a Giemsa stained cytocentrifugation of HD band cells.
Microscopy studies help to understand the chemical and enzymatic steps
Observation of semithin sections of the seabream gut tissue during the different steps of the isolation protocol permitted visualisation of the different degrees of digestion of the tissue as well as the progressive release of immune cells. Treatment with DTT loosened the spaces between mucosal epithelium enterocytes (Fig. 4B) compared to control fragments (Fig. 4A), whereas treatment with collagenase for 60 minutes sufficed to digest the connective tissue that forms the lamina propria of the intestinal epithelium (Fig. 4C).
Fig. 4.
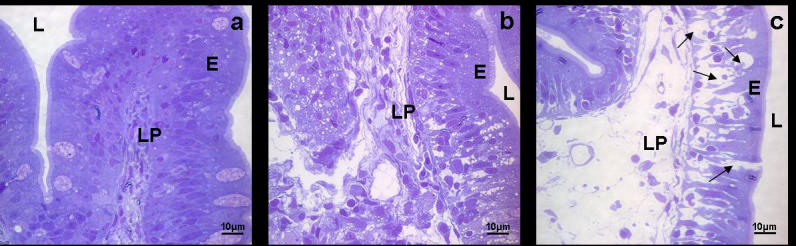
Light micrographs of semithin sections of seabream intestine at different stages of cell extraction. L (lumen), E (enterocytes), LP (lamina propria). (a) Gut semithin section at the beginning of the isolation procedure (control sample, time 0). Bar: 10 μm. (b) Gut semithin section after 10min in DTT. Bar: 10 μm (c) Gut semithin section after 10 min in DTT and 60 min in collagenase (0.15 mg/ml). Note the open interepithelial spaces (arrows) that allow leucocytes to be freed into the media. Bar: 10 μm.
The GALT extraction method here described led us to obtain cell pools that consisted of lymphocytes, granulocytes, macrophages, enterocytes, erythrocytes and caliciform cells. All cell types were seen in the cytocentrifugation samples after Giemsa staining. Caliciform cells were the largest of all the cell types, round and with a cytoplasm mostly occupied by mucus vacuoles. Erythrocyte numbers varied from sample to sample but were always low. Enterocytes were smaller than caliciform cells but bigger than immune cells and contained features of picnocytosis- like vesicles that were intensely stained with Giemsa. Granulocytes and macrophages were scarce in all cases. Lymphocytes were abundant at all times, showing a typical round morphology, small size and big nucleus.
Cytocentrifugation of the three bands obtained by Percoll density separation pointed to the presence of all cell types in LD, along with cell debris (not shown). The ID band contained cell suspensions enriched in enterocytes and caliciform cells (Fig. 3C). Lymphocytes were also found. The HD band corresponding to low SSC and FSC values by flow cytometry was composed of highly homogeneous lymphocyte suspensions according to their morphological and staining properties, as shown in Fig. 3D. Some macrophages and granulocytes were observed but always in very low numbers.
Respiratory burst as an indicator of phagocyte release during isolation
NBT assays corroborated the low abundance of phagocyte (NBT-positive) cells present in the seabream GALT cells obtained by three different protocols. No significant differences were found between mechanical and enzymatic protocols. The use of 0.37 mg/ml collagenase for 120 min produced more NBT-positive cells, however the increase was not statistically significant compared to the other methods. The scarcity of NBT-positive cells was reflected in light micrographs (Fig. 5).
Fig. 5.
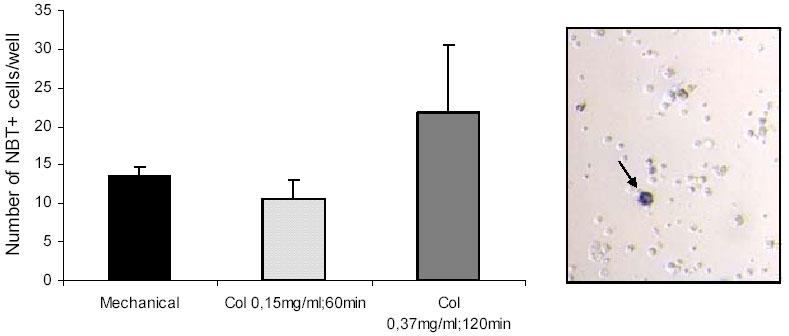
Number of NBT+ cells per well in total seabream GALT cells suspensions obtained after three different purification protocols. Data are expressed as mean ± sd. Representative light micrograph (x400) after NBT assay showing a NBT-positive cell (arrow) containing formazan precipitates.
Positive controls (head kidney suspensions) always contained high numbers of NBT-positive cells (not shown).
Discussion
Traditionally, primary lymphoid organs have received most attention but, at present, there is growing interest in understanding the immunity of mucosal surfaces, since they are strategically placed in areas constantly exposed to external pathogens (20). The study of GALT cellular components, their specific location and their morphological and functional characteristics are all key aspects when trying to understand the role of GALT within the whole fish immune system.
In general, two main immune cell populations can be identified in association with gastrointestinal surfaces: the intraepithelial leucocytes (IEL’s) and the lamina propria leucocytes (LPL’s). In higher vertebrates, IEL’s are exclusively represented by T-cells, whilst the lamina propria hosts T and B lymphocytes, plasma cells, mast cells and macrophages (20). In fish, the lamina propria of gut folds is known to contain the largest population of GALT leucocytes (5). As a consequence, studies based on purification protocols that only free immune cells within the epithelium give a partial view of the total immune defences associated to a particular intestinal surface. It was assumed that lymphoid cells from gut tissue belong to two fractions: the DTT/EDTA-fraction, containing the IEL’s, and the collagenase-fraction, containing the LPL’s (2, 18). Apart from the theoretical basis stated before and the available literature, we further confirmed this assumption by microscopic observation. Whilst we found no degradation of the lamina propria during the chemical treatment, the connective tissue was clearly loosing its integrity due to the action of the collagenase enzyme.
The present study has addressed the enrichment of total cell pools obtained from the seabream digestive tract in immune cells. The heterogeneity of the cell types that make up this tissue and its nature as a secondary lymphoid organ make this a complex task. It is noteworthy that goblet cells are known to vary in number and mucus content in animal intestine mucosa depending on the diet, health status or age (21-22). When high mucus content is present GALT isolation becomes even more troublesome. In this context, we found essential that fish were starved for at least 48h. When some food content was still present in the digestive tract the protocol yielded variable results.
The use of a purely mechanical procedure was the most time and cost effective, providing cell pools of high viability. Previous data on carp GALT (9) found no clear differences between mechanical and enzymatic extraction protocols although no information on the quality of the cell pools obtained in each case was given. In our case, flow cytometry revealed some differences in the FSC and SSC values of the isolated cells. The greater scattering of the R2 region in cell suspensions obtained by this method suggests that not single cells but groups of them, typically enterocytes, were present, as corroborated in the semithin sections. Thus, care should be taken to avoid the presence of small tissue fragments which hinder the use of flow cytometry analysis.
Microscopic observation of fragments during the different isolation steps revealed that DTT and collagenase treatment released immune cell allocated both within the epithelium and in the lamina propria. Viability of the isolated cells was not significantly affected by the collagenase step duration or the collagenase concentration.
Nylon wool columns have been used to enrich T cells based on the differential adherence properties of T-cells, B-cells and other cells to nylon (23). When IEL’s from rainbow trout were isolated after 1h incubation in DTT and then filtered through nylon wool columns (17), this step reduced the epithelial cell content although the cells were not examined by microscopy or flow cytometry. We found both mucus and some rounded cells not further characterised attached to nylon wool fibres under scanning electron microscopy. Their morphological features resembled that of lymphocytes but whether fish B lymphocytes display adherence properties to nylon wool, as their mammalian counterparts, remains to be investigated. The use of these columns eased, nonetheless, subsequent handling and study of the isolated cells without affecting their viability, and is therefore to be recommended.
Density gradients helped us to purify a population of putative lymphocytes, according to flow cytometry and light microscopy features. Similarly, IEL’s from rainbow trout were purified using the same technique (11). We reveal consistently high numbers of lymphocytes in the seabream gut tissue, which is in agreement with previous studies that pointed to a preponderance of T cells in the gut immune system of the sea bass (24) or carp (25). Lymphocytes were abundant in both the ID and HD band although the latter had greater levels of purity, containing almost only lymphocytes. It is possible that the lymphocytes found in each band display differential properties, although this was not morphologically obvious under light microscopy. Rainbow trout IEL’s also appeared in two Percoll density bands, high (HD) and intermediate (LD), the former being the most abundant (17).
The absence of a density band containing phagocytes alone led us to conduct respiratory burst assays using NBT as substrate. Very low percentages of NBT+ cells were found, confirming the data obtained by flow cytometry and cytocentrifugation slides. Moreover, interfish variation was greater than variations due to different isolation protocols, underlining the fact that these cells are present in low numbers in the seabream gut. Low numbers of granulocytes have also been reported in carp GALT (8). Alternatively, seabream phagocytes could have been present in our GALT cell suspensions but not capable of bursting without the presence of a previous stimulus to prime them. Further studies should address the possibility of phagocytic cells migrating into the gut compartment in case of infection.
In summary, we have succeeded in isolating high numbers of seabream GALT cells by using relatively short and low cost protocols. Nylon wool columns and Percoll gradients enabled us to purify abundant gut lymphocytes with high viability (>90%), whereas phagocytic cells (granulocytes and macrophages) were scarce. The present data in conjunction with the use of specific cell markers for each gut leucocyte subset should open up investigations into the functions and biology of seabream GALT cells.
Acknowledgments
I. Salinas wishes to thank Fundación Séneca for a PhD scholarship. The authors wish to thank Dr. P. Muñoz and Dr. A. Cuesta for their valuable help as well as to all the staff from SACE, University of Murcia.
Protocols
Isolation of seabream GALT cells by mechanical stripping
Reagents
Cold RPMI-1640 culture medium with 0.35% sodium chloride (to adjust the medium’s osmolarity to gilthead seabream plasma osmolarity, 353.33 mOs) containing 10U.I./ml heparin (Sigma).
Place 10 ml of media in a Petri dish with the seabream gut already devoid of connective tissue and gut contents.
Use the blunt edge of a scalpel to carefully strip the mucosal layer of the intestine once it is longitudinally opened.
Collect media with a Pasteur pipette and filter through a 100 μm nylon mesh.
Wash twice in sRPMI (400x g, 23°C, 10 min).
Count cells and adjust to 107 cells/ml. This is your “mechanical” cell suspension.
Isolation of seabream GALT cells (IEL’s and LPL’s) by chemical and enzymatic treatmen
Reagents
Phosphate buffer saline (x10, Gibco) Ca and Mg free. Dilute it in distilled water and adjust pH to 7.4.
DL-dithiothreitol (Sigma)
Ethylenediamintetraacetic acid (EDTA)
Hank’s buffer saline solution (HBSS)
Foetal Bovine Serum (Gibco) (FBS)
Streptomicin and penicilin (Gibco)
DNAse I (Sigma)
Collagenase type IV (Sigma)
RPMI-1640 culture medium with 0.35% sodium chloride (to adjust the medium’s osmolarity to gilthead seabream plasma osmolarity, 353.33 mOs)
23°C incubator (5% CO2)
Neubauer chamber
Other common equipment and reagents for cell culture
Prepare a desired volume of solution I by adding DTT (0.145 mg/ml) and EDTA (0.37 mg/ml) to PBS.
Prepare washing media by adding streptomycin and penicillin, 5% FBS and DNAse I (0.05 mg/ml) to HBSS
Solution II: Weigh the collagenase you are going to use the same day and resuspend it in washing media (final concentration 0, 0.15 or 0.37 mg/ml). Keep refrigerated and do not use solution II that has been stored for over one week.
Bleed the specimen, conduct careful dissection in cold PBS and remove al the connective tissue and rinse off any remaining gut contents
Place 1cm long segments (longitudinally opened first) in a 50ml tube containing 15-20ml of solution I. Shake in an orbital shaker at 60 rpm for 10 min.
Collect supernatant and filter it through a 100 μm nylon mesh (S1). Keep S1 in a 23°C incubator, 5% CO2 until S2 is ready.
Wash tissue fragments in a Petri dish with washing media to remove any DTT from solution I.
Place gut fragments in a clean 50 ml tube and add 15ml of solution II. Shake as before for 30, 60 or 120 min.
Collect supernatant, filter it through a 100 μm nylon mesh and strain tissue fragments against the mesh at the same time. Big surface meshes are recommended since they tend to block due to mucus content. Use fresh sRPMI if you need in order to filter your cell suspension. Add the filtered suspension to S1 to obtain S2.
Wash twice in sRPMI (400x g, 23°C, 10 min). Count and adjust cells to 107 cells/ml.
Use of nylon wool columns
We used nylon wool columns that we prepared ourselves with nylon wool fibre (0.5 g Kisker) and 10 ml syringes. Readily usable nylon wool columns are commercially available at a higher price than the ones we produced. The choice of the syringe and the amount of nylon wool was done following instructions provided by manufacturer.
Loading greater volumes of cells or more concentrated cell suspensions clotted the columns and precluded adequate elution of the non-adherent phase.
Load the column with 5 ml of sRPMI for 1h prior to adding the cell suspension.
Add 5 ml of your cell suspension (S2) slowly. The eluted media first loaded will elute and it can be disposed. Incubate for 1h and then wash the column twice with 5 ml of sRPMI to collect non-adherent cells. The nylon fiber with the adherent phase was fixed and used for scanning electron microscopy observation.
Wash twice non-adherent cells in sRPMI and count and adjust to 107 cells/ml. This is your NW suspension.
As shown by scanning electron microscopy, mucus was retained by the fibres which resulted in easier to work cell suspensions. Not only did they not clump together before flow cytometry (in which case they needed to be resuspended with a Pasteur pipette) but also subsequent Percoll separation of different cell populations was achieved in a more efficient way.
References
- Isolation of functionally active intraepithelial lymphocytes and enterocytes from human small and large intestine. Lundqvist C, Hammarström M-L, Athlin L, Hammarström S. J Immunol Methods. 1992;152:253–263. doi: 10.1016/0022-1759(92)90147-L. [DOI] [PubMed] [Google Scholar]
- Intraepithelial and lamina propria lymphocytes show distinct patterns of apoptosis whereas both populations are active in Fas based cytotoxicity in coeliac disease. Di Sabatino A, Ciccocioppo R, D'Alò S, Parroni R, Millimaggi D, Cifone MG, Corazza GR. Gut. 2007;49:380–386. doi: 10.1136/gut.49.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isolation and purification of lymphocyte subsets from gut-associated lymphoid tissue in neonatal swine. Solano-Aguilar GI, Vengroski KG, Beshah E, Lunney JK. J Immunol Methods. 2000;241:185–199. doi: 10.1016/S0022-1759(00)00209-X. [DOI] [PubMed] [Google Scholar]
- Purification of human lamina propria plasma cells by an immunomagnetic selection method. Medina F, Segundo C, Salcedo I, García-Poley A, Brieva JA. J Immunol Methods. 2004;285:129–135. doi: 10.1016/j.jim.2003.11.006. [DOI] [PubMed] [Google Scholar]
- The morphology of the immune system in teleost fishes. Press CM, Evensen O. Fish Shellfish Immunol. 1999;9:309–318. doi: 10.1006/fsim.1998.0181. [DOI] [Google Scholar]
- Gut immunology in fish: a review. Hart S, Wrathmell AB, Harris JE, Grayson TH. Dev Com Immunol. 1998;12:453–480. doi: 10.1016/0145-305X(88)90065-1. [DOI] [PubMed] [Google Scholar]
- Immunocytochemical detection of Ig-positive cells in blood, lymphoid organs and the gut associated lymphoid tissue of the turbot (Scophtalmus maximus). Fournier-Betz V, Quentel C, Lamour F, LeVen A. Fish Shellfish Immunol. 2000;10:187–202. doi: 10.1006/fsim.1999.0235. [DOI] [PubMed] [Google Scholar]
- Histological enzyme and flow cytometry analysis of channel catfish intestinal tract immune cells. Hébert P, Ainsworth AJ, Boyd B. Dev Comp Immunol. 2002;26:53–62. doi: 10.1016/S0145-305X(01)00044-1. [DOI] [PubMed] [Google Scholar]
- The gut-associated lymphoid tissue (GALT) of carp (Cyprinus carpio L.): an immunocytochemical analysis. Rombout JH, Taverne-Tiele AJ, Villena MI. Dev Comp Immunol. 1993;17:55–66. doi: 10.1016/0145-305X(93)90015-I. [DOI] [PubMed] [Google Scholar]
- Dietary vitamin E and rainbow trout (Oncorhynchus mykiss) phagocytes functions: effect on gut and head kidney leucocytes. Clerton P, Troutaud D, Verlhac V, Gabadaudan J, Deschaux P. Fish Shellfish Immunol. 2001;11:1–13. doi: 10.1006/fsim.2000.0287. [DOI] [PubMed] [Google Scholar]
- Phenotypic and functional similarity of gut intraepithelial and systemic T cells in a teleost fish. Bernard D, Six A, Rigottier-Gois L, Messiaen S, Chilmonczyk S, Quillet E, Boudinot P, Benmansour A. J Immunol. 2006;176:3942–3949. doi: 10.4049/jimmunol.176.7.3942. [DOI] [PubMed] [Google Scholar]
- Intestinal adaptations of rainbow trout to changes in dietary carbohydrate. Buddington RK, Hilton JW. Am J Physiol Gastrointest Liver Physiol. 1987;253:G489–G496. doi: 10.1152/ajpgi.1987.253.4.G489. [DOI] [PubMed] [Google Scholar]
- Evans DH. The physiology of fishes. 2nd ed: CRC Press; 1998. p. 43-64.
- Fontaine M. Nutrition des poissons. Paris: Editions du CNRS, 1981. p. 1-20.
- Dietary administration of Lactobacillus delbrüeckii and Bacillus subtilis, single or combined, on gilthead seabream cellular innate immune responses. Salinas I, Cuesta A, Esteban MA, Meseguer J. Fish Shellfish Immunol. 2005;19:66–76. doi: 10.1016/j.fsi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Detection of MHC class II transcripts in lymphoid tissues of the common carp (Cyprinus carpio L.). Rodrigues PNS, Hermsen TT, Rombout JH, Egberts E, Stet RJM. Dev Comp Immunol. 1995;19:483–496. doi: 10.1016/0145-305X(95)00033-P. [DOI] [PubMed] [Google Scholar]
- Isolation of rainbow trout (Oncorhynchis mykiss) intestinal intraepithelial lymphocytes (IEL) and measurement of their cytotoxic activity. McMillan DN, Secombes CJ. Fish Shellfish Immunol. 1997;7:527–541. doi: 10.1006/fsim.1997.0099. [DOI] [Google Scholar]
- Intraepithelial but not lamina propria lymphocytes in the porcine gut are affected by dexamethasone treatment. Schwarz E, Saalmüller A, Gerner W, Claus R. Vet Immunol Immunopathol. 2005;105:125–139. doi: 10.1016/j.vetimm.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Modulation of the in vitro activity of European sea bass (Dicentrarchus labrax L.) phagocytes by the myxosporean parasite Sphaerospora dicentrarchi (Myxosporea: Bivalvulida). Muñoz P, Alvarez-Pellitero P, Sitja-Bobadilla A. Fish Shellfish Immunol. 2000;10:567–581. doi: 10.1006/fsim.2000.0272. [DOI] [PubMed] [Google Scholar]
- The immune-enhancing effects of dietary fibres and prebiotics. Schley PD, Field CJ. Br J Nutr. 2002;87 Suppl 2:S221–S230. doi: 10.1079/BJNBJN/2002541. [DOI] [PubMed] [Google Scholar]
- Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. Faure M, Mettraux C, Moennoz D, Godin JP, Vuichoud J, Rochat F, Breuille D, Obled C, Corthesy-Theulaz I. J Nutr. 2006;136:1558–1564. doi: 10.1093/jn/136.6.1558. [DOI] [PubMed] [Google Scholar]
- The influence of different management systems and age on intestinal morphology, immune cell numbers and mucin production from goblet cells in post-weaning pigs. Brown DC, Maxwell CV, Erf GF, Davis ME, Singh S, Johnson ZB. Vet Immunol Immunopathol. 2006;111:187–198. doi: 10.1016/j.vetimm.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Hathcock KS. T-cell enrichment by nonoadherence to nylon. In: Coligan JE, Kruisbeek AM, David H. Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. New York, 1992.
- Expression of lymphocyte antigenic determinants in developing gut-associated lymphoid tissue of the seabass Dicentrarchus labrax (L.). Picchieti S, Terribili FR, Mastrolia L, Scapigliati G, Abelli L. Anat Embryol. 1997;196:457–463. doi: 10.1007/s004290050113. [DOI] [PubMed] [Google Scholar]
- Indications for a distinct putative T cell population in mucosal tissue of carp (Cyprinus carpio L.). Rombout JH, Joosten PH, Engelsma MY, Vos AP, Taverne N, Taverne-Thiele JJ. Dev Comp Immunol. 1998;22:63–77. doi: 10.1016/S0145-305X(97)00048-7. [DOI] [PubMed] [Google Scholar]


