Abstract
Murine gammaherpesvirus 68 (MHV-68) infection of mice is a potential model with which to address fundamental aspects of the pathobiology and host control of gammaherpesvirus latency. Control of MHV-68 infection, like that of Epstein–Barr virus, is strongly dependent on the cellular immune system. However, the molecular biology of MHV-68 latency is largely undefined. A screen of the MHV-68 genome for potential latency-associated mRNAs revealed that the region encompassing and flanking the genomic terminal repeats is transcriptionally active in the latently infected murine B-cell tumor line S11. Transcription of one MHV-68 gene, that encoding the hypothetical M2 protein, was detected in virtually all latently infected S11 cells and in splenocytes of latently infected mice, but not in lytically infected fibroblasts. Furthermore, an epitope was identified in the predicted M2 protein that is recognized by CD8+ T cells from infected mice and a cytotoxic T lymphocyte line that recognizes this epitope killed S11 cells, indicating that the M2 protein is expressed during latent infection and is a target for the host cytotoxic T lymphocyte response. This work therefore provides essential information for modeling MHV-68 latency and strategies of immunotherapy against gammaherpesvirus-related diseases in a highly tractable animal model.
The gammaherpesviruses are medically important viruses that establish a lifelong latent infection within lymphocytes. Human gammaherpesviruses are the Epstein–Barr virus (EBV) and Kaposi’s sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8. EBV, a virus with demonstrated oncogenic potential, is associated with numerous human tumors, including Burkitt lymphoma, Hodgkin’s disease, nasopharyngeal carcinoma, and immunoblastic lymphomas in immune-compromised individuals (1). Latent KSHV infection is detectable in virtually all Kaposi’s sarcomas and B-cell primary effusion lymphomas, as well as in a high proportion of cases of HIV-associated Castleman’s disease (2, 3), suggesting that KSHV possesses significant oncogenic potential as well.
EBV is capable of growth-transforming B lymphocytes in vitro into permanent lymphoblastoid cells lines that maintain a latent infection. Latency in these cells is characterized by the expression of a subset of 12 EBV genes, the so-called latency-associated genes, that function in maintenance of the viral genome, repression of virus replication, and the promotion and control of cell proliferation (4). During the course of primary infection in vivo, however, such highly proliferative cells induce a potent cytotoxic T lymphocyte (CTL) response to several of the EBV latency-associated proteins (1). In the face of this developing CTL response, EBV enters a more restricted and less immunogenic state of latency within a pool of resting B cells, which serve as the long-term reservoir of EBV-infected cells (5–7). Significant outgrowth of infected B cells as a result of reactivation of the growth program of EBV latency is efficiently limited by this cellular immune response (1). Thus, development of a CTL response to latency-associated viral antigens is important not only for limiting acute EBV infection, but long-term prevention of EBV-induced disease as well.
To a large extent, our knowledge of latent infection has come from analyses of latently infected cell lines. Ultimately, however, latency must be addressed in the context of normal infection, preferably within a natural host. Unfortunately, gammaherpesviruses such as EBV have extremely limited host ranges, and even within infectable New World primates EBV infection does not recapitulate a key aspect of natural infection, namely the establishment of a long-term inapparent infection (8). Experimental infection of Old World primates such as rhesus macaques with the EBV- and KSHV-related viruses that naturally infect these species offers a promising alternative model of infection (9, 10). These models, however, have limited practical use, particularly for addressing fundamental aspects of the host control of herpesvirus latency. A potentially useful model for these studies is the infection of laboratory mice with the murine gammaherpesvirus 68 (MHV-68).
After intranasal inoculation of mice with MHV-68, productive infection occurs in alveolar epithelial cells that is resolved 7–10 days postinfection (p.i.) (11). Concomitant with the resolution of acute infection in the lung, latency is established within splenic B lymphocytes, as well as within lung epithelial cells (12–15). Recent evidence indicates that MHV-68 also can establish latency in peritoneal macrophages (16). Like EBV, MHV-68 induces a CTL response that is critical to the control of latent infection (17–19). However, the molecular biology of MHV-68 latency itself is not well defined. To identify MHV-68 latency-associated genes, we screened the entire MHV-68 genome for the expression of latent-gene mRNAs in a murine B-cell tumor line. One MHV-68 gene identified, that which contains the M2 ORF (20), is expressed within all latently infected cells in vitro, as well as within splenocytes of latently infected mice. Furthermore, we have defined an epitope within the M2 protein that is recognized by CD8+ T cells from infected mice. Thus, this work provides a significant step in the development of the MHV-68 model of infection and establishes a foundation for studies to define mechanisms underlying the control of gammaherpesvirus latency.
MATERIALS AND METHODS
Cells and Virus.
BHK-21 cells were maintained in Glasgow’s MEM supplemented with glucose (4.5 g/liter), 2 mM l-glutamine, 10% tryptose phosphate broth, and 10% newborn calf serum. BALB/c 3T3 cells were maintained in DMEM containing 10% newborn calf serum. S11 tumor cells (21) were maintained in RPMI medium 1640 supplemented with 2 mM l-glutamine, 50 μM β-mercaptoethanol, and 10% defined FBS (HyClone). MHV-68 replication in S11 cells was induced by exposure to 20 ng of 12-O-tetradecanoylphorbol 13-acetate (TPA) per ml and 4 mM sodium butyrate for 24 hr. Stocks of MHV-68 (clone g2.4) were generated in BHK-21 cells as described (11). BHK-21 cells were infected with 10 plaque-forming units of virus per cell. Some infections were done in the presence of either cycloheximide (100 μg/ml) to inhibit early and late viral transcription or phosphonoacetic acid (100 μg/ml) to inhibit late viral transcription.
RNA Analysis.
Poly(A)+ RNA was isolated from S11 cells as described (22) or from infected BHK-21 cells by using an mRNA purification kit (Amersham Pharmacia). Northern blot analysis of MHV-68 gene expression was performed as described (22) with 32P-labeled DNA probes derived from cloned viral restriction fragments [gift of S. Efstathiou, University of Cambridge (U.K.); ref. 23], PCR products, or cDNAs containing a specific ORF(s). Blots were rehybridized to a probe for glyceraldehyde-3-phosphate dehydrogenase or β-actin mRNA to monitor RNA loading. RNA Millennium markers (Ambion, Austin, TX) or an RNA ladder (GIBCO/BRL) were used as size standards.
In Situ Hybridization.
In situ hybridizations were performed on S11 cells as described (15) with minor modifications. Cells were washed twice in PBS, fixed in PLP (75 mM l-lysine hydrochloride/37.5 mM sodium phosphate buffer, pH 7.4/2% paraformaldehyde/10 mM sodium metaperiodate) for 5 min, washed once, and then resuspended in PBS at a concentration of 106 cells/ml. Cells (100 μl) were transferred onto Biobond-treated slides by using a cytocentrifuge (Shandon, Pittsburgh) and fixed again in PLP. Probes were digoxygenin-labeled RNAs generated with an RNA-labeling kit (Boehringer Mannheim) and were detected by using a combination of alkaline phosphatase-conjugated antidigoxygenin and 4-chloro-3-indolyl phosphate/nitro blue tetrazollium (BCIP/NBT tablets; Sigma), followed by counterstaining with neutral red. Sense and antisense transcripts spanning MHV-68 tRNAs 1–4 were generated from pEH 1.4 (24). The probe for the overlapping thymidine kinase/glycoprotein H mRNAs has been described (15). M2 and M3 probes were generated from DNA fragments containing the complete ORF cloned into pBluescript (Stratagene).
Analysis of MHV-68 Gene Expression in Vivo.
BALB/c mice were infected intranasally with 400 plaque-forming units of MHV-68. At 14 and 28 days p.i. cells were harvested from the spleens of three mice, pooled, and disrupted in 20 ml of RNAzol B (Tel-Test, Friendswood, TX) by several passages through an 18-gauge needle. After extraction as specified by the manufacturer, RNA was extracted twice with phenol/chloroform and once with chloroform and precipitated in ethanol. RNA was digested with RQ1 DNase (Promega) and subjected to reverse transcription–PCR (RT-PCR). In brief, 1 μg of poly(A)+ or 2 μg of total RNA was reverse-transcribed by using an oligo(dT) adapter primer; 2 μl of cDNA product then was amplified by PCR in a 50-μl reaction (10 mM Tris⋅HCl, pH 9.0/50 mM KCl/2.5 mM MgSO4/10% DMSO/1 mM each dNTP/25 pmol each of forward and reverse primer specific for the MHV-68 M2, M3, or M8/57 mRNA/2.5 units of Taq DNA polymerase). The PCR profile was 30 cycles × (95°C, 40 s; 50°C, 2 min; 72°C, 3 min) followed by a 15-min extension at 72°C. PCR primers were: M2, CTTCCTTAGCCAGTCTCTTC and ACCTTCACTGTTACTCCTCG; M3, GTGGCACACCAGTGAATGAC and GGTGAAGTGAGAGAATCCAG; M8/57, ATGCTCGAGTGTAGAGGA and TATCTCTAAGCAGAGGAGAG. PCR product then was subjected to Southern blot analysis.
Mapping of CD8+ T Cell Epitopes.
Overlapping 15-mer peptides spanning the M2 ORF were synthesized as reported (25). Synthesis of 8- and 9-mer peptides was performed by using an model 433A peptide synthesizer (Applied Biosystems). Spleen cells from MHV-68-infected BALB/c mice were incubated in vitro with 15-mer (40 μg/ml) or 8/9-mer peptides (1 μg/ml) in the presence of brefeldin A (10 μg/ml) and IL-2 (10 units/ml) for 5 hr. Fc binding then was blocked by using anti-mouse CD16/CD32 (PharMingen), and cells were stained with tricolor-conjugated anti-CD8 antibody (Caltag, Burlingame, CA). Cells were fixed in 2% formaldehyde, permeablized in 0.5% saponin (Sigma), and stained as described (26) with anti-mouse IFN-γ or an isotype control IgG1 antibody (PharMingen) in the presence of 0.5% saponin. Samples were analyzed with a Becton-Dickinson FACScan flow cytometer using cellquest software (Becton-Dickinson Immunocytometry Systems). As a positive control for IFN-γ production, cells were treated with phorbol 12-myristate 13-acetate (50 ng/ml) plus ionomycin (500 ng/ml).
RESULTS
MHV-68 RNA Expression in Latently and Lytically Infected Cells.
The only cells capable of maintaining latent MHV-68 infection in vitro are derived from the murine B-cell tumor line S11 (21). To identify potential MHV-68 latency-associated genes, we screened S11 cells for the expression of viral mRNAs by Northern blot hybridization using a series of DNA probes representing the entire MHV-68 genome. A clonal line of S11 cells was selected that exhibited the lowest level of spontaneous lytic infection among a panel of clones generated by limiting dilution. Although 1% or less of the cells within this S11 line expressed lytic cycle antigens, this level of productive infection can result in substantial production of replicative gene mRNAs. Therefore, to help distinguish between true latency-associated genes and abundantly expressed lytic cycle genes, we compared the RNA from these cells to RNA from cells treated with TPA and sodium butyrate to induce virus replication.
Complete results of the Northern blot screen of the entire MHV-68 genome for potential latency-associated transcripts are published as supplemental data on the PNAS web site (www.pnas.org). As indicated in Table 1, the only transcriptionally active region of the MHV-68 genome in S11 cells that encoded potential mRNAs that did not increase in abundance upon induction of the virus lytic cycle with TPA was the right end of the genome. These were transcripts of 5.6, 3.2, and 1.6 kb detected with a probe spanning the overlapping M12 and M13 ORFs (20) that appeared low in abundance. Noninducible transcripts of 3.2 and 1.6 kb also were detected with a terminal repeat (TR) probe. Because the M12 and M13 ORFs both extend into the TR domain, these are likely to be the same 3.2- and 1.6-kb transcripts detected with the M12/M13 probe. Also detected (with a HindIII-E probe) were the tRNA-like molecules previously shown to be expressed within S11 cells as well as latently infected cells in vivo (15, 24). Poly(A)+ transcripts present within untreated S11 cells, but which increased dramatically upon induction, were detected with probes HindIII-E, -G, -N, -D, and EcoRI-L (Table 1). With the exception of HindIII-E and -D that contain novel ORFs (M1-M3, M10a,b,c, and M11) that could potentially encode latency-associated proteins, HindIII-G, -N, and EcoRI-L span genes previously identified as lytic cycle-specific either by demonstration of actual expression during MHV-68 replication in permissively infected cells or by their significant homology to known lytic cycle genes of other herpesviruses (20, 27–30). We have analyzed a full-length cDNA (coordinates 97,780–93,847) of the TPA-inducible 4.2-kb transcript detected in uninduced S11 cells with the HindIII-D probe (as well as the overlapping EcoRI-D) and found that this mRNA does not encode the novel ORFs 10a,b,c, or M11 within HindIII-D, but ORFs 67, 66, and M9. However, M9 recently was noted to be the probable counterpart of the herpes simplex virus capsid protein gene UL34 (A. Davison, personal communication). Thus, transcripts detected in untreated S11 cells with the HindIII-G, -N, -D, and EcoRI-L probes were most likely abundant lytic cycle transcripts expressed within a minor population of cells supporting virus replication.
Table 1.
MHV-68 transcripts detected in uninduced S11 cells
| Probe | Genomic coordinates* | ORFs* | Transcripts, kb |
|---|---|---|---|
| HindIII-E | 107–6,261 | M1, M2, M3 | 1.4, tRNAs |
| HindIII-G | 11,100–16,237 | 6, 7 | 4.3, 5.65 |
| HindIII-N | 16,238–19,868 | 8, 9 | 2.9 |
| EcoRI-L | 80,645–84,996 | 61, 62, 63 | 3.9 |
| HindIII-D | 95,678–104,034 | 67, 68, 69, M10a,b,c, 72, M11, 73 | 4.2 |
| M12/M13 | 117,979–118,787 | M12, M13 | 1.6, 3.2, 5.6 |
| TR† | 118,237–119,450 | M12, M13, M14 | 1.6, 3.2 |
Transcripts in bold type were not induced with TPA. Complete Northern blot data are available as supplemental data on the PNAS web site, www.pnas.org.
Probe coordinates and ORFs are according to Virgin, et al. (20); only ORFs overlapping the probe by ≥100 bp are listed.
TR probe was a 1.2-kbp PstI fragment spanning each TR.
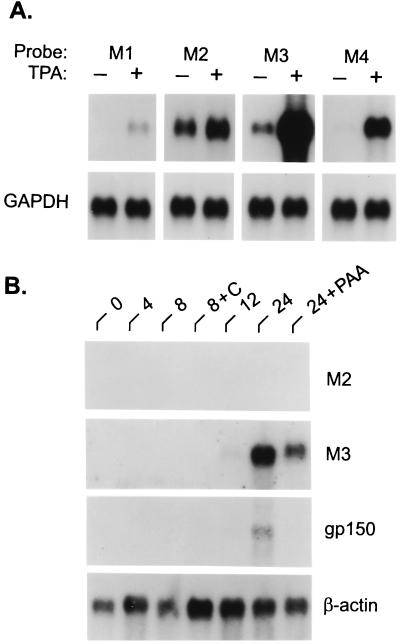
At the extreme left end of the genome, expression of an apparent single abundant yet highly inducible transcript of 1.4 kb was detected with the HindIII-E probe, which contains ORFs M1, M2, and a portion of M3 (Table 1) (20). However, only a very low level of transcript was detected in uninduced cells with the adjacent HindIII-J probe (see the supplemental data at www.pnas.org), which also contains part of M3 as well as M4 and ORF4 (20). This finding suggested that M1 and/or M2 may encode a latency-associated transcript. To address this possibility, we hybridized Northern blots to probes specific for M1, M2, M3, and M4. As demonstrated in Fig. 1A, each probe recognized a transcript of ≈1.4 kb. However, only the M2-specific transcript was expressed in untreated S11 cells and not appreciably induced by TPA. Thus, this M2-containing transcript, in addition to those encoded by the right end of the genome, appeared to represent the only truly latency-specific genes expressed in S11 cells. Within infected BHK cells, which are completely permissive for MHV-68 replication, no expression of an M2 transcript was detected (Fig. 1B). By contrast, M3, which was expressed at a low level in untreated S11 cells, was abundantly expressed in BHK cells and in the presence of phosphonoacetic acid, an inhibitor of herpesvirus DNA polymerase, indicating that the M3 gene is expressed as an early gene during virus replication.
Figure 1.
Expression of unique MHV-68 genes M1-M4 during latent and lytic infection. (A) Detection of M1, M2, M3, and M4 RNAs in S11 cells with gene-specific probes. Each RNA blot contained poly(A)+ RNA (2.5 μg per lane) from untreated (−) or TPA-treated (+) S11 cells; all transcripts detected were ≈1.4 kb. (B) Poly(A)+ RNA isolated from lytically infected BHK cells at time points up to 24 hr p.i. was analyzed by successive Northern blot hybridization for expression of the M2, M3, and gp150 genes; cells infected in the presence of cycloheximide (C) or phosphonoacetic acid (PAA) are indicated. All blots were stripped and rehybridized to a probe for either glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or β-actin to control for differences in RNA loading.
Characterization of MHV-68 Latency-Associated Transcripts.
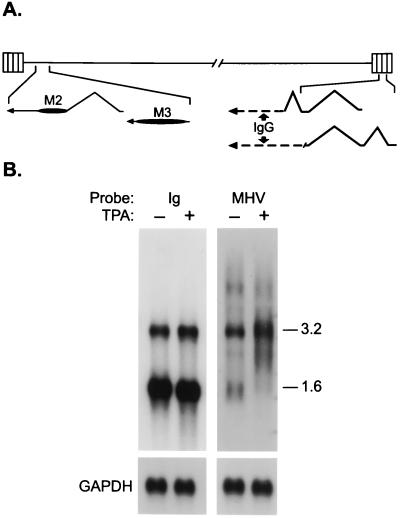
We next determined the nucleotide sequence of cDNAs isolated from an S11 cDNA library by screening with HindIII-E, M12/M13, and TR probes. Several M2 and M3 cDNAs were obtained, the structures of which are illustrated in Fig. 2A. These represent leftward-oriented polyadenylated transcripts of 1.4 kb, consistent with our Northern blot analysis. Whereas the M3 transcript is unspliced, the M2 transcript is composed of two exons, a noncoding 110-nt 5′ exon and a 1,235-nt 3′ exon that contains the M2 ORF and a predicted 656-nt 3′ untranslated region. Because of the splice acceptor site used, the M2 ORF present in the cDNA is 7 aa shorter than the genomic ORF (20). Proteins of the expected size were expressed from the M2 and M3 cDNAs when transcribed and translated in vitro (data not shown).
Figure 2.
Characterization of potential MHV-68 latency-associated transcripts. (A) Structures of transcripts as determined by cDNA nucleotide sequence relative to genomic sequence. Genomic coordinates, presented 5′ to 3′ with respect to the mRNA, are according to Virgin et al. (20): M2 mRNA, 5924–5815 (exon 1); 4609–3375 (exon 2); M3 mRNA, 7291–6007. Two cDNAs derived from MHV-68/IgG fusion transcripts contained one complete and one partial copy each of a 91-nt exon (118, 695–118, 605) encoded within each TR; the longer cDNA (lower-most structure) also contained a 179-nt exon (119, 373–119,195) derived from a TR and was fused directly to mouse IgG-2A heavy chain via an incomplete copy of the 91-nt exon, whereas in the shorter cDNA a complete copy of the 91-nt exon was spliced to an IgG-2A exon. All cDNAs contained a poly(A) tract at their 3′ end. (B) Northern blot analysis of poly(A)+ RNA from S11 cells by using Ig- and MHV-specific probes derived from the fusion cDNAs.
We also isolated two overlapping cDNAs representing leftward transcripts originating within the TRs (Fig. 2A). Surprisingly, these cDNAs contained at their 5′ end two or three short exons encoded by MHV-68 followed by sequence derived from a mouse IgG heavy chain gene. Thus, these transcripts most likely arose from a copy of the MHV-68 genome that had integrated into the host chromosome. Neither cDNA contained an apparent functional ORF within the MHV-68-derived exons. Northern blot analysis using the murine Ig and MHV-68 segments of these cDNAs as probes (Fig. 2B) indicated that the 3.2-kb transcript detected with the M12/M13 and TR probes (Table 1) contained Ig as well as MHV-68 sequences and is likely to be a fusion transcript(s) from which these cDNAs were derived. A 1.6-kb transcript was detected with the Ig probe that was not detected with equal intensity by the MHV-68 probe. Thus, a 5.6-kb transcript and possibly the lower abundance 1.6-kb RNA detected with the MHV-68 probe (Fig. 2B) may be encoded by a functional MHV-68 gene within the standard viral genome. However, attempts thus far to isolate a cDNA of this putative gene have been unsuccessful.
The M2 Gene Is Latency Associated.
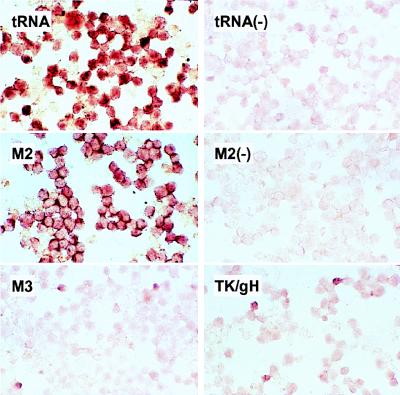
To determine whether the M2 gene was indeed a latency-associated gene, i.e., expressed in all latently infected cells, we performed in situ hybridizations on S11 cells with a probe specific for the M2 transcript. Also included were probes for the MHV-68 tRNA-like molecules expressed in latently infected cells (15, 24), as well as probes for M3 and two known lytic cycle genes, thymidine kinase (TK) and glycoprotein H (gH) (29, 30). As shown in Fig. 3, the antisense, but not sense, tRNA probe hybridized to virtually every cell as expected, as did the M2-specific probe. By contrast, the expression of M3 and TK/gH (the same probe hybridizes to both mRNAs) was detected in only a small percentage of cells, as expected for lytic cycle genes.
Figure 3.
The M2 gene, but not M3, is expressed in virtually all latently infected cells in vitro. S11 cells were hybridized in situ to digoxygenin-labeled antisense RNA probes specific for MHV-68 tRNAs 1–4, M2, M3, and dually for the lytic cycle genes thymidine kinase (TK) and glycoprotein H (gH); hybridizations with sense tRNA and M2 probes were included as negative controls (−).
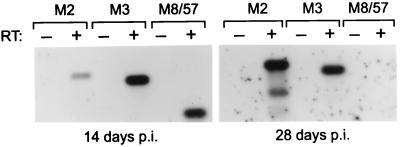
To determine whether the M2 gene was expressed during latency in vivo, RNA was isolated from the spleens of BALB/c mice at 14 and 28 days p.i. and subjected to RT-PCR with primers specific for the M2 mRNA, as well for M3 and a previously characterized lytic cycle transcript that encodes a homolog of the EBV “SM” transactivator. This is a 1.5-kb early gene mRNA in which MHV-68 ORFs M8 and 57 are spliced in-frame within the same transcript (30). At 14 days p.i., when a low level of virus replication is detectable in the spleen (11), expression of all three transcripts was evident (Fig. 4, Left). However, at 28 days p.i., when latency has been established and virus replication is not routinely detectable (11), only the M2 and M3 transcripts were detected (Fig. 4, Right). Note that we failed to detect expression of the M8/57 transcript even after 2 × 30 cycles of amplification. Identical results were obtained in C57BL/6 mice (data not shown). Thus, although M3 was not expressed during latent infection in S11 cells (Fig. 3), M2 as well as M3 appeared to be expressed during latency in vivo. This apparent discrepancy with respect to M3 expression may indicate that there are different patterns or programs of MHV-68 latent gene expression, perhaps analogous to the different latency programs of EBV. Alternatively, the sensitivity of detection of M3 expression by RT-PCR simply may be greater than that of M8/57, and thus the M3 signal obtained at 28 days p.i. may have resulted from an ability to detect a very low level of lytic cycle transcription by this assay. Nonetheless, the data presented in Figs. 3 and 4 provide strong evidence that the M2 gene of MHV-68 is expressed during latency, both in vitro and in vivo.
Figure 4.
M2 and M3 mRNAs are expressed in splenocytes of latently infected mice. RNA isolated from spleens of infected BALB/c mice at 14 and 28 days p.i. was subjected to RT-PCR followed by Southern blot hybridization to detect expression of the MHV-68 M2, M3, and M8/57 mRNAs. After the initial 30 cycles of amplification of cDNA generated from the RNA isolated at 28 days p.i., the PCR product was diluted 50-fold and reamplified to confirm that expression of the M8/57 lytic cycle-specific mRNA was undetectable. Product sizes are 650 bp (M2), 589 bp (M3), and 340 bp (M8/57); the respective sizes of the M2 and M3 fragments from 14 and 28 days p.i. samples are identical.
The M2 Protein Contains a CD8+ T Cell Epitope Recognized During MHV-68 Infection.
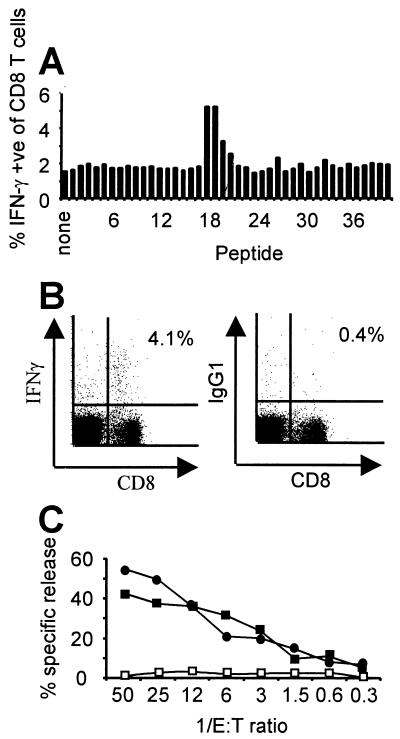
We next addressed whether the M2 protein elicits a CD8+ T cell response in infected mice. Spleen cells were harvested from BALB/c mice at 18 days p.i. and tested for their ability to recognize a panel of overlapping 15-mer peptides spanning the entire predicted M2 protein. As illustrated in Fig. 5A, three consecutive peptides, 18–20, induced IFN-γ synthesis in CD8+ T cells, suggesting the presence of a CTL epitope in this region of M2 (26). We then synthesized and tested all possible 8-mer and 9-mer peptides from the region covered by 15-mers 18–20 and found that a 9-aa peptide, M291–99 (GFNKLRSTL) was sufficient to stimulate synthesis of IFN-γ in 4.1% of CD8+ T lymphocytes (Fig. 5B). Not surprisingly, this peptide contains a classic H-2Kd binding motif (X[Y,F]XXXXXX[I,L,V]) (31). We also have generated a CD8+ CTL cell line reactive to S11 cells from splenocytes of BALB/c mice harvested 24 days p.i. that recognizes the same M2 epitope. As shown in Fig. 5C, this CTL line effectively killed S11 cells as well as BALB/c 3T3 cells pulsed with the M291–99 peptide, but not unpulsed 3T3 cells, indicating that the M2 protein is both expressed in S11 cells and a target of the host CTL response. Furthermore, this data (Fig. 5C) in conjunction with our observations that virtually all S11 cells are latently infected and expressing M2 RNA (Fig. 3) and that the M2 gene is not transcribed in cells completely permissive for MHV-68 replication (Fig. 1B) provides strong evidence that the M2 gene does indeed encode a latency-associated protein.
Figure 5.
The M2 protein contains an epitope recognized by CD8+ T cells. (A) Each 15-aa peptide from a panel of overlapping peptides encompassing the entire M2 protein was incubated with pooled splenocytes harvested from two BALB/c mice 18 days p.i. The percentage of CD8+ T cells producing IFN-γ in response to each peptide was measured by flow cytometry. Data are representative of two independent experiments. Phorbol 12-myristate 13-acetate plus ionomycin stimulation resulted in production of IFN-γ by 47% of CD8+ T cells (not shown). (B) IFN-γ production by splenocytes from a BALB/c mouse (representative of three mice tested) at 18 days p.i. in response to the 9-mer peptide GFNKLRSTL (M291–99) that is present within peptides 18–20 in A; staining with an isotype-matched control antibody is also shown (Right). Splenocytes from uninfected mice did not respond to M291–99 (not shown). (C) A CD8+ CTL line kills S11 cells (●) and BALB/c 3T3 cells pulsed with M291–99 (■), but not unpulsed 3T3 cells (□). Results are from a standard 5-hr 51Cr-release assay (32) at various effector to target (E:T) ratios and are representative of six independent experiments.
DISCUSSION
MHV-68 infection of laboratory mice offers a biologically relevant and tractable model with which to define mechanisms of immune control of herpesvirus infections. As such, it is a potentially valuable tool for development of strategies to disrupt or prevent the establishment of latency by the human gammaherpesviruses EBV and KSHV, which possess significant pathogenic potential. Here we have demonstrated that within a murine B-cell tumor line, latency-specific expression of MHV-68 mRNAs is limited to the region of the genome within and flanking the TR elements. The transcripts identified consist of singly spliced mRNA from which the M2 protein is expressed in latently infected cells and at least one multiply spliced transcript that is partially encoded by the TRs and extends leftward into the unique sequence domain of the genome. A third and unspliced transcript containing the M3 ORF was found to be highly expressed in lytically infected S11 and BHK cells in vitro, but also may be expressed during latency in vivo. Thus, relative to the type III or growth program of EBV latency within EBV-immortalized B lymphoblastoid cell lines, in which at least 10 EBV proteins are expressed (1, 34), latently infected S11 cells express a limited repertoire of MHV-68 genes. Recently, an RT-PCR-based screen for MHV-68 RNAs in the spleens and peritoneal cells of persistently infected mice by Virgin et al. (33) identified three regions of the MHV-68 genome potentially expressed during latency. In spleen cells these regions contained ORFs M2, M3, and M9, in agreement with data presented here on S11 cells (the TR region was not evaluated), and ORFs M2, M11, 73, and 74 in peritoneal cells (most likely macrophages; ref. 16). Although M9 is most likely a lytic-cycle gene (see above), definitive identification of latency-associated expression of ORFs M11, 73, and 74, as with M3, remains to be demonstrated.
Whereas EBV efficiently immortalizes B lymphocytes in vitro through the actions of several viral proteins expressed during the EBV growth program of latency, attempts to immortalize lymphocytes with either MHV-68 or the more closely related KSHV have proven unsuccessful. The apparent lack of growth-transforming capability by MHV-68 and KSHV may indicate that the life cycles of these gammaherpesviruses do not include an EBV-like growth program. MHV-68 latent infection in S11 cells therefore may be functionally equivalent to the alternative and more restricted forms of EBV latency believed to mediate long-term maintenance of EBV infection in resting B lymphocytes in vivo (7), in which the expression of EBV proteins that promote cell growth is repressed (1, 5, 6). The MHV-68 latency program in S11 tumor cells therefore may be analogous to the restricted EBV latency program (type I latency) manifested in Burkitt lymphoma cells, in which the only viral proteins expressed are the genome maintenance and origin-binding protein EBNA-1, and at least one as yet uncharacterized protein (RK-BARF0) encoded by the BamHI-A rightward transcripts (1, 34).
Analysis of the mRNAs partially encoded by the TRs revealed that at least one of these is a 3.2-kb transcript containing both Ig and MHV-68 exons, indicating that it is derived from an integrated MHV-68 genome or one that has acquired cellular genetic material. Although the MHV-68-specific nucleotide sequences at the 5′ ends of these cDNAs do not appear to contain a functional ORF, they do contain MHV-68 exons and thus it is highly likely that there is a bona fide MHV-68 gene encompassing the TRs. This gene would be located in the same relative position and orientation in the MHV-68 genome as are the latency-associated LMP-2A and -2B genes in the EBV genome (35). Furthermore, analysis of the “K15” transcripts expressed from this region of the KSHV genome indicates these are multiexon mRNAs predicted to encode an LMP-2-like protein (T. Schulz, personal communication). Thus, based on the data presented here and comparative characterizations of latency-associated gene expression of two related gammaherpesviruses, we predict such a gene also exists within the MHV-68 genome.
A major interest in the MHV-68 model of infection is its potential utility in testing hypotheses formulated to broaden understanding of pathogenicity and the establishment, maintenance, and host control of latent infection by the medically relevant human gammaherpesviruses EBV and KSHV that cannot be directly or easily tested within humans or a primate model. Of particular interest is the practical knowledge to be gained from delineating the immunologic control of MHV-68 latency. In this context, a mouse model is uniquely useful because the host immune system is relatively well defined and can be manipulated genetically, facilitating identification of factors and the dissection of pathways that determine the outcome of a gammaherpesvirus infection. Obviously, identification of viral proteins expressed during latency in vivo is an important prerequisite for studies of this nature. The M2 protein has been definitively linked to MHV-68 latency, both in vitro and in vivo, and contains an CD8+ T cell epitope recognized in infected mice. The immune response to cells expressing the M2 protein therefore may be pivotal in the maintenance of the long-term association that this gammaherpesvirus has with its host. Because expression of the M2 gene clearly persists within latently infected mice, it will be particularly important to define the mechanism(s) that MHV-68 has evolved to evade the host cellular immune surveillance, as have EBV and other herpesviruses. Thus, in addition to significantly enhancing our understanding of MHV-68 latency, this work provides an important foundation for future studies relevant to human gammaherpesvirus infections. Of immediate interest is whether induction of an anti-M2 CTL response in naive animals can prevent or ameliorate sequela associated with acute infection or prevent the establishment of latency itself. Such a strategy, if successful, may provide a generally applicable means with which to target the pathogenic potential of the gammaherpesviruses.
Supplementary Material
Acknowledgments
We thank M. Wakeling and D. Henson for excellent technical assistance and C. Sample and I. Ruf for critical review of the manuscript. This work was supported by U.S. Public Health Service Grants AI37597 (to D.L.W.), AI42927 (to M.A.B.), and CA56639 (to J.T.S.), Cancer Center (CORE) Grant CA21765, the American Lebanese Syrian Associated Charities (ALSAC), the Medical Research Council (United Kingdom), The Royal Society (London), and a Biomedical Research Collaboration Grant from the Wellcome Trust (United Kingdom). J.P.S. is a Royal Society University Research Fellow.
ABBREVIATIONS
- MHV-68
murine gammaherpesvirus 68
- EBV
Epstein–Barr virus
- KSHV
Kaposi’s sarcoma-associated herpesvirus
- p.i.
postinfection
- TR
terminal repeat
- RT-PCR
reverse transcription-PCR
- CTL
cytotoxic T lymphocyte
- TPA
12-O-tetradecanoylphorbol 13-acetate
References
- 1.Rickinson A B, Kieff E. In: Fields Virology. Fields B N, Knipe D M, Howley P M, editors. New York: Lippincott; 1996. pp. 2397–2446. [Google Scholar]
- 2.Ganem D. Cell. 1997;91:157–160. doi: 10.1016/s0092-8674(00)80398-0. [DOI] [PubMed] [Google Scholar]
- 3.Boshoff C, Weiss R A. In: Advances in Cancer Research. Vande Woude G F, Klein G, editors. London: Academic; 1998. pp. 57–86. [DOI] [PubMed] [Google Scholar]
- 4.Kieff E. In: Fields Virology. Fields B N, Knipe D M, Howley P M, editors. New York: Lippincott; 1996. pp. 2343–2396. [Google Scholar]
- 5.Tierney R J, Steven N, Young L S, Rickinson A B. J Virol. 1994;68:7374–7385. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorley-Lawson D A, Miyashita E M, Khan G. Trends Microbiol. 1996;4:204–208. doi: 10.1016/s0966-842x(96)90020-7. [DOI] [PubMed] [Google Scholar]
- 7.Miyashita E M, Yang B, Babcock G J, Thorley-Lawson D A. J Virol. 1997;71:4882–4891. doi: 10.1128/jvi.71.7.4882-4891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shope T, Dechairo D, Miller G. Proc Natl Acad Sci USA. 1973;70:2487–2491. doi: 10.1073/pnas.70.9.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moghaddam A, Rosenzweig M, Lee-Parritz D, Annis B, Johnson R P, Wang F. Science. 1997;276:2030–2033. doi: 10.1126/science.276.5321.2030. [DOI] [PubMed] [Google Scholar]
- 10.Desrosiers R C, Sasseville V G, Czajak S C, Zhang X, Mansfield K G, Kaur A, Johnson R P, Lackner A A, Jung J U. J Virol. 1997;71:9764–9769. doi: 10.1128/jvi.71.12.9764-9769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sunil-Chandra N P, Efstathiou S, Arno J, Nash A A. J Gen Virol. 1992;73:2347–2356. doi: 10.1099/0022-1317-73-9-2347. [DOI] [PubMed] [Google Scholar]
- 12.Sunil-Chandra N P, Efstathiou S, Nash A A. J Gen Virol. 1992;73:3275–3279. doi: 10.1099/0022-1317-73-12-3275. [DOI] [PubMed] [Google Scholar]
- 13.Usherwood E J, Stewart J P, Robertson K, Allen D J, Nash A A. J Gen Virol. 1996;7:2819–2825. doi: 10.1099/0022-1317-77-11-2819. [DOI] [PubMed] [Google Scholar]
- 14.Weck K E, Barkon M L, Yoo L I, Speck S H, Virgin H W. J Virol. 1996;70:6775–6780. doi: 10.1128/jvi.70.10.6775-6780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart J P, Usherwood E J, Ross A J, Dyson H, Nash A A. J Exp Med. 1998;187:1941–1951. doi: 10.1084/jem.187.12.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weck K E, Kim S S, Virgin H W, Speck S H. J Virol. 1999;73:3273–3283. doi: 10.1128/jvi.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehtisham S, Sunil-Chandra N P, Nash A A. J Virol. 1993;67:5247–5252. doi: 10.1128/jvi.67.9.5247-5252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart J P, Usherwood E J, Dutia B M, Nash A A. In: Herpesviruses and Immunity. Medveczky P G, Friedman H, Bendinelli M, editors. New York: Plenum; 1998. pp. 149–163. [Google Scholar]
- 19.Stevenson P G, Belz G T, Altman J D, Doherty P C. Eur J Immunol. 1999;29:1059–1067. doi: 10.1002/(SICI)1521-4141(199904)29:04<1059::AID-IMMU1059>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 20.Virgin H W, Latreille P, Wamsley P, Hallsworth K, Weck K E, Dal Canto A J, Speck S H. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Usherwood E J, Stewart J P, Nash A A. J Virol. 1996;70:6516–6518. doi: 10.1128/jvi.70.9.6516-6518.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nonkwelo C, Ruf I K, Sample J. J Virol. 1997;71:6887–6897. doi: 10.1128/jvi.71.9.6887-6897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Efstathiou S, Ho Y M, Minson A C. J Gen Virol. 1990;71:1355–1364. doi: 10.1099/0022-1317-71-6-1355. [DOI] [PubMed] [Google Scholar]
- 24.Bowden R J, Simas J P, Davis A, Efstathiou S. J Gen Virol. 1997;78:1675–1687. doi: 10.1099/0022-1317-78-7-1675. [DOI] [PubMed] [Google Scholar]
- 25.Geysen H M, Meleon R H, Barteling S J. Proc Natl Acad Sci USA. 1984;81:3992–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 27.Stewart J P, Janjua N J, Sunil-Chandra N P, Nash A A, Arrand J R. J Virol. 1994;68:6496–6504. doi: 10.1128/jvi.68.10.6496-6504.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart J P, Janjua N J, Pepper S deV, Bennion G, Mackett M, Allen T, Nash A A, Arrand J R. J Virol. 1996;70:3528–3535. doi: 10.1093/benz/9780199773787.article.b00034574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pepper S deV, Stewart J P, Arrand J R, Mackett M. Virology. 1996;219:475–479. doi: 10.1006/viro.1996.0274. [DOI] [PubMed] [Google Scholar]
- 30.Mackett M, Stewart J P, Pepper S deV, Chee M, Efstathiou S, Nash A A, Arrand J R. J Gen Virol. 1997;78:1425–1433. doi: 10.1099/0022-1317-78-6-1425. [DOI] [PubMed] [Google Scholar]
- 31.Rammensee H G, Friede T, Stevanoviic S. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 32.Cole G A, Hogg T L, Coppola M A, Woodland D L. J Immunol. 1997;158:4301–4309. [PubMed] [Google Scholar]
- 33.Virgin H W, Presti R M, Li X-Y, Liu C, Speck S H. J Virol. 1999;73:2321–2332. doi: 10.1128/jvi.73.3.2321-2332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fries K L, Sculley T B, Webster-Cyriaque J, Rajadurai P, Sadler R H, Raab-Traub N. J Virol. 1997;71:2765–2771. doi: 10.1128/jvi.71.4.2765-2771.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sample J, Liebowitz D, Kieff E. J Virol. 1989;63:933–937. doi: 10.1128/jvi.63.2.933-937.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.