Abstract
Lysyl oxidase is required for the normal biosynthesis and maturation of collagen and elastin. It is expressed by vascular smooth muscle cells, and its increased expression has been previously found in atherosclerosis and in models of balloon angioplasty. The lysyl oxidase propeptide (LOX-PP) has more recently been found to have biological activity as a tumor suppressor, and it inhibits Erk1/2 Map kinase activation. We reasoned that LOX-PP may have functions in normal non-transformed cells. We, therefore, investigated its effects on smooth muscle cells, focusing on important biological processes mediated by Erk1/2-dependent signaling pathways including proliferation and matrix metalloproteinase-9 (MMP-9) expression. In addition, we investigated whether evidence for accumulation of LOX-PP could be found in vivo in a femoral artery injury model. Recombinant LOX-PP was expressed and purified, and was found to inhibit primary rat aorta smooth muscle cell proliferation and DNA synthesis by more than 50%. TNF-α-stimulated MMP-9 expression and Erk1/2 activation were both significantly inhibited by LOX-PP. Immunohistochemistry studies carried out with affinity purified anti-LOX-PP antibody showed that LOX-PP epitopes were expressed at elevated levels in vascular lesions of injured arteries. These novel data suggest that LOX-PP may provide a feedback control mechanism that serves to inhibit properties associated with the development of vascular pathology.
Tumor necrosis factor-α (TNF-α) contributes to the development of atherosclerotic lesions [15]. Important among its many activities, TNF-α promotes the proliferation of smooth muscle cells (SMCs) [15] and it stimulates matrix metalloproteinase (MMP) production [6]. Thinning of the fibrous cap by MMPs is undesirable due to the release of sequestered factors that leads to thrombosis [15]. TNF-α regulation of MMP-9 is of particular importance in atherosclerotic lesion development and in fibrous cap thinning [3, 20]. Thus, up-regulation of MMP-9 expression by TNF-α can promote development of atherosclerotic lesions by multiple mechanisms that include stimulation of SMC proliferation and MMP-9 production.
Lysyl oxidase is required for the maturation of collagen and elastin precursors in the biosynthesis of a functional extracellular matrix [13]. Lysyl oxidase catalyzes the oxidative deaminination of lysine residues in elastin precursors and of lysine and hydroxylysine residues in fibrillar collagen precursors to form peptidyl-aldehydes. Formation of these aldehydes by lysyl oxidase is the final enzymatic step required for the formation of essential lysine-derived cross-linkages [13]. It is now understood that lysyl oxidase enzymes are encoded by a five-member gene family: lysyl oxidase itself (LOX), and lysyl oxidase-like proteins (LOXL1 – LOXL4) [5]. These proteins all have a conserved C-terminal domain that encodes the catalytic enzyme region, and an N-terminal propeptide region. The LOX propeptide (LOX-PP) is unique in structure and has little sequence identity to LOXL1 and no sequence similarity to the other LOXL proteins [5]. LOX-PP contains the tumor suppressor functionality of LOX and it inhibits ras-dependent signaling, including Erk1/2 Map kinase activation [10, 24]. LOX is expressed by vascular SMCs, and LOX deficient mice die at parturition and exhibit major cardiovascular defects [18]. Lysyl oxidase enzyme activity and expression are increased in atherosclerosis, and in models of restenosis [2, 12, 22].
Due to the presence of LOX in vascular tissues and smooth muscle cells, we asked whether LOX-PP might have biological activities in vascular SMCs. Because LOX-PP is an effective inhibitor of Erk1/2 Map kinases [19, 28], and this Map kinase mediates TNF-α stimulated SMC proliferation and MMP-9 expression [20], we determined whether LOX-PP inhibits vascular SMC proliferation and TNF-α stimulated MMP-9 expression, and whether LOX-PP directly inhibits the MEK/Erk enzymes. Finally, we determined whether LOX-PP is detectable in vivo in femoral arteries that had been subjected to guidewire injury and in sham controls. Findings implicate a novel role for LOX-PP to provide a feedback mechanism to control vascular SMC proliferation and MMP production.
MATERIALS AND METHODS
Cell culture
Primary rat aorta smooth muscle cells (SMC) were isolated from 2 day old rats [23], and were cultured in Dulbecco’s Modified Eagles Medium containing 10% fetal bovine serum, 250 units/ml penicillin, 250 μg/ml streptomycin, 0.1 mM non-essential amino acids, and 1 mM sodium pyruvate. Cells were utilized after passage 1 [21, 29], as indicated.
Recombinant lysyl oxidase propeptide
The full length rat LOX-PP (residues 22 to 162) containing the osteonectin (BM40) signal peptide replacing the natural LOX signal peptide was cloned into a tetracycline inducible plasmid to produce LOXPP/pcDNA4/TO/myc-His. The construct itself was confirmed by DNA sequencing. Stable transfectants were generated by Lipofectamine 2000 mediated transfection of TREX 293 cells (Invitrogen), and selected with 5 μg/ml blasticidin and 150 μg/ml zeocin. Resistant cells were induced with 1 μg/ml doxycycline for 48 hours, and media containing LOX-PP was purified on a nickel affinity column (BioRad Bio-Scale Mini Profinity IMAC Cartridge) essentially as described [9], followed by further purification by ultrafiltration. Urea was removed by exhaustive dialysis against water or PBS. Nanospray LC/MS/MS mass spectroscopy of tryptic digests confirmed that rat LOX-PP is produced. SDS PAGE stained with Coomassie Blue R250 revealed a single band with an apparent molecular size of 35 kDa that is fully consistent with our published studies [7]. In selected experiments LOX-PP expressed in bacteria was utilized [9].
Cell growth assay
SMC were grown in 12-well plates to 60% visual confluence and then placed in media containing 0.2% serum for 24 hours. Cells were treated with fresh 0.2% serum-containing medium in the presence or absence of LOX-PP (10 μg/ml) alone, or vehicle alone (control). Cells were collected after 48 hours by treatment with trypsin/EDTA, centrifugation at 1000 × g. Cells were suspended in 50 μl DMEM and stained with 0.2% trypan blue, and viable cells were counted using a hemocytometer. Data are from three independent cultures per group per time point.
DNA synthesis assay
Cells were grown in 12 well plates and grown for 4 days to near visual confluence in DMEM containing 10% serum, and then cultured in serum free medium containing 0.1% bovine serum albumin (BSA) for 24 hours. Cells were treated with LOX-PP for 2.5 hrs before TNF-α (20 ng/ml) addition for 24 hours. Cells were labeled with 2μCi/ml [3H]-thymidine for 18 hours. Experiments were terminated by washing the cells with ice cold PBS, precipitation with 10% TCA, and extraction of the DNA with 0.2 M NaOH, 0.1% SDS. Radioactivity was measured by liquid scintillation counting.
RNA isolation and real time PCR
Total RNA was isolated from SMC cultures and purified using RNeasy mini-RNA purification kits (Qiagen). Intact RNA samples (1 μg) were reverse-transcribed using random primers and (Applied Biosystems Reverse Transcription kit). Aliquots were then utilized in qPCR reactions with TaqMan probes (Applied Biosystems) for MMP-9 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in an Applied Biosystems GeneAmp Prism 7500 System. Data were analyzed using the 2−ΔΔ ct method and mRNA levels were normalized to GAPDH mRNA [1].
Erk1/2 map kinase phosphorylation assay
SMC were grown to near confluence and then placed in serum-free medium containing 0.1% BSA for 24 hours. TNF-α (20 ng/ml) was then added with or without LOX-PP or vehicle in serum-free medium. Cells were lysed with ice-cold buffer: 50 mM Tris-Cl pH 7.5 , 100 mM NaCl, 5 mM EDTA, 1% Triton-X-100, PMSF 0.5 mM, DTT 1 mM, PNPP 10 mM, NaF 10 mM, β-glycerophosphate 10 mM, protease inhibitor and phosphatase inhibitor. Samples were then subjected to 10% SDS-PAGE and Western blotting for phosphorylated Erk1/2 and for total Erk1/2 with primary antibodies from Cell Signaling [1]. Signals were quantitated using a digital densitometry system (Versadoc; BioRad).
MEK/Erk2 activity assays
The ability of LOX-PP to directly inhibit enzymes in the Erk1/2 Map kinase pathway was measured. Recombinant purified and active MEK1, MEK2 and inactive Erk2 enzymes were purchased from Upstate Biotechnology. MEK1 and MEK2 activity assays were performed according to the kinase assay protocols provided by Upstate in incubations done in the presence or absence of LOX-PP. In stage 1, MEK1, or MEK2, respectively, were incubated with inactive Erk2 in the presence or absence of LOX-PP in a final volume of 25 μl. Aliquots of Stage I reactions are then assayed for the amount of active Erk2 resulting from MEK phosphorylation and activation of Erk2, using [γ32P]-ATP and myelin basic protein as substrate. In some experiments, LOX-PP was added to Stage II reactions to determine if LOX-PP can inhibit Erk2 activity directly.
Femoral artery injury and immunohistochemistry
Eleven week-old mice were subjected to guidewire-induced femoral artery injury or sham surgery [11, 25]. After 14 days, animals were sacrificed, femoral arteries were fixed and embedded in paraffin [11]. Block biopsies were sectioned (6 μm) and subjected to immunohistochemistry with anti-LOX-PP antibody. Sections were deparaffinized and antigen retrieval was performed by microwave heating of sections in 10 mM sodium citrate, pH 6. LOX-PP antibody was raised against a synthetic peptide corresponding to murine lysyl oxidase residues 78 – 115, and affinity purified against using an antigen affinity column. Antibody staining was performed with 1 μg/ml LOX-PP primary antibody according to methods previously utilized [27]. Sections were counterstained with hematoxylin. No immunostaining was observed in sections stained with non-immune IgG instead of primary LOX-PP antibody.
RESULTS
LOX-PP inhibits SMC proliferation
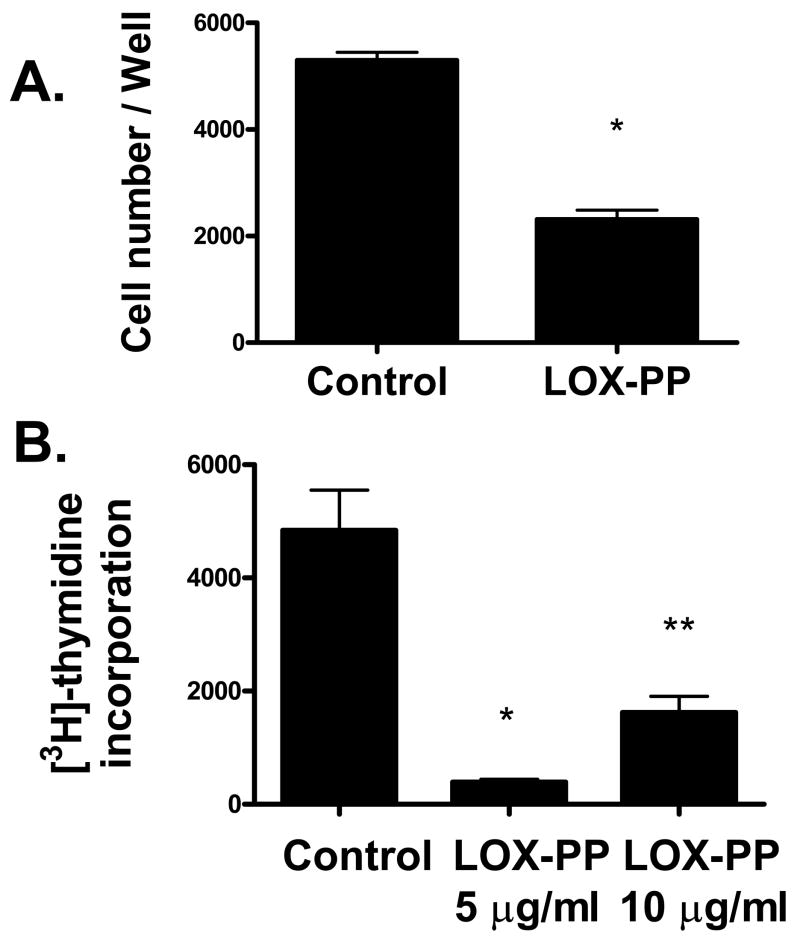
Pro-LOX, mature LOX, and therefore LOX-PP are expressed in vascular smooth muscle cells [26]. Here, we examined the potential of purified LOX-PP to influence SMC properties in order explore possible novel experimental therapeutic strategies for vascular disease. The effect of LOX-PP on the growth of vascular SMCs was first determined. Primary vascular SMCs were grown with LOX-PP (10 μg/ml) or vehicle was then the number of viable cells per culture determined after 48 hours. The concentration of LOX-PP was based on preliminary dose response- and published studies [19, 24, 28]. Figure 1A shows that LOX-PP significantly inhibits the growth of SMC cultures. We next measured LOX-PP inhibition of DNA synthesis. SMCs were cultured and treated with LOX-PP or vehicle for 2.5 hours. Tritiated thymidine incorporation was then determined. Figure 1B shows that LOX-PP highly significantly inhibits thymidine incorporation in SMC.
Figure 1.
LOX-PP inhibits growth of SMC in cultures. In A, primary neonatal rat aortic SMCs were grown as described in the presence of LOX-PP (10 μg/ml) or vehicle and the number of viable cells per culture determined on day 2 n = 3; *, p < 0.0001). In B, Rat aortic SMCs were cultured, LOX-PP or vehicle was added to cultures for 24 hours. Tritiated thymidine incorporation was then determined. Values shown are the averages of 3 independent cultures (*, p < 0.005; **, p < 0.05).
LOX-PP inhibits TNF-α-stimulated MMP-9 expression
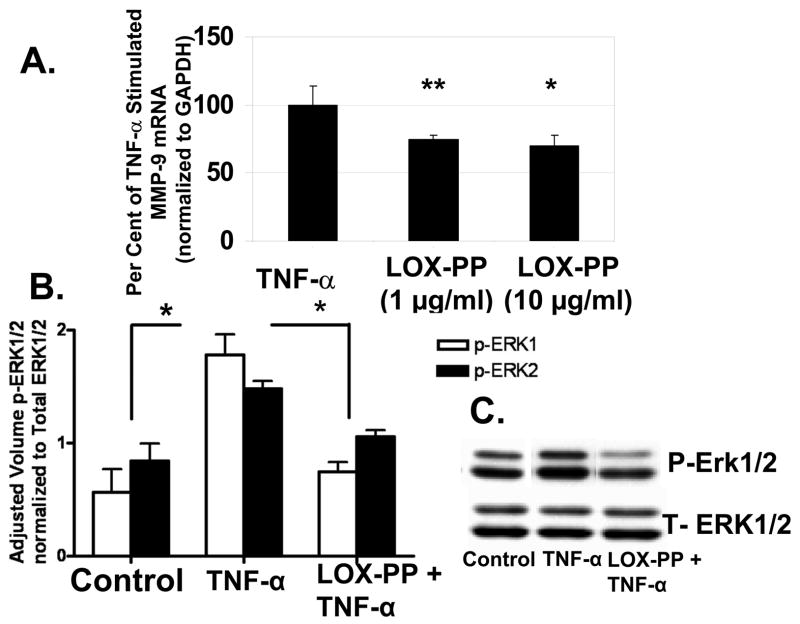
TNF-α up-regulates MMP-9 expression in human SMCs and this is mediated by Erk1/2 Map kinase activation [20]. We have confirmed this in rat vascular SMCs (Figure S1). Thus, LOX-PP could inhibit TNF-α-stimulated MMP-9 production via Erk1/2. SMCs were treated with 1 μg/ml or 10 μg/ml LOX-PP or vehicle, followed by TNF-α. RNA was isolated and analyzed by qPCR. Figure 2A shows that both concentrations of LOX-PP reduce MMP-9 mRNA levels, with somewhat greater inhibition occurring at 10 μg/ml.
Figure 2.
LOX-PP inhibits (A) TNF-α stimulation of MMP-9 mRNA levels, and (B and C) Erk1/2 activation. In A, primary neonatal rat aortic SMCs were treated with or without 20 ng/ml TNF-α in the presence and absence of LOX-PP. MMP-9 and GAPDH mRNA levels were determined by qPCR with Taqman reagents. Data are pooled from two different experiments with three independent cultures each, and are expressed as a per cent of TNF-α-stimulated MMP-9 mRNA levels +/− SE, normalized to GAPDH. *, p<0.05; **, p<0.004, n=3. Primary neonatal rat aortic SMCs were cultured for 24 h in the presence or absence of 10 μg/ml LOX-PP or vehicle. Cells were then stimulated with 20 ng/ml TNF-α or vehicle (PBS) for 15 minutes. Cells were extracted and aliquots subjected to Western blotting for phosphorylated, and total Erk1/2, respectively. Panel (B) shows densitometric quantitation of phosphorylated Erk 1 and Erk 2 levels normalized to total Erk 1 and 2. *, p<0.05, n=3. Panel (C) contains a representative Western blot.
We next determined whether LOX-PP inhibits TNF-α stimulated Erk1/2 activation in SMCs. Cells were treated with 10 μg/ml LOX-PP or vehicle for 24 hours, and then with TNF-α or vehicle for 15 min, based on preliminary time course studies (not shown). Figures 2B and 2C show that LOX-PP inhibits TNF-α-stimulated Erk1/2 activation. LOX-PP had no significant effect on the basal level of phosphorylated Erk1/2 (data not shown).
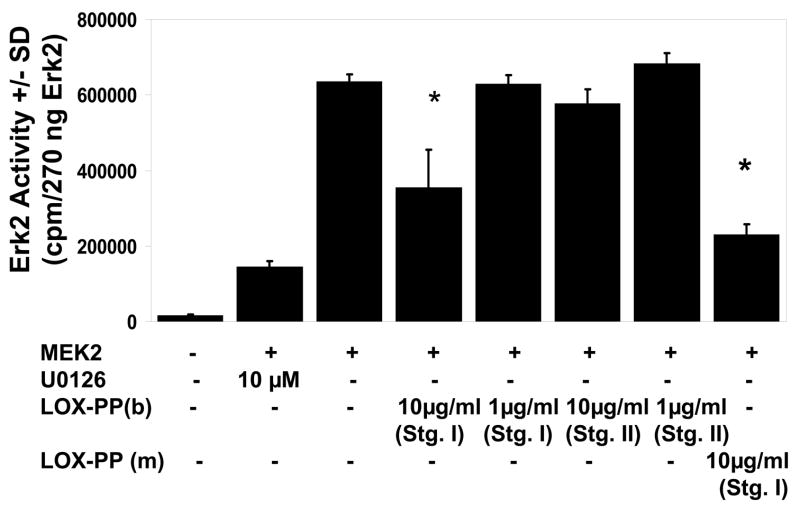
Previous studies have shown that LOX-PP is taken-up by mesenchymal cells [7]. LOX-PP could, therefore, directly inhibit Erk1/2 activation. The pathway of Erk1/2 activation occurs by MEK1 and MEK2 phosphorylation of Erk1 and Erk2. Phosphorylated and activated Erk1 and Erk2 then phosphorylate target effector proteins [10]. Thus, MEK1 or MEK2, respectively, was incubated in the presence or absence of LOX-PP and inactive Erk2. This reaction mixture is designated the stage I reaction. In the stage II reaction, an aliquot of stage I is then assayed for the amount of Erk2 activity utilizing myelin basic protein as substrate in the presence of [γ32P]-ATP, measuring the amount of phosphorylation of myelin basic protein. Figure 3 shows that recombinant LOX-PP (10 μg/ml) in the stage I reaction inhibits MEK2 by about 40%. Experiments indicate that recombinant LOX-PP expressed in bacteria and LOX-PP expressed in mammalian cells inhibits MEK2 to the same degree (Figure 3). LOX-PP added only to stage II does not inhibit the assay, indicating that LOX-PP inhibits the activity of MEK2 and not Erk2. Interestingly, LOX-PP does not inhibit MEK1 activation of Erk2. Thus, although LOX-PP can significantly inhibit MEK2 activity, data additional mechanisms of action of LOX-PP occur in live cells to inhibit Erk1/2 activation.
Figure 3.
LOX-PP directly inhibits MEK2 activity, and not Erk2. Recombinant active MEK2 ( 1 ng) was incubated with 6.7 μg of inactive Erk2 in the presence or absence of recombinant LOX-PP purified from a bacterial expression system [LOX-PP (b)] [9] or from a mammalian expression system [LOX-PP (m)] at 30o C for 15 min. This incubation is stage I (Stg. I). Aliquots of Stage I reactions were then assayed for the activity of Erk2 by measuring the phosphorylation of myelin basic protein in the presence of [γ32P]-ATP [Stage II reaction (Stg. II)]. Some reactions contained LOX-PP only in the Stage II reaction. Data are means +/− SD of triplicate assays, and are from a representative experiment done three times with similar results (*, p<0.05).
LOX-PP is highly expressed in vascular lesions
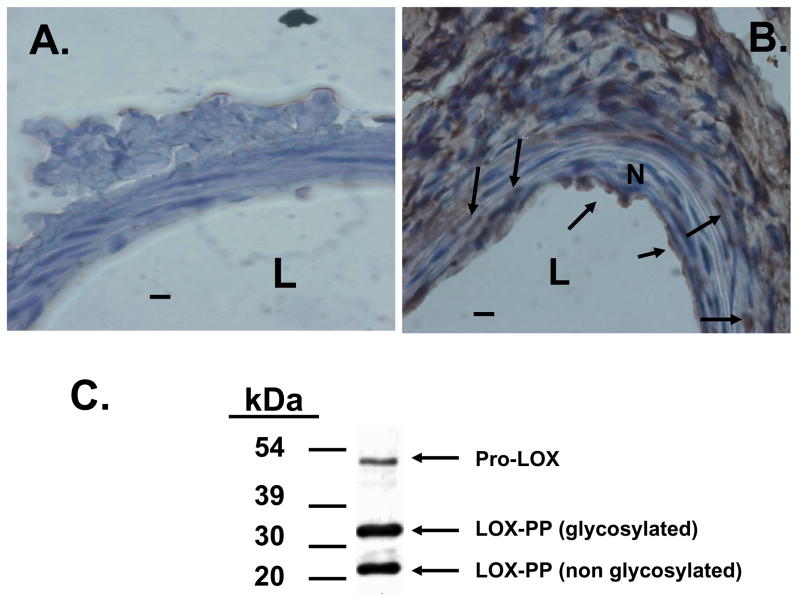
To assess for the presence in vivo of LOX-PP in vascular tissues, we performed immunohistochemistry on murine femoral arteries injured by guidewire scraping of the luminal surface and in sham operated control animals two weeks after injury. The affinity purified antibody utilized in this study was raised against a peptide derived from the unique LOX-PP region of LOX. Data in Figure 4A and 4B show abundant expression of the LOX-PP epitope in injured femoral arteries and little or no staining in uninjured arteries. The observed immunoreactivity must also be due to released LOX-PP, as pro-LOX is processed by vascular aortic smooth muscle cells [26]. Data in Figure 4C are consistent with this finding, and data indicate that all forms of LOX-PP are expressed by vascular SMCs. Figure 4C confirms the specificity of the LOX-PP antibody.
Figure 4.
LOX-PP epitopes occur at elevated levels in guidewire-injured femoral arteries. Eleven week-old mice were subjected to guidewire-induced femoral artery injury or sham surgery as described [11, 25] and sacrificed after 14 days. Sections were subjected to immunohistochemistry with anti-LOX-PP antibody. Arrows mark positive signals in smooth muscle cells. Micrographs are from (A) sham-, and (B) injured-, femoral arteries (bar = 10 μm; n = 4); (L, lumen; N, neointima). (C) Western blot of a cultured neonatal rat aorta smooth muscle cell extract assayed with the LOX-PP antibody showing pro-lysyl oxidase (50 kDa), glycosylated LOX-PP (35 kDa) [7], and non-glycosylated LOX-PP (20 kDa) [9].
DISCUSSION
LOX is critical for the biosynthesis of extracellular matrix proteins [13]. LOX is also a tumor suppressor and it inhibits ras-dependent transformation of fibroblasts [4, 8, 14]. The tumor suppressor activity of LOX has been mapped to the pro-peptide region [24] and LOX-PP inhibits tumor formation by breast cancer cells in mice [19]. In normal osteoblasts, LOX-PP accumulates in cultures [9] and recombinant LOX-PP is taken up by cells and accumulates over time associated with microtubules [7]. LOX is critically important for development of a normal cardiovascular system [17]. LOX is highly expressed in atherosclerotic tissues, and in models of balloon angioplasty [2, 12, 22]. It is, therefore, of considerable interest to determine LOX-PP biological functions in vascular cells. Here, we have asked whether LOX-PP inhibits Erk1/2-dependent biological activities in aortic smooth muscle cells, and if LOX-PP accumulates in a model of arterial restenosis. Data support that LOX-PP inhibits SMC proliferation and TNF-α-stimulated MMP-9 synthesis, and that these effects are mediated by both indirect and direct inhibition of the Erk1/2 Map kinase pathway. Moreover, data show that LOX-PP epitopes accumulate in arteries in which the endothelium has been injured.
Deposition of a fibrous extracellular matrix in atherosclerosis was formerly considered to be deleterious to disease outcome. Occlusion of the lumen of arteries due to excess extracellular matrix accumulation and accretion on lesions was seen to be a major factor in restricting blood flow leading to heart damage, but more recent findings indicate that thinning of extracellular matrix by MMP-driven proteolysis in atherosclerotic lesions leads to thrombolytic events that are more dangerous than artery occlusion [16]. Proliferation and migration of SMCs also contributes to atherosclerotic lesion development. We speculate that increased lysyl oxidase enzyme levels and increased LOX-PP levels in atherosclerosis are each potentially of benefit to clinical outcomes. Active lysyl oxidase enzyme contributes to collagen and elastin maturation that is required for structural integrity and function of both proteins, thereby contributing to the biosynthesis and stabilization of the extracellular matrix. The present study implicates LOX-PP as a feedback inhibitor of SMC proliferation and MMP-9 production, thereby serving to limit further SMC-dependent growth of restenotic and/or atherosclerotic lesions. Thus, LOX may have two different potentially beneficial anti-atherogenic biological activities derived, respectively from LOX-PP and LOX enzyme. Our novel findings stimulate future studies of new in vivo models to further understand the biological activities and mechanisms of action of LOX-PP in the context of vascular pathologies.
Supplementary Material
Acknowledgments
We are grateful to Dr. Erdjan Salih and Dr. Frank Oppenheim, Department of Periodontology and Oral Biology, Boston University, for LC/MS/MS mass spectral analysis of recombinant LOX-PP and Ning Zhang and Leah Mycoff Yu for SMC tissue culture preparation and assistance with the vascular injury model. This work was supported by the following NIH grants: HL13262 to KR and by DE14066 and DE11004 to PCT and by CA082742.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Black SA, Jr, Palamakumbura AH, Stan M, Trackman PC. Tissue-specific mechanisms for CCN2/CTGF persistence in fibrotic gingiva: interactions between cAMP and MAPK signaling pathways, and prostaglandin E2-EP3 receptor mediated activation of the c-JUN N-terminal kinase. J Biol Chem. 2007;282:15416–29. doi: 10.1074/jbc.M610432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brasselet C, Durand E, Addad F, Al Haj Zen A, Smeets MB, Laurent-Maquin D, Bouthors S, Bellon G, de Kleijn D, Godeau G, Garnotel R, Gogly B, Lafont A. Collagen and elastin cross-linking: a mechanism of constrictive remodeling after arterial injury. Am J Physiol Heart Circ Physiol. 2005;289:H2228–33. doi: 10.1152/ajpheart.00410.2005. [DOI] [PubMed] [Google Scholar]
- 3.Cho A, Reidy MA. Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ Res. 2002;91:845–51. doi: 10.1161/01.res.0000040420.17366.2e. [DOI] [PubMed] [Google Scholar]
- 4.Contente S, Kenyon K, Rimoldi D, Friedman RM. Expression of gene rrg is associated with reversion of NIH 3T3 transformed by LTR-c-H-ras. Science. 1990;249:796–8. doi: 10.1126/science.1697103. [DOI] [PubMed] [Google Scholar]
- 5.Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;70:1–32. doi: 10.1016/s0079-6603(01)70012-8. [DOI] [PubMed] [Google Scholar]
- 6.Dollery CM, Libby P. Atherosclerosis and proteinase activation. Cardiovasc Res. 2006;69:625–35. doi: 10.1016/j.cardiores.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Guo Y, Pischon N, Palamakumbura AH, Trackman PC. Intracellular distribution of the lysyl oxidase propeptide in osteoblastic cells. Am J Physiol Cell Physiol. 2007;292:C2095–102. doi: 10.1152/ajpcell.00613.2006. [DOI] [PubMed] [Google Scholar]
- 8.Hajnal A, Klemenz R, Schafer R. Up-regulation of lysyl oxidase in spontaneous revertants of H-ras- transformed rat fibroblasts. Cancer Res. 1993;53:4670–5. [PubMed] [Google Scholar]
- 9.Hong HH, Pischon N, Santana RB, Palamakumbura AH, Chase HB, Gantz D, Guo Y, Uzel MI, Ma D, Trackman PC. A role for lysyl oxidase regulation in the control of normal collagen deposition in differentiating osteoblast cultures. J Cell Physiol. 2004;200:53–62. doi: 10.1002/jcp.10476. [DOI] [PubMed] [Google Scholar]
- 10.Jeay S, Pianetti S, Kagan HM, Sonenshein GE. Lysyl oxidase inhibits ras-mediated transformation by preventing activation of NF-kappa B. Mol Cell Biol. 2003;23:2251–63. doi: 10.1128/MCB.23.7.2251-2263.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones MR, Zhao Z, Sullivan CP, Schreiber BM, Stone PJ, Toselli PA, Kagan HM, Cohen RA, Ravid K. A(3) adenosine receptor deficiency does not influence atherogenesis. J Cell Biochem. 2004;92:1034–43. doi: 10.1002/jcb.20122. [DOI] [PubMed] [Google Scholar]
- 12.Kagan HM, Raghavan J, Hollander W. Changes in aortic lysyl oxidase activity in diet-induced atherosclerosis in the rabbit. Arteriosclerosis. 1981;1:287–91. doi: 10.1161/01.atv.1.4.287. [DOI] [PubMed] [Google Scholar]
- 13.Kagan HM, Trackman PC. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol. 1991;5:206–10. doi: 10.1165/ajrcmb/5.3.206. [DOI] [PubMed] [Google Scholar]
- 14.Kenyon K, Contente S, Trackman PC, Tang J, Kagan HM, Friedman RM. Lysyl oxidase and rrg messenger RNA. Science. 1991;253:802. doi: 10.1126/science.1678898. [DOI] [PubMed] [Google Scholar]
- 15.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 16.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–8. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 17.Maki JM, Kivirikko KI. Cloning and characterization of a fourth human lysyl oxidase isoenzyme. Biochem J. 2001;355:381–7. doi: 10.1042/0264-6021:3550381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–9. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 19.Min C, Kirsch KH, Zhao Y, Jeay S, Palamakumbura AH, Trackman PC, Sonenshein GE. The tumor suppressor activity of the lysyl oxidase propeptide reverses the invasive phenotype of Her-2/neu-driven breast cancer. Cancer Res. 2007;67:1105–12. doi: 10.1158/0008-5472.CAN-06-3867. [DOI] [PubMed] [Google Scholar]
- 20.Moon SK, Cha BY, Kim CH. ERK1/2 mediates TNF-alpha-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kappaB and AP-1: Involvement of the ras dependent pathway. J Cell Physiol. 2004;198:417–27. doi: 10.1002/jcp.10435. [DOI] [PubMed] [Google Scholar]
- 21.Nagata Y, Jones MR, Nguyen HG, McCrann DJ, St Hilaire C, Schreiber BM, Hashimoto A, Inagaki M, Earnshaw WC, Todokoro K, Ravid K. Vascular smooth muscle cell polyploidization involves changes in chromosome passenger proteins and an endomitotic cell cycle. Exp Cell Res. 2005;305:277–91. doi: 10.1016/j.yexcr.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 22.Nuthakki VK, Fleser PS, Malinzak LE, Seymour ML, Callahan RE, Bendick PJ, Zelenock GB, Shanley CJ. Lysyl oxidase expression in a rat model of arterial balloon injury. J Vasc Surg. 2004;40:123–9. doi: 10.1016/j.jvs.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 23.Oakes BW, Batty AC, Handley CJ, Sandberg LB. The synthesis of elastin collagen glycosaminoglycans by high density primary cultures of neonatal rat aortic smooth muscle. An ultrastructural and biochemical study. Eur J Cell Biol. 1982;27:34–46. [PubMed] [Google Scholar]
- 24.Palamakumbura AH, Jeay S, Guo Y, Pischon N, Sommer P, Sonenshein GE, Trackman PC. The propeptide domain of lysyl oxidase induces phenotypic reversion of ras-transformed cells. J Biol Chem. 2004;279:40593–600. doi: 10.1074/jbc.M406639200. [DOI] [PubMed] [Google Scholar]
- 25.Roque M, Fallon JT, Badimon JJ, Zhang WX, Taubman MB, Reis ED. Mouse model of femoral artery denudation injury associated with the rapid accumulation of adhesion molecules on the luminal surface and recruitment of neutrophils. Arterioscler Thromb Vasc Biol. 2000;20:335–42. doi: 10.1161/01.atv.20.2.335. [DOI] [PubMed] [Google Scholar]
- 26.Trackman PC, Bedell-Hogan D, Tang J, Kagan HM. Post-translational glycosylation and proteolytic processing of a lysyl oxidase precursor. J Biol Chem. 1992;267:8666–71. [PubMed] [Google Scholar]
- 27.Uzel MI, Kantarci A, Hong HH, Uygur C, Sheff MC, Firatli E, Trackman PC. Connective tissue growth factor in drug-induced gingival overgrowth. J Periodontol. 2001;72:921–31. doi: 10.1902/jop.2001.72.7.921. [DOI] [PubMed] [Google Scholar]
- 28.Wu M, Min C, Wang X, Yu Z, Kirsch KH, Trackman PC, Sonenshein GE. Repression of BCL2 by the tumor suppressor activity of the lysyl oxidase propeptide inhibits transformed phenotype of lung and pancreatic cancer cells. Cancer Res. 2007;67:6278–85. doi: 10.1158/0008-5472.CAN-07-0776. [DOI] [PubMed] [Google Scholar]
- 29.Yaar R, Cataldo LM, Tzatsos A, Francis CE, Zhao Z, Ravid K. Regulation of the A3 adenosine receptor gene in vascular smooth muscle cells: role of a cAMP and GATA element. Mol Pharmacol. 2002;62:1167–76. doi: 10.1124/mol.62.5.1167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.