Abstract
The heterotrimeric G protein α subunit (Gα) functions as a molecular switch by cycling between inactive GDP-bound and active GTP-bound states. When bound to GDP, Gα interacts with high affinity to a complex of the β and γ subunits (Gβγ), but when bound to GTP, Gα dissociates from this complex to activate downstream signaling pathways. Gα's state is communicated to other cellular components via conformational changes within its switch I and II regions. To identify key determinants of Gα's function as a signaling pathway molecular switch, a Bayesian approach was used to infer the selective constraints that most distinguish Gα and closely related Arf family GTPases from distantly related translational and metabolic GTPases. The strongest of these constraints are imposed on seven residues within or near the switch II region. Likewise, constraints imposed on Gα but not on other, closely related molecular switches correspond to four nearby residues. These constraints are explained by a proposed mechanism for GTP-induced dissociation of Gα from Gβγ where an Arg–Trp pair senses the presence of bound GTP leading to conformational retraction of a nearby lysine and to disruption of an aromatic cluster. Within a complex of Giα, Giβγ, and GDP, this lysine establishes greater surface contact with Giβ than does any other residue in Giα, whereas the aromatic cluster packs against a highly conserved tryptophan in Giβ that establishes greater surface contact with Giα than does any other residue in Giβ. Other structural features associated with Gα functional divergence further support the proposed mechanism.
Keywords: structure/function studies, G-proteins (as signal transducers), Arf, Arl, CHAIN analysis
Heterotrimeric GTP-binding proteins (or G proteins) (McCudden et al. 2005; Milligan and Kostenis 2006) are key components of various signaling pathways that are associated with diverse biological activities, including, for example, the sensation of odor, taste, vision, and pain (Dong et al. 2001), embryonic development (Malbon 2005), various physiological processes (Hubbard and Hepler 2006), microtubule assembly (Zheng 2004), and cell division (Knust 2001). G proteins consist of an α subunit (Gα), which binds guanine nucleotides and possesses intrinsic GTPase activity, and β and γ subunits, which together form an inseparable Gβγ complex that binds to Gα. The Gα subunit regulates cellular processes by serving as a molecular switch that cycles between an inactive (GDP-bound) state and an active (GTP-bound) state. In the GDP-bound state, Gα binds to Gβγ with high affinity. An appropriate cellular signal induces a membrane-associated G protein-coupled receptor to bind to the Gα/Gβγ complex and to catalyze within Gα the replacement of GDP with GTP. Due to a dramatically decreased affinity for Gβγ, GTP-bound Gα dissociates from Gβγ and binds to effector proteins, thereby activating downstream signaling pathway components. (Some heterotrimeric G proteins may not dissociate in this way, however; see Bunemann et al. 2003.) The intrinsic GTPase activity of Gα returns it to the inactive GDP-bound state, so that it again may bind to Gβγ.
Gα subunits are related to small Ras-like GTPases, which, like Gα, function as molecular switches within various signaling pathways by cycling between GDP- and GTP-bound states. Ras-like GTPases include members of the Ras, Ran, Rab, and Rho families (Takai et al. 2001; Vetter and Wittinghofer 2001; Wennerberg et al. 2005) and of the ADP-ribosylation factor (Arf) family (D'Souza-Schorey and Chavrier 2006), which also includes Arf-like (Arl) GTPases (Burd et al. 2004). Gα subunits also are distantly related to other GTPases that are not known to regulate signaling pathways and which include, for example, dynamin, septin, and translation initiation and elongation factors (Leipe et al. 2002). Gα and Ras-like GTPases harbor two structural regions, termed switch I and II, that undergo major conformational changes when transitioning between active and inactive states. These conformational changes mediate recognition by and interaction with effector molecules and thus play key roles in communicating the state of these proteins to downstream cellular components. Unlike Ras-like GTPases, Gα also harbors a third switch region, termed switch III. For a review of the structural principles underlying G protein function, see Oldham and Hamm (2006).
Many residue positions are highly conserved within individual Gα and Ras-like GTPase subfamilies, but only a few key residues' positions (primarily those involved in guanine nucleotide binding) are conserved across all GTPases (Hall 2000). However, certain groups of subfamilies (such as the Gα and Arf family GTPases examined here) share additional conserved residue positions—suggesting that each such group shares sequence-encoded properties absent from GTPases outside of that group. Given that these GTPases are present in organisms that have diverged over a billion years ago, these conserved properties are likely to maintain important underlying functions or mechanisms. If so, then nonrandom patterns of sequence conservation and divergence among various GTPases should be, at least to some extent, covariant with the conservation and divergence of corresponding underlying mechanisms. Hence, sequence patterns that are conserved across multiple subfamilies will reflect mechanistic similarities, whereas patterns that are conserved within certain subfamilies but not others reflect mechanistic differences between those groups of subfamilies.
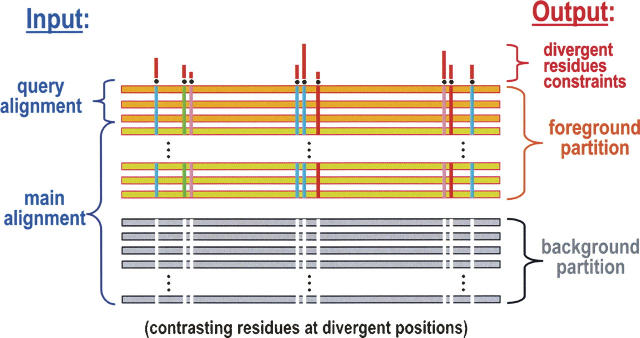
To access such implicit mechanistic information from available sequence data, we devised a Bayesian statistical procedure (Neuwald et al. 2003) that is described briefly in Figure 1. This procedure identifies and quantifies the selective pressures (or constraints) distinguishing certain proteins (termed the “foreground”) from other, functionally divergent proteins (termed the “background”). As an aid to biological interpretation, various categories of constraints determined in this way are then mapped to available crystal structures. (For a review of this approach, which is termed Contrast Hierarchical Alignment and Interaction Network [CHAIN] analysis, see Neuwald 2006.) As applied here, this approach reveals similarities and differences between the switch II-associated mechanisms of Gα and Arf family GTPases, and suggests a plausible mechanism for linking the binding of GTP to the dissociation of Gα from Gβγ.
Figure 1.
Schematic representation of the input and output for the Bayesian partitioning with pattern selection (BPPS) procedure (Neuwald et al. 2003) used here to characterize Gα functional divergence. The input consists of a “query alignment” of representative sequences of interest and a “main alignment” containing either all or a subset of the available sequences related to the query set. The main alignment typically contains hundreds or thousands of sequences, which need to be aligned very accurately, as previously described (Neuwald and Liu 2004). The output is defined statistically by the BPPS procedure, which optimally partitions the main alignment into a “foreground” and a “background,” such that the foreground (which includes the query sequences) exhibits a strikingly conserved pattern that is strikingly nonconserved within (and thus contrasts with) the background. For this reason the output is termed a Constrast Hierarchical (CH) alignment. In the figure, horizontal bars represent aligned sequences. Vertical colored bars represent foreground residues that strikingly diverge from the background at those positions; the colors reflect the different types of amino acids conserved. The histogram above the alignment represents the estimated strengths of the selective pressures imposed on divergent residue positions. For an overview of this approach, see Neuwald (2006). (Figure adapted from Neuwald 2006 with permission from Elsevier © 2006.)
Results and Discussion
CHAIN analysis of Gα
In order to characterize the selective constraints imposed on Gα subunits, CHAIN analysis was performed on various subgroups of related GTPases. The most basic analysis is shown in Figure 2A, which compares Gα and other P-loop GTPases with all other proteins. This analysis and the other analyses described here focus on GTPase alignments starting from just before the Walker A “GK[TS]” motif and ending just beyond the switch II region. The positions subject to the strongest constraints in Figure 2A correspond to residues playing key roles in guanine nucleotide binding, namely the residues of the Walker A GK[TS] motif and the glycine of a D.G motif, all of which bind to the phosphate moieties of GTP (Vetter and Wittinghofer 2001), and the aspartate of the D.G motif, which coordinates with the Mg++ ion associated with bound GTP. These residues, whose roles are well understood, serve as landmarks for the two other analyses described here.
Figure 2.

CH alignments characterizing three categories of functionally divergent constraints imposed on Gα GTPases. The proteins whose constraints are being compared are indicated above each alignment using the format “foreground vs. background.” The bars directly below each alignment correspond to the structural regions shown in Figures 3 and 4 and are color coded as follows: Walker A, purple; switch I, red; and switch II, orange. Directly below the bars, the most conserved residue patterns at each position in the foreground are shown, and directly below these the corresponding (weighted) frequencies are shown in integer tenths, where a “9,” for example, indicates that the corresponding residue occurs in 90%–100% of the sequences. Below this (in B and C) the most conserved residue patterns and their frequencies at each position in the background are shown in light gray. The relative selective pressures imposed on divergent residues are indicated by the histogram above each alignment using an approximately logarithmic scale. For the analyses in B and C, the strongest constraints in these categories all occur within the aligned region shown. (A) CH-alignment revealing the locations of key catalytic residues generally conserved within all P-loop GTPases. (B) CH-alignment showing divergent residues distinguishing the Gα and Arf families from distantly related GTPases that, like Gα and Arf, belong to the TRAFAC class (Leipe et al. 2002). The background includes the translational GTPases EF-Tu/EF-1α, IF2/eIF5B, eIF2γ/SelB, EF-G/EF-2, eRF3, LepA (Qin et al. 2006), and TypA/BipA (Owens et al. 2004) and the sulfur and iron metabolic GTPases CysN (Mougous et al. 2006) and FeoB (Cartron et al. 2006), respectively. The BPPS procedure (Neuwald et al. 2003) was used to identify and categorize these families prior to their inclusion in this analysis. (C) CH-alignment showing divergent residues distinguishing Gα from Arf family GTPases.
Figure 2B shows a comparison of the Gα and Arf families with distantly related GTPases associated with translation and metabolism. This comparison was performed for two reasons: First, preliminary analyses reveal that Gα GTPases are more closely related to—and thus share more critical features with—Arf family GTPases than is the case for other Ras-like GTPases. Second, my primary goal was to identify key structural features responsible for the roles of Gα and closely related GTPases as molecular switches within signaling pathways—roles that translational and metabolic GTPases presumably lack. The most distinguishing feature of Gα and Arf/Arl GTPases in this regard is a cluster of strikingly co-conserved residues that is structurally within or near the switch II region, and which I term the Gα/Arl–switch II component. These residues consist of a threonine within the switch I region and of residues forming the pattern [WF].G.Q…R.W…[YF], which starts within the β-strand preceding the switch II region and ends just beyond the switch II region. Important roles have been identified previously for two of these residues, which thus serve as internal controls: a switch I threonine (Thr181 in Fig. 2B) that coordinates with the GTP-bound Mg++ ion, and a glutamine within the switch II region (Gln204 in Fig. 2B) that is believed to play a key role in catalysis by stabilizing the transition state (Vetter and Wittinghofer 2001). This pattern also corresponds to a wDvGGqxxxRxxW sequence signature previously noted for Arf family GTPases (Pasqualato et al. 2002).
Other GTPases that function as molecular switches in signaling pathways—namely, members of the Ran, Ras, Rho, and Rab families and certain atypical members of the Arf family, such as SAR—conserve degenerate forms of this Gα/Arl pattern, presumably due to additional functional divergence. In order to focus on aspects of these conserved features most relevant to Gα's function, these GTPases were omitted from the alignment in Figure 2B and, instead, will be examined elsewhere.
To explore the possible role of the Gα/Arl–switch II component specifically within Gα GTPases, Figure 2C examines the functional constraints most distinguishing Gα subunits from those Arf family GTPases harboring the canonical Gα/Arl pattern. This analysis reveals four distinguishing residues: (1) an arginine (Arg178) within the switch I region, (2) a nearby isoleucine or valine (Ile184), (3) a glutamate (Glu207), and (4) a lysine (Lys210) within the switch II region. The switch I arginine (Arg178) functions as an “arginine finger” that plays a key role in catalysis by stabilizing the transition state (Vetter and Wittinghofer 2001). The roles of the other co-conserved residues in Figure 2C have, up to now, been unclear.
Structural implications
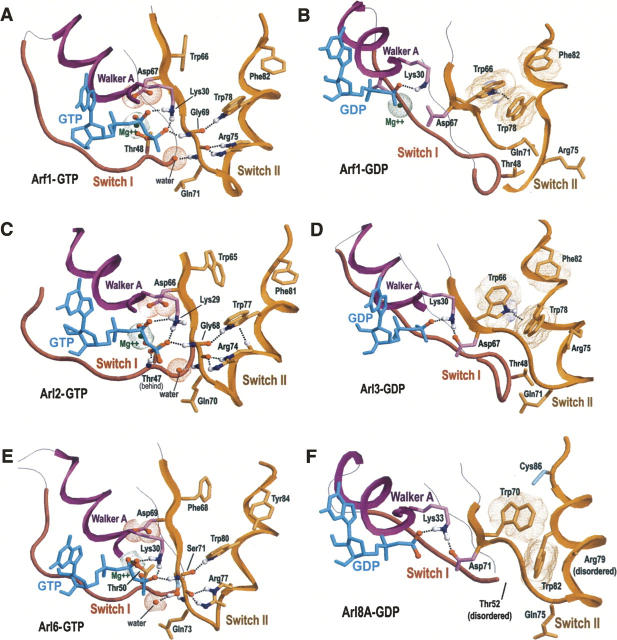
An examination of the Gα/Arl–switch II component within available crystal structures helps explain why the co-conserved residues in Figure 2B and C are subject to strong selective pressures. Figure 3 shows the structural conformations of the Gα/Arl switch II component of representative Arf and Arl GTPases bound to either GTP (Fig. 3A,C,E) or GDP (Fig. 3B,D,F). (Similar conformations typically were observed for structures of other Arf family GTPases that are not shown here.) Figure 4A and B likewise show the GTP- and GDP-bound forms, respectively, of Giα. Collectively, these figures reveal two important features: (1) In the GTP-bound state the conserved arginine and tryptophan within the switch II region form hydrogen bonds to key catalytic regions (as was previously noted by Pasqualato et al. 2002), but (2) in the GDP-bound state these hydrogen bonds are disrupted and an aromatic cluster is formed. (Aromatic–aromatic interactions were determined based on the criteria of Burley and Petsko 1985, 1986).
Figure 3.
Structural conformations of the Gα/Arl–switch II component in Arf and Arl GTPases. Color scheme for backbone traces: Walker A motif and the helix that follows it, purple; switch I region, red; switch II region and C-terminal end of the preceding β-strand, orange. Residues with magenta colored side chains distinguish all P-loop GTPases from other proteins (see Fig. 2A); residues with orange side chains distinguish the Gα and Arf families from translational and metabolic GTPases (Fig. 2B); residues with cyan side chains distinguish individual Arl subfamilies from other Arf subfamilies (corresponding CH-alignment not shown). Oxygen, nitrogen, and (predicted) hydrogen atoms that establish hydrogen bonds or ionic interactions are colored red, blue, and white, respectively. Hydrogen bonds are shown as dotted lines; ionic and aromatic–aromatic interactions are shown as dot clouds. Hydrogen atoms were added using the program REDUCE (Word et al. 1999). Figures were generated using RasMol (Sayle and Milner-White 1995). (A) Arf1 + GTP (pdb id: 1J2JA) (Shiba et al. 2003). (B) Arf1 + GDP (pdb id: 1HURA) (Amor et al. 1994). (C) Arl2 + GTP (pdb id: 1KSJA) (Hanzal-Bayer et al. 2002). (D) Arl3 + GDP (pdb id: 1FZQA) (Linari et al. 1999). (E) Arl6 + GTP (pdb id: 2H57A) (Wang et al. 2006). The canonical glycine residue of the Gα/Arl component (Gly69 in Arf1) has been replaced in Arl6 by a conserved serine (Ser71), the side chain—OH group of which appears to hydrogen bond to a buried water near the γ-phosphate of GTP. This serine thus may help position and activate this water for catalysis and presumably reflects a key (functionally divergent) feature of Arl6 GTPases. (F) Arl8A + GDP (pdb id: 2H18A) (Atanassova et al. 2006).
Figure 4.
Structural conformations of the Gα/Arl–switch II component of Gα GTPases. Residues with green side chains distinguish Gα from Arf family GTPases (see Fig. 2C). For other descriptions see the legend to Figure 3. (A) Giα + GTP analog (pdb id: 1CIPA) (Coleman and Sprang 1999). The GTP analog is designated as “GTP.” (B) Giα + GDP + PO4 3− (pdb id: 1GITA) (Berghuis et al. 1996). (C) Gia + GDP + AlF4, the transition state conformation (pdb id: 1GFIA) (Coleman et al. 1994). (D) Giα + GDP bound to the Gβγ complex (pdb id: 1GG2A) (Wall et al. 1995). Regions of the Gβ subunit are shown as grayish blue.
Active state Arg–Trp hydrogen bonds
In the GTP-bound state, the Gα/Arf–switch II tryptophan and arginine each hydrogen bond to a switch II backbone oxygen (Figs. 3A,C,E, 4A,C). The backbone oxygen that hydrogen bonds with the tryptophan (Trp211 in Fig. 2B) corresponds to the Gα/Arl–switch II glycine (Gly202 in Fig. 2B). Mutation of this glycine in Gαs inhibited the ability of GTP to activate adenylyl cyclase (Osawa and Johnson 1991), suggesting that this glycine plays a role in communicating Gα's GTP-bound state to downstream signaling factors. The backbone oxygen that hydrogen bonds with the arginine (Arg208 in Fig. 2B) corresponds to the D.G motif glycine that is characteristic of all GTPases (Gly203 in Fig. 2A). The high backbone flexibility associated with these glycines may facilitate precise 3D positioning of these hydrogen bonds, and consequently, of the associated peptide bonds. These peptide bonds form a connection to backbone –NH groups that protrude into the GTP binding site and that are well situated to form hydrogen bonds with the γ-phosphate group of GTP and with a nearby water molecule that is poised for nucleophilic attack on the γ-phosphorous atom of GTP. By contrast, the GDP-bound forms of these GTPases typically lack the Arg–Trp-associated hydrogen bonds, presumably due to the absence of a γ-phosphate group in GDP. Proteolytic analysis supports a dramatic conformational change associated with these residues upon deactivation: Both the arginine (Fung and Nash 1983; Mazzoni and Hamm 1996) and the tryptophan (Mazzoni and Hamm 1993) are protected from proteolysis in the GTP-bound state but not the GDP-bound state. Taken together, these observations suggest a key role for the arginine and tryptophan in coupling the binding of GTP to switch II conformational changes and thus to recognition of the active state by downstream effector proteins. Moreover, the arginine and tryptophan also seem likely to play key roles in positioning both the γ-phosphate of GTP and the attacking (activated) water molecule for catalysis, as suggested by the transition state structure of Giα (Coleman et al. 1994), which is shown in Figure 4C. In either case, the exceptionally strong evolutionary constraints imposed on these three residues (Gly202, Arg208, and Trp211 in Fig. 2B) suggest that they play functional roles roughly equivalent in importance to the proposed transition state stabilization roles of the switch I threonine (Thr181) and of the switch II glutamine (Gln204) (Vetter and Wittinghofer 2001).
Inactive state aromatic cluster
The canonical aromatic residues of the Gα/Arl–switch II component typically form aromatic–aromatic interactions in the GDP-bound state, but not in the GTP-bound state (Figs. 3, 4A,B). Thus, the strong selective pressures imposed on these aromatic residues may be partly due to their roles in stabilizing the inactive switch II conformation. Within Arf and Arl GTPases these aromatic interactions may also help mediate an “interswitch toggle”—a forward slippage of the β-strand preceding the switch II region that has been proposed to mediate a front to back communication from the N terminus to the guanine nucleotide binding site (Pasqualato et al. 2002).
The GTP-associated disruption of the aromatic cluster may be due, in part, to formation of the switch II hydrogen bonds established by the Arg and Trp sensors inasmuch as this event repositions the tryptophan sensor relative to the other canonical aromatic residues. However, this change also may be due to a more direct communication between bound GTP or GDP and the canonical aromatic residue located within the β-strand directly preceding the switch II region (e.g., Phe199–Giα in Figs. 2B, 4, Trp66–Arf1 in Fig. 3A,B). This aromatic residue is sequence adjacent and thus covalently attached to the aspartate of the D.G motif (Asp200–Giα; Asp67–Arf1); this aspartate coordinates with the Mg++ ion that, in turn, coordinates with the β- and γ-phosphate groups of bound GTP. Hence, upon transition of Gα to the GTP-bound state, this arrangement could propagate conformational changes via the Mg++ ion and the aspartate to the aromatic cluster, thereby helping to disrupt the cluster.
The roles of canonical residues in Gα–Gβγ dissociation
The preceding observations also shed light on the four canonical residues that distinguish Gα from Arf family GTPases (Fig. 2C), and as a result, suggest a link between GTP-binding and dissociation of Gα from Gβγ. Within the Gα sequence the switch II lysine (Lys210–Giα in Fig. 2C) is located directly between the Gα/Arl–switch II arginine and tryptophan (Arg208–Giα and Trp211–Giα). Moreover, based on the crystal structure of the Giα–Gβγ complex (Wall et al. 1995), this lysine is also the most buried residue (120 Å2) in Gα upon binding to Gβ and, in doing so, it interacts with hydrophobic and acidic residues that are highly conserved within Gβ (alignment not shown): The hydrophobic region of the Lys210–Giα side-chain interacts with conserved hydrophobic residues, whereas the basic –NH3 group of Lys210–Giα interacts with conserved acidic residues (Fig. 4D). In addition, the most buried residue in Giβ upon binding to Giα, namely Trp99–Giβ (149 Å2), forms an aromatic cluster with two of the Gα/Arl–switch II aromatic residues (Phe199–Giα and Phe215–Giα in Fig. 4D). Likewise, within the Giα–Gβγ complex, the canonical isoleucine/valine (Ile184–Giα in Figs. 2C, 4D) forms van der Waals interactions with Phe199–Giα and Phe215–Giα, with Trp99–Gβ, and with the second most buried residue in Giβ upon binding to Giα (Leu117–Giβ; 109 Å2) (not shown). The residue positions corresponding to Trp99–Giβ and Leu117–Giβ are highly conserved across Gβ subunits from diverse organisms.
Together these observations suggest the following mechanism by which binding of GTP to Gα could mediate its dissociation from the Gβγ complex. First note that the high degree of surface contact between Lys210–Giα and Giβ and between Trp99–Giβ and Giα are associated with the two proposed structural elements of the Gα/Arl–switch II component: the GTP-sensing Arg–Trp pair and the aromatic cluster, respectively. When bound to Gβ both of these elements are in their inactive, GDP-bound states. Upon binding to GTP, however, the canonical Gα lysine (Lys210–Giα in Figs. 2C, 4) seems likely to be retracted from its position of interaction with Gβ due to formation of hydrogen bonds with backbone oxygens by the arginine and tryptophan sensors, which lie on either side of this lysine in the switch II sequence. Notably, in the active GTP-bound state, this lysine forms a salt bridge with the canonical glutamate that is specifically conserved in Gα (Glu207–Giα in Fig. 2C); thus, this salt bridge may ensure proper formation of the active switch II conformation by sequestering lysine out of the way so as to avoid interfering interactions. Breakup of the Gα/Arf aromatic cluster upon binding to GTP (as discussed above) likewise would appear to disrupt the interaction of Trp99–Giβ with Giα and, together with the lysine retraction, lead to dissociation of Gα from Gβγ.
Conclusion
Even though previous studies have examined extensively the structure and function of Gα's switch regions (Oldham and Hamm 2006, and references therein), the empirically based statistical analysis described here provides a fresh perspective and direction for future research by examining two categories of selective pressures imposed on Gα subunits. Within the first category are identified seven residues within the Gα switch I and II regions that most distinguish Gα and Arf family GTPases from evolutionarily related, yet functionally distinct GTPases (Fig. 2B). Two of these residues, a threonine and a glutamine within the switch I and II regions, respectively, are well characterized and are believed to perform important catalytic roles. The five remaining residues appear to play key roles in mediating alternative switch II conformations: In the GTP-bound state a canonical Arg–Trp pair appears to form a hydrogen-bonding network (via switch II backbone oxygen atoms) with the γ phosphate of bound GTP, whereas in the GDP-bound state this network is disrupted and, instead, mutual interactions are formed between canonical aromatic residues. Within a second category are four residues, also within the Gα switch I and II regions, that most distinguish Gα GTPases from the otherwise relatively similar Arf family GTPases. The most distinguishing of these residues correspond to the well-characterized “arginine finger” and to a lysine implicated here in GTP-regulated association and dissociation of Gα and Gβγ. Hence, just as the arginine finger is critical for Gα's inherent GTPase activity, this lysine, in conjunction with these other canonical residues, appears to play an equally critical role in heterotrimeric G protein signaling.
Acknowledgments
I thank Andrey Tovtchigretchko for critical reading of the manuscript. This work was funded by National Library of Medicine Grant LM06747 and by the National Institutes of Health Division of General Medicine Grant GM078541.
Footnotes
Reprint requests to: Andrew F. Neuwald, The Institute for Genome Sciences, The University of Maryland School of Medicine, 20 Penn Street, Baltimore, MD 21201, USA; e-mail: aneuwald@som.umaryland.edu; fax: (410) 706-1482.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.073098107.
References
- Amor J.C., Harrison, D.H., Kahn, R.A., and Ringe, D. 1994. Structure of the human ADP-ribosylation factor 1 complexed with GDP. Nature 372: 704–708. [DOI] [PubMed] [Google Scholar]
- Atanassova A., Tempel, W., Dimov, S., Yaniw, D., Arrowsmith, C., Edwards, A., Sundstrom, M., Weigelt, J., Bochkarev, A., and Park, H. 2006. Structure of human Adp-ribosylation factor-like 10b (Arl10b). Structural Genomics Consortium, Toronto, Canada.
- Berghuis A.M., Lee, E., Raw, A.S., Gilman, A.G., and Sprang, S.R. 1996. Structure of the GDP–Pi complex of Gly203 → Ala giα1: A mimic of the ternary product complex of gα-catalyzed GTP hydrolysis. Structure 4: 1277–1290. [DOI] [PubMed] [Google Scholar]
- Bunemann M., Frank, M., and Lohse, M.J. 2003. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc. Natl. Acad. Sci. 100: 16077–16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C.G., Strochlic, T.I., and Gangi Setty, S.R. 2004. Arf-like GTPases: Not so Arf-like after all. Trends Cell Biol. 14: 687–694. [DOI] [PubMed] [Google Scholar]
- Burley S.K. and Petsko, G.A. 1985. Aromatic–aromatic interaction: A mechanism of protein structure stabilization. Science 229: 23–28. [DOI] [PubMed] [Google Scholar]
- Burley S.K. and Petsko, G.A. 1986. Amino–aromatic interactions in proteins. FEBS Lett. 203: 139–143. [DOI] [PubMed] [Google Scholar]
- Cartron M.L., Maddocks, S., Gillingham, P., Craven, C.J., and Andrews, S.C. 2006. Feo–transport of ferrous iron into bacteria. Biometals 19: 143–157. [DOI] [PubMed] [Google Scholar]
- Coleman D.E. and Sprang, S.R. 1999. Structure of Giα1.GppNHp, autoinhibition in a gα protein–substrate complex. J. Biol. Chem. 274: 16669–16672. [DOI] [PubMed] [Google Scholar]
- Coleman D.E., Berghuis, A.M., Lee, E., Linder, M.E., Gilman, A.G., and Sprang, S.R. 1994. Structures of active conformations of Gi α1 and the mechanism of GTP hydrolysis. Science 265: 1405–1412. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C. and Chavrier, P. 2006. ARF proteins: Roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 7: 347–358. [DOI] [PubMed] [Google Scholar]
- Dong X., Han, S., Zylka, M.J., Simon, M.I., and Anderson, D.J. 2001. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106: 619–632. [DOI] [PubMed] [Google Scholar]
- Fung B.K. and Nash, C.R. 1983. Characterization of transducin from bovine retinal rod outer segments. II. Evidence for distinct binding sites and conformational changes revealed by limited proteolysis with trypsin. J. Biol. Chem. 258: 10503–10510. [PubMed] [Google Scholar]
- Hall A. 2000. GTPases. Oxford University Press, New York.
- Hanzal-Bayer M., Renault, L., Roversi, P., Wittinghofer, A., and Hillig, R.C. 2002. The complex of Arl2–GTP and PDE delta: From structure to function. EMBO J. 21: 2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard K.B. and Hepler, J.R. 2006. Cell signalling diversity of the Gqα family of heterotrimeric G proteins. Cell. Signal. 18: 135–150. [DOI] [PubMed] [Google Scholar]
- Knust E. 2001. G protein signaling and asymmetric cell division. Cell 107: 125–128. [DOI] [PubMed] [Google Scholar]
- Leipe D.D., Wolf, Y.I., Koonin, E.V., and Aravind, L. 2002. Classification and evolution of P-loop GTPases and related ATPases. J. Mol. Biol. 317: 41–72. [DOI] [PubMed] [Google Scholar]
- Linari M., Hanzal-Bayer, M., and Becker, J. 1999. The delta subunit of rod specific cyclic GMP phosphodiesterase, PDE delta, interacts with the Arf-like protein Arl3 in a GTP specific manner. FEBS Lett. 458: 55–59. [DOI] [PubMed] [Google Scholar]
- Malbon C.C. 2005. G proteins in development. Nat. Rev. Mol. Cell Biol. 6: 689–701. [DOI] [PubMed] [Google Scholar]
- Mazzoni M.R. and Hamm, H.E. 1993. Tryptophan207 is involved in the GTP-dependent conformational switch in the β subunit of the G protein transducin: Chymotryptic digestion patterns of the GTP γS and GDP-bound forms. J. Protein Chem. 12: 215–221. [DOI] [PubMed] [Google Scholar]
- Mazzoni M.R. and Hamm, H.E. 1996. Interaction of transducin with light-activated rhodopsin protects It from proteolytic digestion by trypsin. J. Biol. Chem. 271: 30034–30040. [DOI] [PubMed] [Google Scholar]
- McCudden C.R., Hains, M.D., Kimple, R.J., Siderovski, D.P., and Willard, F.S. 2005. G-protein signaling: Back to the future. Cell. Mol. Life Sci. 62: 551–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. and Kostenis, E. 2006. Heterotrimeric G-proteins: A short history. Br. J. Pharmacol. 147: Suppl. 1: S46–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougous J.D., Lee, D.H., Hubbard, S.C., Schelle, M.W., Vocadlo, D.J., Berger, J.M., and Bertozzi, C.R. 2006. Molecular basis for G protein control of the prokaryotic ATP sulfurylase. Mol. Cell 21: 109–122. [DOI] [PubMed] [Google Scholar]
- Neuwald A.F. 2006. Bayesian shadows of molecular mechanisms cast in the light of evolution. Trends Biochem. Sci. 31: 374–382. [DOI] [PubMed] [Google Scholar]
- Neuwald A.F. and Liu, J.S. 2004. Gapped alignment of protein sequence motifs through Monte Carlo optimization of a hidden Markov model. BMC Bioinformatics 5: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwald A.F., Kannan, N., Poleksic, A., Hata, N., and Liu, J.S. 2003. Ran's C-terminal, basic patch and nucleotide exchange mechanisms in light of a canonical structure for Rab, Rho, Ras and Ran GTPases. Genome Res. 13: 673–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham W.M. and Hamm, H.E. 2006. Structural basis of function in heterotrimeric G proteins. Q. Rev. Biophys. 39: 117–166. [DOI] [PubMed] [Google Scholar]
- Osawa S. and Johnson, G.L. 1991. A dominant negative Gαs mutant is rescued by secondary mutation of the α chain amino terminus. J. Biol. Chem. 266: 4673–4676. [PubMed] [Google Scholar]
- Owens R.M., Pritchard, G., Skipp, P., Hodey, M., Connell, S.R., Nierhaus, K.H., and O'Connor, C.D. 2004. A dedicated translation factor controls the synthesis of the global regulator Fis. EMBO J. 23: 3375–3385. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pasqualato S., Renault, L., and Cherfils, J. 2002. Arf, Arl, Arp and Sar proteins: A family of GTP-binding proteins with a structural device for “front–back” communication. EMBO Rep. 3: 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Polacek, N., Vesper, O., Staub, E., Einfeldt, E., Wilson, D.N., and Nierhaus, K.H. 2006. The highly conserved LepA is a ribosomal elongation factor that back-translocates the ribosome. Cell 127: 721–733. [DOI] [PubMed] [Google Scholar]
- Sayle R.A. and Milner-White, E.J. 1995. RASMOL: Biomolecular graphics for all. Trends Biochem. Sci. 20: 374–376. [DOI] [PubMed] [Google Scholar]
- Shiba T., Kawasaki, M., Takatsu, H., Nogi, T., Matsugaki, N., Igarashi, N., Suzuki, M., Kato, R., Nakayama, K., and Wakatsuki, S. 2003. Molecular mechanism of membrane recruitment of GGA by ARF in lysosomal protein transport. Nat. Struct. Biol. 10: 386–393. [DOI] [PubMed] [Google Scholar]
- Takai Y., Sasaki, T., and Matozaki, T. 2001. Small GTP-binding proteins. Physiol. Rev. 81: 153–208. [DOI] [PubMed] [Google Scholar]
- Vetter I.R. and Wittinghofer, A. 2001. The guanine nucleotide-binding switch in three dimensions. Science 294: 1299–1304. [DOI] [PubMed] [Google Scholar]
- Wall M.A., Coleman, D.E., Lee, E., Iniguez-Lluhi, J.A., Posner, B.A., Gilman, A.G., and Sprang, S.R. 1995. The structure of the G protein heterotrimer Gi α1 β1 γ2. Cell 83: 1047–1058. [DOI] [PubMed] [Google Scholar]
- Wang J.S.Y., Tempel, W., Landry, R., Arrowsmith, C.H., Edwards, A.M., Sundstrom, M., Weigelt, J., Bochkarev, A., and Park, H. 2006. Crystal structure of human Adp-ribosylation factor-like 6. Structural Genomics Consortium, Toronto, Canada.
- Wennerberg K., Rossman, K.L., and Der, C.J. 2005. The Ras superfamily at a glance. J. Cell Sci. 118: 843–846. [DOI] [PubMed] [Google Scholar]
- Word J.M., Lovell, S.C., Richardson, J.S., and Richardson, D.C. 1999. Asparagine and glutamine: Using hydrogen atom contacts in the choice of side-chain amide orientation. J. Mol. Biol. 285: 1735–1747. [DOI] [PubMed] [Google Scholar]
- Zheng Y. 2004. G protein control of microtubule assembly. Annu. Rev. Cell Dev. Biol. 20: 867–894. [DOI] [PubMed] [Google Scholar]