Abstract
The mechanism by which the sedative and amnestic recreational drug gamma hydroxybutyric acid (GHB) acts is controversial. Some studies indicate that it acts at its unique receptor, while others demonstrate effects mediated through the GABAB receptor. We examined the effect of GHB on evoked GABAA receptor mediated mono- and polysynaptic IPSCs as well as on NMDA and AMPA mediated EPSCs in layers II/III pyramidal cells of the frontal cortex of rat brain. One millimolar (mM) GHB suppressed monosynaptic IPSCs by 20%, whereas polysynaptic IPSCs were reduced by 56%. GHB (1mM) also produced a significant suppression of NMDA-mediated EPSCs by 53% compared to 27% suppression of AMPA-mediated EPSCs. All effects of GHB on IPSCs and EPSCs were reversed by the specific GABAB antagonist CGP62349, but not by the GHB receptor antagonist NCS 382. Consistent with a presynaptic site of action, GHB reduced the frequency but not the amplitude of AMPA receptor mediated mEPSCs and had no effect on postsynaptic currents evoked by direct application of NMDA. Finally, even though GHB appeared to be acting at presynaptic GABAB receptors, GHB and the GABAB agonist baclofen appeared to have opposite potencies for depression of NMDA vs AMPA mediated EPSCs. GHB showed a preference for depressing NMDA responses while baclofen more potently suppressed AMPA responses. The suppression of NMDA more than AMPA responses by GHB at intoxicating doses may make it attractive as a recreational drug and may explain why GHB is abused and baclofen is not.
Keywords: GHB, baclofen, NMDA, AMPA, presynaptic
Gamma hydroxybutyric acid (GHB) has multiple roles as a putative neurotransmitter (Maitre, 1997), a widely used drug of abuse (Miotto et al., 2001) and a therapeutic agent in conditions such as narcolepsy and alcohol abuse (Wong et al., 2003). The specific receptors involved in all of its actions are not entirely clear although most of the behavioral and electrophysiological effects seen after high doses in experimental animals appear to be mediated by the GABAB receptor (Wong et al., 2004a; Carai et al., 2005;Xie and Smart, 1992;Jensen and Mody, 2001) with notable exceptions (Berton et al., 1999;Cammalleri et al., 2002). The bulk of the data suggest that the GABAB receptor may mediate many of GHB effects seen clinically during abuse of the drug (Kaupmann et al., 2003). A recent report suggested that GABAB receptors are actively involved in GHB induced physical dependence (Weerts et al., 2005). Given this evidence, it has been unclear why the GABAB agonist baclofen does not have the abuse potential that is seen with GHB, although recent evidence that GHB and baclofen may have differing effects on ventral tegmental neurons (Cruz et al., 2004) may help to explain this paradox.
GHB reduces both inhibitory and excitatory neurotransmission in the hippocampus (Xie and Smart, 1992;Berton et al., 1999;Cammalleri et al., 2002) and midbrain (Madden and Johnson, 1998;Brancucci et al., 2004b;Brancucci et al., 2004a), but less is known about its effects in the cortex (Jensen and Mody, 2001;Godbout et al., 1995). Its clinical effects of lethargy, sleep, disinhibited behavior and ultimately coma suggest depression of excitatory transmission. Furthermore, the prominence of anterograde amnesia in GHB abusers (Schwartz et al., 2000;Varela et al., 2004) suggests the hypothesis that GHB may potently depress cortical N-methyl-D-aspartate (NMDA) receptor mediated neurotransmission.
The experiments described below were designed to investigate the effects of GHB on excitatory and inhibitory synaptic currents in pyramidal neurons of layers II/III of rat neocortical slices. We observed that GHB reduces polysynaptic GABAA inhibition much more than monosynaptic GABAA inhibition and that the effect on polysynaptic inhibition may be due to GHB depression of both NMDA and AMPA receptor mediated excitatory transmission. Finally, even though GHB appeared to be acting at presynaptic GABAB receptors, its effects differed from the classical GABAB agonist baclofen. GHB more potently depressed NMDA receptor mediated responses whereas baclofen more potently depressed AMPA receptor mediated responses. Therefore, although GHB effects can be blocked by GABAB receptor antagonists, there remain subtle unexplained differences in the presynaptic effects of GHB and baclofen suggesting that these two ligands might indeed act at slightly different sites. Such differences may contribute to our understanding of the differing abuse potential of GHB vs. the traditional GABAB agonist, baclofen.
Experimental Procedures
Slice preparation
Frontal cortical slices were prepared from male, Sprague-Dawley rats (PD20-30, Charles River Laboratories, Raleigh NC). Animals were handled and housed according to the guidelines of the National Institutes of Health Committee on Laboratory Animal Resources. All experimental procedures or protocols were approved by Animal Care and Use Committee of Duke University and Durham VA Medical Center. The minimum number of animals needed to obtain statistically significant results was used. The rats were anesthetized with isoflurane and decapitated. The brains were quickly removed from the skulls and placed in a cold (4°C) standard artificial cerebrospinal fluid (aCSF) containing (in mM) 120 NaCl, 3.3 KCl, 1.23 NaH2PO4, 26 NaHCO3, 1.2 MgSO4, 1.8 CaCl2 and 10 D-Glucose at pH 7.3, previously saturated with 95%O2/5%CO2. Cortical slices (300μm thickness) from rats were cut on a vibratome (100PLUS, Sectioning System, Ted Pella, Inc., Redding CA) and incubated in a holding chamber containing aCSF continuously bubbled with 95% O2/5% CO2 at room temperature (20–24°C).
For recording, patch pipettes were pulled from borosilicate glass capillary tubing (1.5mm O.D., 1.05 mm I.D., World Precision Instruments, Sarasota, FL) on a Flaming-Brown horizontal microelectrode puller (Model P-97, Sutter Instrument Co, Novato, CA). The pipettes were filled with an intracellular solution containing (in mM) 130 Cs-Gluconate, 7 CsCl, 10 HEPES, 4 Mg-ATP, 0.3 Tris-GTP and 4 QX-314 (pH=7.25). Osmolarity was adjusted to 285 mOsm. The pipette resistances were in the range of 4–7 Mohm.
After one hour of incubation in the holding chamber, slices were transferred into a small submersion recording chamber and secured in place with a bent piece of platinum wire resting on the top of the slice. Unless otherwise indicated, most recordings were made at room temperature (20–24°C). Whole cell voltage-patch clamp recordings were made from the visualized pyramidal cells in the layer II/III of the frontal cortex. Individual cells were visualized using an infrared differential interference contrast (IR-DIC) Zeiss Axioskop microscope and a 40X water immersion objective. After the establishment of the whole-cell recording configuration, stable long lasting tight-seal recordings were achieved in most cases. IPSCs and EPSCs were recorded continuously using an Axopatch 200-B amplifier (Molecular Devices, Union City, CA). Output current signals were DC-coupled to a digital oscilloscope (Tektronix TDS-2014, Beaverton, OR). Series resistance was monitored throughout the recording and cells were discarded if the series resistance changed > 20%. The digitized data were also acquired and stored using Strathclyde Electrophysiology Software, Whole Cell Program (WINWCP) (Courtesy of Dr. John Dempster) with an interface (BNC-2090, National Instruments, Austin, TX) to a PC-computer. Additionally, real–time measurements of the amplitude of evoked IPSCs or EPSCs were performed and displayed simultaneously on the second PC-computer using a custom-written program developed with Labview (Version 6i, National Instrument) by Dr. Maeng-Hee Kang-Park in our laboratory.
Monosynaptic and polysynaptic GABAA IPSCs
In these experiments, the cell was held at 0mV. GABAA receptor-mediated monosynaptic IPSCs were pharmacologically isolated using aCSF containing D-(−)2-amino-5-phosphonovaleric acid (D-AP5) (50μM), and 6,7-dinitroquinoxaline-2,3-dione (DNQX) (20μM) to block excitatory glutamatergic transmission. Monosynaptic responses were evoked with an electrode placed 50–75 μm lateral to the recording pipette. Stimuli were current pulses with 100-μs duration and stimulation frequency was 0.05 Hz. Polysynaptic GABAA IPSCs were evoked from cells clamped at the holding potential of 0mV and no excitatory synaptic blockers were added to aCSF. In addition, responses were evoked with an electrode placed 150–200 μm lateral to the recording pipette. Stimuli were current pulses with 100-μs duration and stimulation frequency was 0.05 Hz.
Evoked NMDA or AMPA EPSCs
NMDA receptor mediated EPSCs were recorded from cortical cells held at −40mV in the presence of picrotoxin (75μM) and DNQX (20μM). AMPA receptor-mediated EPSCs were recorded at −70mV in the presence of picrotoxin (75μM) and APV (50μM). Current pulses with 100-μs duration were delivered to the slice at a frequency of 0.05 Hz. GHB (0.3, 1, 3 and 30 mM) or baclofen (0.1, 1, 10 and 100 μM) were bath applied to the cells. CGP62349, a specific GABAB receptor antagonist, was bath applied to reverse the effects of GHB or baclofen. To determine if the high affinity specific GHB receptor was necessary for depression of EPSCs, these experiments were repeated while the slices were continuously superfused with 1 mM NCS 382.
AMPA Miniature EPSCs
AMPA receptor-mediated mEPSCs were isolated from cortical cells held at −70mV after adding 1μM tetrodotoxin (TTX) into the aCSF to block Na+-dependent action potential. In order to facilitate spontaneous mEPSCs, the temperature in recording chamber was maintained at 34°C throughout the experiments.
NMDA Application
A pipette filled with 500μM NMDA was positioned near the dendrites of the recorded cell held at −60mV. Whole cell NMDA-induced postsynaptic currents were produced every 60 seconds by direct pressure application (2–6 psi for 20–100ms) of NMDA using a pressure injecting apparatus (Picospritzer IID, General Valve, Fairfield, New Jersey) controlled by a computer. The bathing solution contained TTX (1μM) and 2.9 mM CaCl2,. MgCl2 was accordingly lowered to 0.1 mM to reduce the Mg++ block of NMDA receptor mediated currents.
Drugs and Data Analysis
GHB (Sigma-Aldrich, St Louis, MO) was prepared fresh prior to the experiments. Bicuculline, APV, CNQX, tetrodotoxin (TTX), (2E)-5-hydroxy-5,7,8,9-tetrahydro-6H-benzo[a][7]annulen-6-ylidene ethanoic acid (NCS 382), NMDA, GHB, sodium valproate and baclofen were purchased from Sigma-Aldrich (St. Louis, MO). The GABAB receptor blocker CGP 62349 was kindly provided by Novartis (Basel, Switzerland).
Evoked IPSCs or EPSCs of cortical pyramidal cells were low-pass filtered at 2 kHz and analyzed off-line using Clampfit 9.2 (Axon Instrument Inc., Union City, CA). AMPA receptor mediated mEPSCs were detected and analyzed using Mini Analysis Programs (Synapsoft, v 6.0.3, Decatur, GA) followed by the visual inspection of recording traces to ensure the accuracy of measurements. Cumulative probability distributions were statistically compared with the Komolgorov-Smirov test. Data are expressed as Mean ± SE. For other statistical analysis, we used Student t-test or ANOVA with repeated measures and statistical significance is considered to be P<0.05.
Results
GHB Effects on mono-synaptic IPSCs
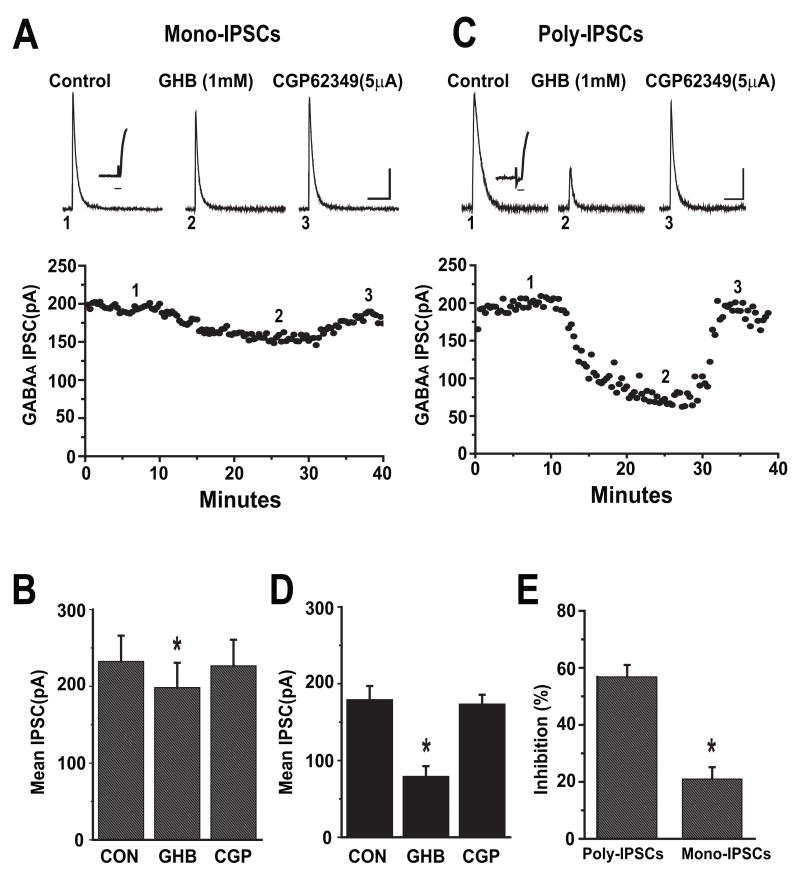
Evoked mono-synaptic IPSCs were recorded from cortical pyramidal cells held at 0mV in the presence of the excitatory amino acid antagonists APV (50μM) and DNQX (20μM). Bicuculline sensitive outward currents were continuously observed and the GHB effect was assessed. As shown in Figure 1A, bath application of GHB (1mM) for 20 minutes decreased the peak amplitude of IPSCs by 22% of control. The GHB effects were reversed after addition of the GABAB receptor antagonist CGP 62349 (5μM). The GHB effects were maximal effects 10–15 minutes after their onset. Figure 1B illustrates the average effect of 1mM GHB on monosynaptic IPSCs (n = 9 cells) where mean peak amplitudes in control, GHB and CGP 62349 were 232 ± 34pA, 198 ± 32pA and 226 ± 34pA respectively (Paired t-test, p<0.05).
Figure 1.
GHB has more potent inhibitory effects on evoked polysynaptic IPSCs than monosynaptic IPSCs in cortical pyramidal cells. (A). Upper panel, average traces of mono-synaptic IPSCs isolated pharmacologically from a prefrontal cortical pyramidal cell held at 0mV are shown. 1 mM GHB reduced the peaks of IPSCs and was reversed by bath application of 5 μM CGP 62349. Number placements correspond to the traces evoked at the time shown in the time course of the responses in the lower panel. Scale bar: 100 ms/50 pA. Inset trace illustrating the latency from stimulation artifact to the onset of the evoked IPSCs. Time scale: 6ms. Lower panel: Time course of GHB induced inhibition of IPSCs recorded from the same cell.
(B) Bar graph shows the effects of GHB on mean amplitude of monosynaptic IPSCs (n= 9). * denotes p<0.05.
(C) Upper panel, average traces (5–7) of polysynaptic IPSCs isolated from a cortical pyramidal cell held at 0mV are shown. 1 mM GHB reduced the peaks of IPSCs and was reversed by bath application of 5 μM CGP 62349. Number placements correspond to the traces evoked at the time shown in the time course of the responses in the lower panel. Scale bar: 100 ms/50 pA. Inset trace illustrating the latency from stimulation artifact to the onset of the evoked IPSCs. Time scale: 6ms. Lower panel: Time course of GHB induced inhibition of IPSCs recorded from the same cell.
(D) Bar graph showing the effects of GHB on mean amplitude of polysynaptic IPSCs (n=7). * denotes p <0.05.
(E) Bar graph showing the average percent inhibition of GHB on the mean amplitude of polysynaptic IPSCs and monosynaptic IPSCs. GHB had more potent inhibitory effects on polysynaptic IPSCs than monosynaptic IPSCs (unpaired t-test, p<0.001). * denotes p <0.001.
GHB Effects on Polysynaptic IPSCs
Polysynaptic IPSCs were recorded similarly in the absence of APV and DNQX. Again, outward postsynaptic currents were routinely isolated and blocked by bath application of 20μM bicuculline (data not shown), indicating that these synaptic currents were mediated by GABAA receptors as previously reported (Li et al., 2002). As was expected, the latency of poly-synaptic IPSCs (7.26 ± 0.35 ms) was significantly (unpaired t-test, p<0.001, n=11) greater than that for mono-synaptic IPSCs (2.082 ± 0.27ms) and furthermore, poly-synaptic IPSCs were more susceptible to GHB. Figure 1C shows representative responses of poly-synaptic IPSCs to bath application of GHB followed by CGP 62349. After establishment of a stable baseline for about 10 minutes, application of GHB (1mM) decreased IPSC amplitude in 2 minutes and reached its maximal effect after 8 minutes. As with mono-synaptic IPSCs, GHB’s inhibitory effect was completely reversed by bath application of CGP 62349 (5μM). In 9 cells, the mean peak amplitudes of the polysynaptic IPSC in control, GHB (1mM) and CGP 62349 were 179 ± 13pA, 79 ± 18pA and 173 ± 12 pA respectively (Figure 1D) (One-way ANOVA, p<0.001). The inhibition by GHB (1mM) of mono- and poly-synaptic IPSCs was 20 ± 4 % (n=9) vs. 56 ± 9 % (n=7), respectively (unpaired t-test, p<0.01) (Figure 1E).
GHB Effects on EPSCs
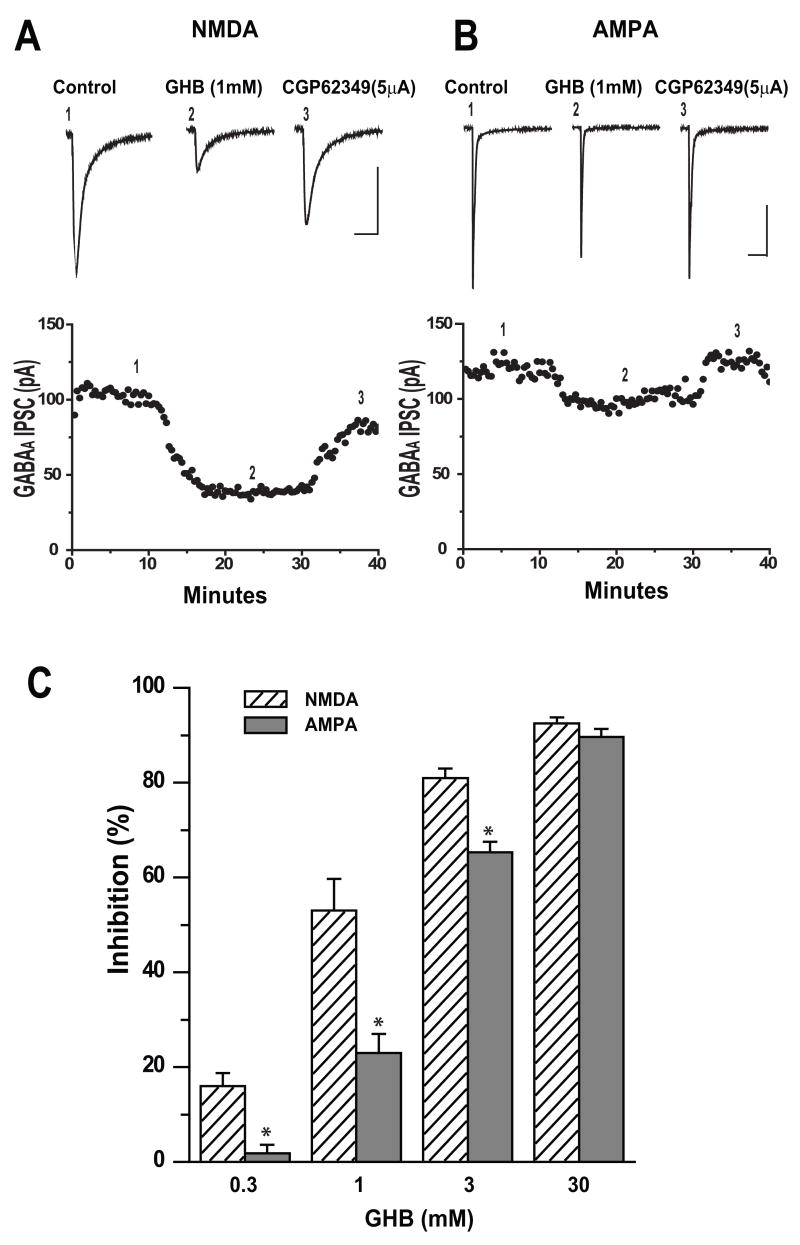
The greater sensitivity of polysynaptic IPSCs suggested that GHB was suppressing excitatory transmission as well as inhibitory in the cortical circuitry and we therefore assessed the effects of GHB on NMDA and AMPA receptor mediated EPSCs in pyramidal cells of layer II-III. Figure 2A shows a typical example of depression of the NMDA EPSC by GHB and reversal by CGP 62349 5μM. Application of GHB (1mM) resulted in a decrease in amplitude of NMDA EPSCs to 67% of control and was reversed by co-application of CGP 62349 (5μM). Figure 2B shows representative traces of the much smaller effect of GHB on evoked AMPA EPSCs and reversal by CGP62349. Depression of the EPSCs usually occurred within 7–10 minutes after bath application of GHB and was rapidly and fully reversed within 3–5 minutes post bath application CGP62349. A summary of the effects of GHB on NMDA and AMPA EPSCs in Figure 2C demonstrates the greater depression of NMDA EPSCs at different concentrations of GHB. Application of GHB 0.3, 1, 3 and 30 mM significantly reduced peak NMDA EPSC amplitude by 16.4 (n=7), 52.9 (n=7), 81.0 (n=5) and 92.5% (n=6) respectively (One-way ANOVA, p<0.05). However, application of GHB 0.3, 1, 3 and 30mM reduced peak AMPA EPSC amplitudes by only 1.8 (n=5), 23.3 (n=7), 65.3 (n=4) and 89.6% (n=5) respectively (One-way ANOVA, p<0.05,). The concentration of GHB that significantly suppressed the amplitude of NMDA EPSCS more than that of AMPA EPSCs was between 0.3 and 3mM (t-test, p<0.05). Whereas 30mM GHB depressed the amplitude of NMDA EPSCS similar to that of AMPA EPSCs (t-test p>0.05). These data indicate that GHB at intoxicating doses more potently inhibits NMDA than AMPA EPSCs (unpaired t-test, p<0.05).
Figure 2.
GHB at intoxicating doses Inhibits NMDA EPSCs more than AMPA EPSCs in cortical pyramidal cells. (A) Upper panel, average traces (5–7) of NMDA EPSCs isolated from a cell held at −40mV are shown. 1 mM GHB reduced the peaks of EPSCs and was reversed by bath application of 5μM CGP 62349. Number placements correspond to the traces evoked at the time shown in the time course of the responses in the lower panel. Scale bar: 100ms/50pA. Lower panel: Time course of GHB induced inhibition on the amplitude of EPSCs recorded from the same cell.
(B) Upper panel, average traces (5–7) of AMPA receptor-mediated EPSCs isolated from cell held at −70mV are shown. 1 mM GHB reduced the peaks of EPSCs and was reversed by bath application of 5μM CGP 62349. Number placements correspond to the traces evoked at the time shown in the time course of the responses in the lower panel. Scale bar: 100ms/50pA. Lower panel: Time course of GHB induced inhibition on the amplitude of EPSCs recorded from the same cell.
(C) Bar graph showing the GHB causes a dose-dependent inhibition of mean amplitude of NMDA EPSCs (0.3 (n=7), 1 (n=7), 3(n=5) and 30mM (n=6)) and AMPA EPSCs (0.3 (n=5); 1(n=7), 3 (n=4) and 30mM(n=5) (One-way ANOVA. p<0.05). * denotes p <0.05.
NCS 382 Did Not Prevent Depression of EPSCs by GHB
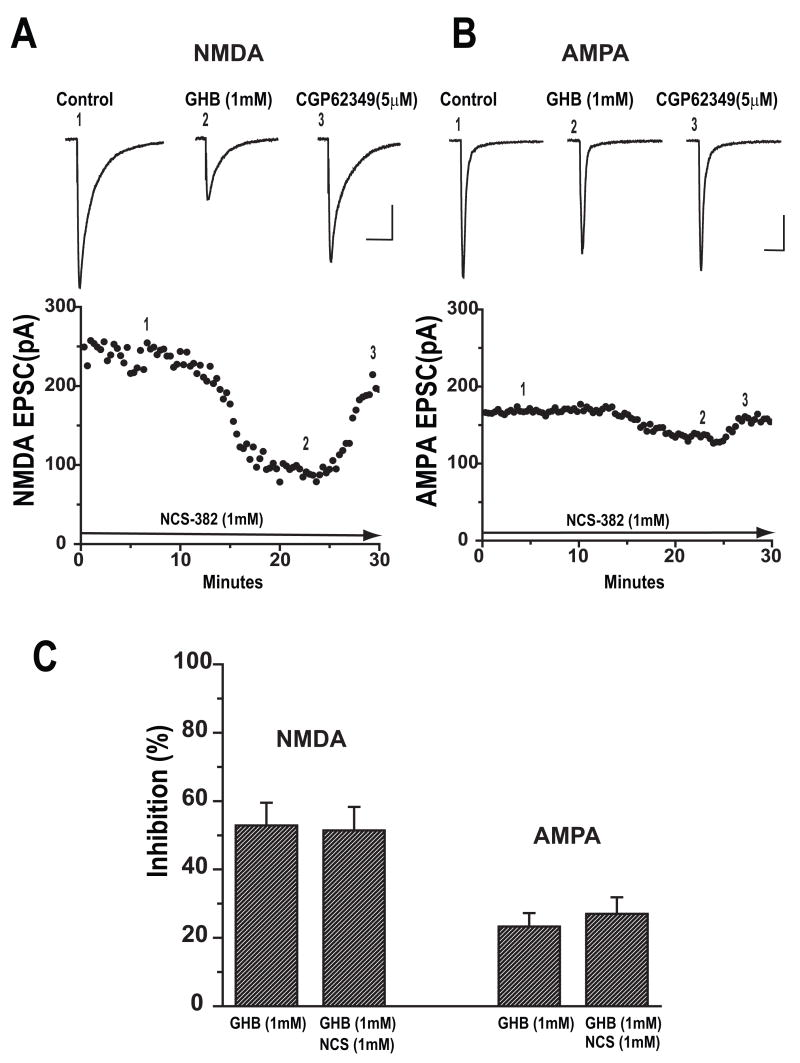
To determine whether GHB specific NCS 382 sensitive receptors contributed to depression of NMDA or AMPA EPSCs by GHB, the slices were continuously superfused with aCSF containing 1 mM NCS 382. After baseline recording, GHB (1mM) was added to aCSF for 15–20 min followed by CGP62349 (10μM). As shown in Figure 3A for NMDA EPSCs and Figure 3B for AMPA EPSCs, bath application of GHB 1mM decreased the amplitudes of EPSCs in the presence of NCS 382 (1mM). On average, GHB (1mM) reduced mean amplitudes of NMDA EPSCs by 51.4 ± 6.9% (n=5) and AMPA EPSCs by 27 ± 6.6 % (n=4) in the presence of NCS 382. There was no significant difference between EPSC suppression by GHB (1mM) in aCSF containing NCS 382 vs. normal aCSF (unpaired t-test, p=0.58; Figure 3C).
Figure 3.
GHB inhibits NMDA and AMPA EPSCs in the presence of endogenous GHB receptor antagonist NCS 382. Sample traces and time course of responses of NMDA (A) and AMPA EPSC (B) before, during bath application of 1 mM GHB and co-application of 5μM CGP 62349 are shown. Scale bar: 100ms/50pA. (C) Bar graph showing the average percent inhibition of GHB on the mean amplitude of NMDA EPSCs and AMPA EPSCs recorded in the presence and absence of GHB receptor blocker NCS 382 (unpaired t-test, p>0.05).
Baclofen Effects on EPSCs
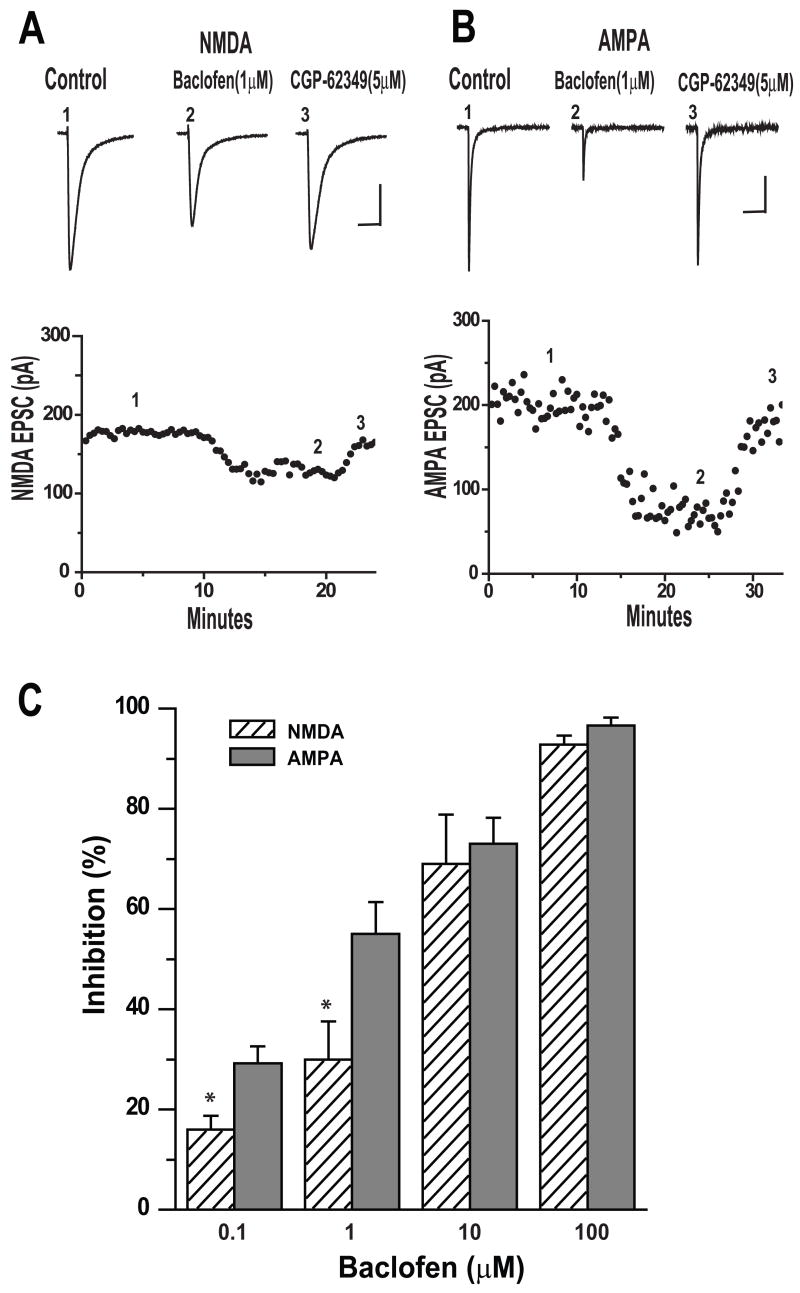
Our data indicated that GHB at intoxicating doses suppressed NMDA receptor mediated EPSCs more than AMPA EPSCs. Additionally, the clear reversal of all GHB effects by CGP62349 but not by GHB receptor antagonist NCS 382 suggested that GHB was acting solely through GABAB receptors. Therefore we applied baclofen, a well known selective GABAB receptor agonist, to determine if it had effects similar to GHB on EPSCs. Representative examples of responses of NMDA EPSCs and AMPA EPSCs to bath application of baclofen (1 μM) are shown in Figure 4A and B. In Figure 4A, after establishment of baseline (control), bath application of baclofen (1 μM) resulted in a decrease in amplitude of NMDA EPSCs by 23% of control and was reversed by CGP 62349 (5 μM). On the other hand, baclofen 1μM reduced the amplitude of AMPA EPSCs by 65% of control and was reversed by CGP 62349 (5μM) as shown in Figure 4B. For NMDA EPSCs, bath application of 0.1, 1.0, 10 and 100μM baclofen reduced peak NMDA EPSC amplitudes by 16 (n=4), 30 (n=6), 69.3 (n=6) and 92.8% (n=6) respectively; while for AMPA EPSCs, baclofen reduced peak AMPA EPSC amplitudes by 29.2 (n=5), 55.7 (n=4), 73.7 (n=7) and 96.6 %(n=5), respectively. The concentration of baclofen that significantly suppressed the amplitude of AMPA EPSCS more than that of NMDA EPSCs was between 0.1 and 1μM (t-test, p<0.05). However, when 10 and 100μM baclofen were applied, it depressed the amplitude of AMPA EPSCS similar to that of NMDA EPSCs (t-test p>0.05). These results were summarized in Figure 4C. These data indicate that the GABAB agonist baclofen, like GHB, clearly suppressed EPSCs, but unlike GHB, the baclofen in low concentrations suppressed the AMPA receptor mediated EPSCs more than the NMDA receptor mediated responses.
Figure 4.
Baclofen has more potent inhibitory effects on evoked AMPA EPSCs than NMDA EPSCs. (A). Upper panel, average traces (5–7) of NMDA receptor-mediated EPSCs isolated from cell held at −40mV are shown. 1 μM baclofen reduced the peaks of EPSCs and was reversed by bath application of 5μM CGP 62349. Number placements correspond to the traces evoked at the time shown in the time course of the responses in the lower panel. Lower panel. Scale bar: 200ms/50pA. Time course of baclofen induced inhibition of EPSCs recorded from the same cell. (B). Upper panel, average traces (5–7) of AMPA receptor-mediated EPSCs isolated from a cell held at −70mV are shown. 1 μMbaclofen reduced the peaks of EPSCs and was reversed by bath application of 5μM CGP 62349. Number placements correspond to the traces evoked at the time shown in the time course of the responses in the lower panel. Scale bar: 100ms/50pA. Lower panel: Time course of GHB induced inhibition on the amplitude of IPSCs recorded from the same cell (C) Bar graph shows the baclofen causes a dose-dependent inhibition on mean amplitude of NMDA-EPSCs (0.1 (n=4); 1 (n=6); 10 (n=6) and 100μM (n=6) and AMPA-EPSCs (0.1 (n=5); 1(n=4); 10(n=7) and 100μM(n=5)) ( One-way ANOVA, p<0.05), respectively. * denotes p <0.05.
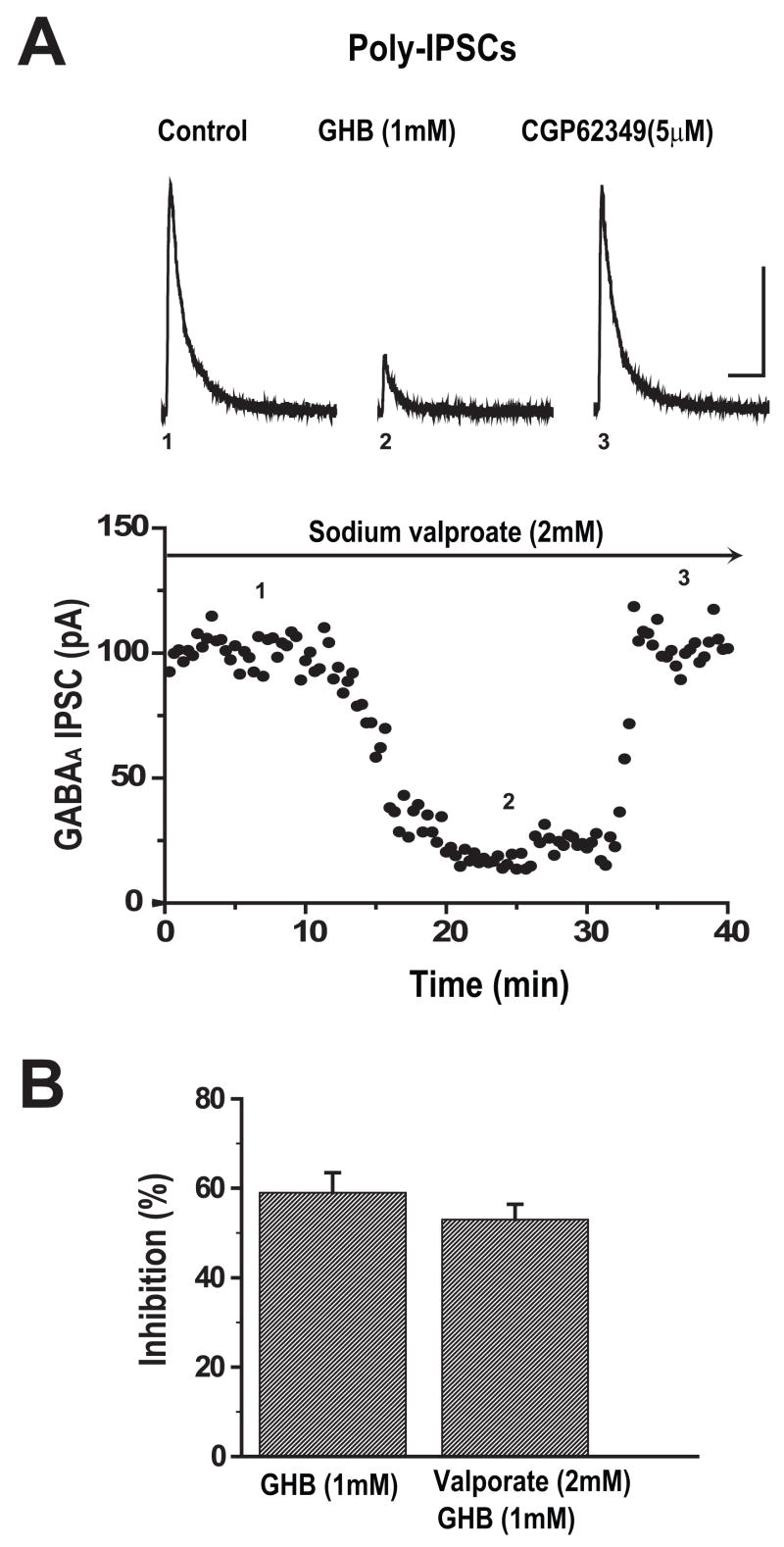
GHB Suppression of IPSCs Not Blocked by Sodium Valproate
It has been suggested that some of the effects of GHB are due to its conversion into GABA. To test this possibility in our system, we used valproate, which inhibits this conversion of GHB to GABA in synaptosomal preparations (Hechler et al., 1997). Slices were bathed in aCSF containing 2 mM sodium valproate for one hour prior to and throughout recordings and polysynaptic IPSCs were recorded from cortical cells held at 0mV. As shown in Figure 5A, after 10 minutes of stable baseline recordings, bath applied GHB (1mM) reduced the peak amplitude of IPSC by 69% of control and the effect was completely reversed by CGP 62349 (5μM). These responses were similar to those obtained under the condition whereby slices were not pre-treated with sodium valproate. Figure 5B shows that GHB reduced mean amplitude of IPSCs by 53 ± 4.5% (n=8) in the presence of sodium valproate and by 59 ± 5.6% in control (n=9) (unpaired t-test, p=0.71).
Figure 5.
Valproate does not reduce GHB mediated suppression of IPSCs in cortical pyramidal cells. (A) Sample traces and time course of responses of polysynaptic IPSCs isolated from a cortical cell held at 0mV in the presence of 2 mM sodium valproate are shown before, during bath application of 1 mM GHB and co-application of 5μM CGP 62349. Number placements in the upper panel correspond to the traces evoked at the time shown in the time course of the responses in the lower panel. Scale bar: 100ms/50pA.
(B) Bar graph showing the average percent inhibition of GHB on the mean amplitude of polysynaptic IPSCs recorded in the presence and absence of 2 mM sodium valproate (unpaired t-test, p>0.05).
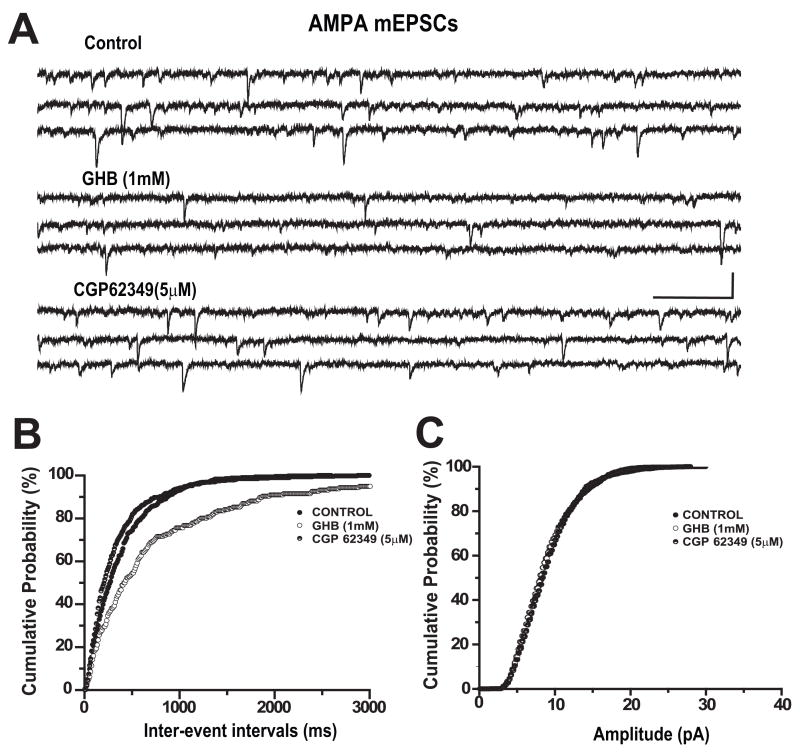
GHB Suppresses AMPA Mediated mEPSCs
We have assumed that the observed effects of GHB and baclofen were due to presynaptic mechanisms because activation of GABAB receptor linked potassium conductances should be blocked by our intracellular perfusion solution containing cesium and QX 314 (Gähwiler and Brown, 1985;Otis et al., 1993;Andrade, 1991). To obtain more direct evidence of presynaptic effects, we evaluated the effect of GHB on AMPA receptor-mediated mEPSCs recorded in TTX. mEPSCs were continuously monitored for 50 minutes at a holding potential of −70mV (Figure 6A) before (control), during bath application of GHB and after addition of CGP 62349. GHB (1mM) decreased the frequency of mEPSCs and was reversed by CGP62349 (5μM) but had no effect on their amplitude. In this example, the frequency of mEPSCs was averaged for 3 minutes after each treatment and the mean mEPSCs frequency was reduced from 2.64 Hz (control) to 1.12 Hz by GHB and recovered to 2.95 Hz after application of CGP62349. Furthermore, GHB (1mM) significantly prolonged inter-event intervals of mEPSC (K-S test, p<0.0001) but has no effect on amplitudes of mEPSCs of this cell (see Figure-6B and C). In 7 cells tested, GHB (1mM) significantly increased the inter-event intervals of mIPSCs but had no effects on amplitude (n=7, K-S test p<0.05).
Figure 6.
GHB reduced spontaneous frequency of AMPA receptor mediated mEPSCs. mEPSCs were recorded from neurons held at −70mV in the presence of 1μM TTX and at recording temperature of 35°C. (A) Bath application of 1 mM GHB reduced the frequency of AMPA receptor mediated mEPSCs and was reversed by CGP 62349. Scale bar: 200ms/20pA. Cumulative probability analysis indicated that GHB decreased frequency of mEPSCs (B, K-S test p<0.01) but had no effect on the amplitude of mEPSCs (C, K-S test, p>0.05).
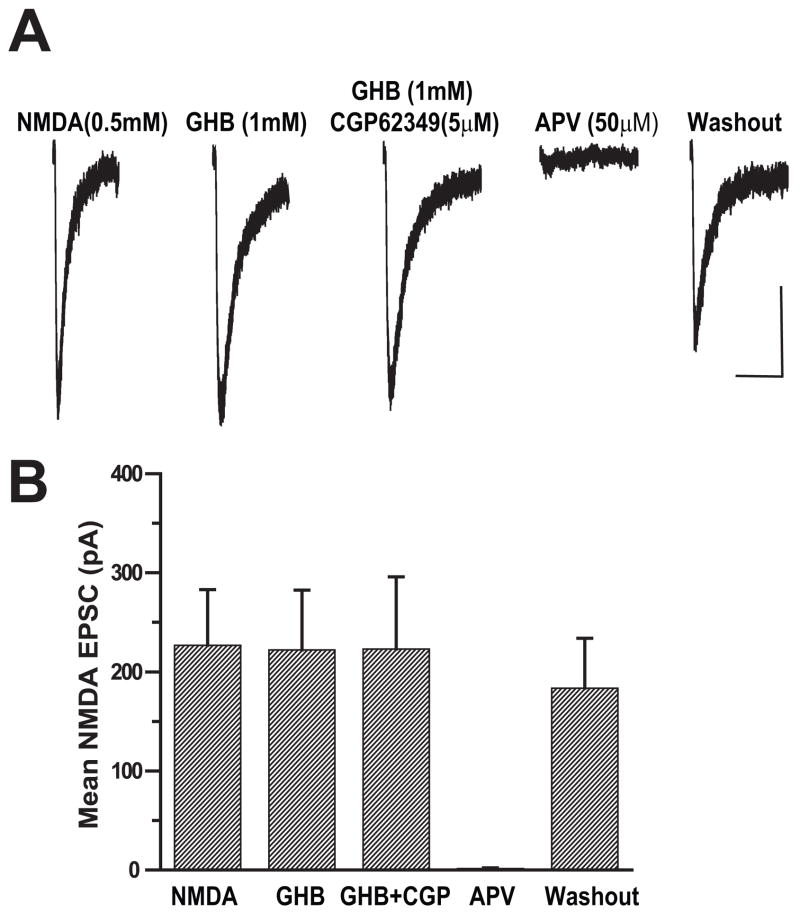
GHB Did Not Depress Responses to Applied NMDA
Attempts to record NMDA mEPSCs were unsuccessful due to the low frequency and small size of these events in our preparation. As an alternative, whole cell NMDA currents were elicited in the presence of TTX by puffing 0.5 mM NMDA directly onto the dendrites near the soma of the recorded cell. In this set of experiments, the responses were recorded from cortical cells held at −60mV in the slices superfused with aCSF containing 0.1 mM Mg++ to reduce magnesium blockade of NMDA channels at this holding potential. When aCSF was pressure-applied directly to the recorded cells no responses were observed (data not shown). Application of 0.5 mM NMDA at intervals of 60 seconds produced inward currents that were completely blocked by APV (50 μM) (Figure 7A). The amplitudes of whole cell NMDA currents recorded from this cortical cell were unchanged after bath application of 1mM GHB for 10 minutes, but were completely inhibited by APV (50 μM) and recovered following washout. The results from 6 such experiments are summarized in Figure 7B. There was no statistical difference in mean amplitude of whole cell NMDA currents before and after bath application of GHB (paired t-test, p=0.96). This indicates that the GHB suppression of NMDA EPSPs occurs at a presynaptic site.
Figure 7.
GHB has no effect on the post-synaptic NMDA currents evoked by direct pressure application of NMDA. (A) Average traces of whole cell currents elicited by puffing 0.5 mM NMDA onto the dendrites of recorded cell before, during bath application of 1 mM GHB and during 10 μM CGP 62349 are shown. APV (50μM completely blocked NMDA-evoked currents. Scale bar: 10s/50pA.
(B) Bar graph showing that GHB has no effect the mean amplitudes of APV-sensitive NMDA-induced whole cell currents (n=6, paired t-test p>0.05).
DISCUSSION
In the rat neocortical slice we found that GHB reduced monosynaptic evoked GABAA IPSCs in agreement with previously demonstrated reductions of spontaneous action potential-dependent IPSCs (Jensen and Mody, 2001) and reductions of GABA release as measured using microdialysis (Hu et al., 2000). The greater reduction of polysynaptic IPSCs is also expected if GHB is reducing synaptic excitation of interneurons and perhaps hyperpolarizing interneurons (Jensen and Mody, 2001). Thus GHB appears to act at multiple sites in the polysynaptic neocortical inhibitory circuit to suppress inhibition.
It has been suggested that inhibitory effects of GHB might be mediated in part by conversion of GHB to GABA (Hechler et al., 1997), a process that is blocked by sodium valproate. However, application of sodium valproate at a concentration expected to block this process had no detectable effect on the suppression of polysynaptic IPSCs by GHB. Therefore, if GHB is converted to GABA in our slice preparation, this process does not seem necessary for the suppression of the inhibitory circuitry by GHB. In a similar vein, Ren and Mody (2003) demonstrated inhibition of MAP kinase phosphorylation by GHB in vivo and also found that sodium valproate had no effect on this process.
In keeping with prior observations (Jensen and Mody, 2001), we also found GHB potently reduced pyramidal cell EPSCs. However, when we compared GHB effects on NMDA vs. AMPA receptor mediated EPSCs, we observed that NMDA EPSCs were depressed by GHB at relatively low concentrations (300 μM). This is a relatively low concentration compared to several reports examining synaptic effects of the drug at 500 μM to 1 mM or more (Jensen and Mody, 2001;Xie and Smart, 1992;Brancucci et al., 2004a). We could find only one report (Berton et al., 1999) comparing the effects of GHB on NMDA and AMPA mediated transmission in the hippocampus where, unlike our experiments in the neocortex, 600 μM GHB had similar depressant effects on both components.
In humans, amnesia has been reported as a prominent effect of GHB intoxication (Schwartz et al., 2000;Varela et al., 2004) and is seen at doses (Wong et al., 2004b) below those producing sleep or coma, consistent with NMDA antagonistic effects (Eckstein et al., 1999). Similarly, it has been reported that low doses of GHB impair learning and reduce [3H]MK-801 binding in frontal cortex (Sircar and Basak, 2004) of adolescent rats. It seems possible, therefore, that GHB abuse with ingestion of moderate amounts of drug could reduce the NMDA component of excitatory transmission much more than the AMPA component in cortex, thus producing amnesia as well as the dissociative effects associated with NMDA antagonists.
In contrast to GHB, baclofen more potently reduced the AMPA mediated component of the EPSC than the NMDA mediated component. These findings in neocortex contrast with reports of baclofen selectively reducing the NMDA receptor mediated component of excitatory transmission in the hippocampus (Morrisett et al., 1991) and in dopamine neurons of the midbrain (Wu et al., 1999).
At this point, we cannot offer an explanation for this differential action of baclofen vs. GHB. First, we have no evidence that either drug altered postsynaptic conductances in our preparation. The GABAB receptor activated postsynaptic potassium conductance should be blocked by cesium and QX-314 (Gähwiler and Brown, 1985;Otis et al., 1993;Andrade, 1991). Furthermore GHB did not reduce the amplitude of AMPA mEPSCs or the amplitude of currents evoked by pressure-applied NMDA. Secondly, we conclude that both drugs are acting at GABAB receptors based on reversal by CGP62349. The lack of effect of NCS 382 in the present study is also indirect evidence for action at the GABAB receptor although agonistic effects of NCS-382 (King et al 1997, Carai et al 2005, Carter et al 2003,) and GHB receptors insensitive to this antagonist have been reported (Andriamampandry et al 2003). Assuming that GHB and baclofen are both acting at presynaptic GABAB receptors, the differing effect on NMDA currents could be explained if the drugs were acting at different populations of GABAB receptors or at different populations of synapses, however, the ability of high doses of baclofen and GHB to exert similar effects on AMPA and NMDA currents is more in keeping with one population of receptors. At this point, we do not have any explanation of the differing sensitivity of AMPA vs. NMDA currents at low levels of the drugs, but we do suggest that this difference in sensitivity might have implications for the differing clinical effects of the drugs.
It has never been clear why baclofen and GHB have such differing abuse potentials, given that both seem to mediate most of their effects on behavior through GABAB receptors. A recent study of baboons fed high doses of GHB for a month or more by Goodwin et al. (2005) confirmed that a typical GHB withdrawal syndrome was triggered by administration of the GABAB antagonist CGP36742 providing more evidence that physical dependence seen during high dose GHB regimens is mediated by the GABAB receptor. A potential mechanism explaining why GHB but not baclofen is addictive was proposed by Cruz et al. (2004). They showed that GHB and baclofen resulted in disinhibition and inhibition, respectively, of ventral tegmental dopamine neurons when applied at clinically relevant concentrations. As discussed by Snead and Gibson (2005) this evidence suggests that GHB would be more likely than baclofen to activate the mesolimbic dopamine system that has been implicated in the actions of other addicting substances.
Our novel finding that GHB in intoxicating doses preferentially inhibits NMDA-mediated cortical synaptic activity more than AMPA-mediated responses is of particular interest because it raises the additional possibility that the dissociative effects of NMDA antagonism, which some recreational drug abusers find attractive, are more likely with use of GHB compared to baclofen. As baclofen tends to block AMPA responses at lower concentrations than NMDA EPSPs, achieving NMDA antagonism with baclofen would require powerful suppression of AMPA-mediated functions and that would likely result in death.
Two other commonly used recreational drugs, ketamine (Morgan et al., 2004) and ethanol (Nagy, 2004), have NMDA antagonist properties, produce dissociative effects, and also produce amnesia. If GHB inhibits NMDA-mediated more than AMPA mediated synaptic transmission in the human brain, this may explain not only its abuse potential, but also some of the difficult problems users encounter as they withdraw from this drug. We are now initiating studies to address the issues of tolerance and withdrawal from GHB.
Acknowledgments
The authors wish to acknowledge the generous support of the National Institute of Drug Abuse (DA 014931)
Abbreviations
- aCSF
artifical cerebrospinal fluid
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- EPSC
excitatory postsynaptic current
- GHB
Gamma hydroxybutyric acid
- GABA
gamma amino butyric acid
- IPSC
inhibitory post synaptic current
- NCS -382
(2E)-5-hydroxy-5,7,8,9-tetrahydro-6H-benzo[a][7]annulen-6-ylidene ethanoic acid
- NMDA
N-methyl-D-aspartate
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Andriamampandry C, Taleb O, Viry S, Muller C, Humbert JP, Gobaille S, Aunis D, Maitre M. Cloning and characterization of a rat brain receptor that binds the endogenous neuromodulator gamma-hydroxybutyrate (GHB) The FESEB. 2003;17:1691–1693. doi: 10.1096/fj.02-0846fje. [DOI] [PubMed] [Google Scholar]
- Andrade R. Blockade of neurotransmitter-activated K+ conductance by QX-314 in the rat hippocampus. Eur J Pharmacol. 1991;199:259–262. doi: 10.1016/0014-2999(91)90467-5. [DOI] [PubMed] [Google Scholar]
- Berton F, Brancucci A, Beghè F, Cammalleri M, Demuro A, Fracesconi W, Gessa G. γ-Hydroxybutyrate inhibits excitatory postsynaptic potentials in rat hippocampal slices. Eur J Pharmacol. 1999;380:109–116. doi: 10.1016/s0014-2999(99)00515-4. [DOI] [PubMed] [Google Scholar]
- Brancucci A, Berretta N, Mercuri NB, Francesconi W. Gamma-hydroxybutyrate and ethanol depress spontaneous excitatory postsynaptic currents in dopaminergic neurons of the substantia nigra. Brain Res. 2004a;997:62–66. doi: 10.1016/j.brainres.2003.10.046. [DOI] [PubMed] [Google Scholar]
- Brancucci A, Berretta N, Mercuri NB, Francesconi W. Presynaptic modulation of spontaneous inhibitory postsynaptic currents by gamma-hydroxybutyrate in the substantia nigra pars compacta. Neuropsychopharmacol. 2004b;29:537–543. doi: 10.1038/sj.npp.1300344. [DOI] [PubMed] [Google Scholar]
- Cammalleri M, Brancucci A, Berton F, Loche A, Gessa GL, Francesconi W. Gamma-hydroxybutyrate reduces GABA(A)-mediated inhibitory postsynaptic potentials in the CA1 region of hippocampus. Neuropsychopharmacol. 2002;27:960–969. doi: 10.1016/S0893-133X(02)00378-0. [DOI] [PubMed] [Google Scholar]
- Carai MA, Colombo G, Gessa GL. Resuscitative effect of a gamma-aminobutyric acid B receptor antagonist on gamma-hydroxybutyric acid mortality in mice. Ann Emerg Med. 2005;45:614–619. doi: 10.1016/j.annemergmed.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Carter LP, Flores LR, Wu H, Chen W, Unzeitig AW, Coop A, France CP. The role of GABAB receptors in the discriminative stimulus effects of gamma-hydroxybutyrate in rats: time course and antagonism studies. J Pharmacol Exp Ther. 2003;305:668–674. doi: 10.1124/jpet.102.047860. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Luscher C. Bidirectional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Eckstein M, Henderson SO, DelaCruz P, Newton E. Gamma hydroxybutyrate (GHB): report of a mass intoxication and review of the literature. Prehosp Emerg Care. 1999;3:357–361. doi: 10.1080/10903129908958969. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Brown DA. GABAB -receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. PNAS. 1985;82:1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout R, Jelenic P, Labrie C, Schmitt M, Bourguignon JJ. Effect of gamma-hydroxybutyrate and its antagonist NCS 382 on spontaneous cell firing in the prefrontal cortex of the rat. Brain Res. 1995;673:157–160. doi: 10.1016/0006-8993(94)01461-p. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Froestl W, Weerts EM. Involvement of gamma-hydroxybutyrate (GHB) and GABA-B receptors in the acute behavioral effects of GHB in baboons. Psychopharmacology. 2005;180:342–351. doi: 10.1007/s00213-005-2165-y. [DOI] [PubMed] [Google Scholar]
- Hechler V, Ratomponirina C, Maitre M. γ-hydroxybutyrate conversion into GABA induces displacement of GABAB binding that is blocked by valproate and ethosuximide. J Pharmacol Exp Ther. 1997;281:753–760. [PubMed] [Google Scholar]
- Hu RQ, Banerjee PK, Snead OCI. Regulation of γ-aminobytyric acid (GABA) release in cerebral cortex in the γ-hydroxybutyric acid (GHB) model of absence seizures in rat. Neuropharmacol. 2000;39:427–439. doi: 10.1016/s0028-3908(99)00152-5. [DOI] [PubMed] [Google Scholar]
- Jensen K, Mody I. GHB depresses fast excitatory and inhibitory synaptic transmission via GABA(B) receptors in mouse neocortical neurons. Cereb Cortex. 2001;11:424–429. doi: 10.1093/cercor/11.5.424. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Cryan JF, Wellendorph P, Mombereau C, Sansig G, Klebs K, Schmutz M, Froestl W, van der PH, Mosbacher J, Brauner-Osborne H, Waldmeier P, Bettler B. Specific gamma-hydroxybutyrate-binding sites but loss of pharmacological effects of gamma-hydroxybutyrate in GABA(B)(1)-deficient mice. Eur J Neurosci. 2003;18:2722–2730. doi: 10.1111/j.1460-9568.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- King MA, Thinschmidt JS, Walker DW. Gammahydroxybutyrate (GHB) receptor ligand effects on evoked synaptic field potentials in CA1 of the rat hippocampal slice. J Neural Transm. 1997;104:1177–1193. doi: 10.1007/BF01294719. [DOI] [PubMed] [Google Scholar]
- Li Q, Clark S, Lewis DV, Wilson WA. NMDA receptor antagonists disinhibit rat posterior cingulate and retrosplenial cortices: a potential mechanism of neurotoxicity. J Neurosci. 2002;22:3070–3080. doi: 10.1523/JNEUROSCI.22-08-03070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden TE, Johnson SW. Gamma-hydroxybutyrate is a GABAB receptor agonist that increases a potassium conductance in rat ventral tegmental dopamine neurons. J Pharmacol Exp Ther. 1998;287:261–265. [PubMed] [Google Scholar]
- Maitre M. The γ-hydroxybutyrate signalling system in brain: Organization and functional implications. Prog Neurobiol. 1997;51:337–361. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Miotto K, Darakjian J, Basch J, Murray S, Zogg J, Rawson R. Gamma-hydroxybutyric acid: patterns of use, effects and withdrawal. Am J Addict. 2001;10:232–241. doi: 10.1080/105504901750532111. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Riccelli M, Maitland CH, Curran HV. Long-term effects of ketamine: evidence for a persisting impairment of source memory in recreational users. Drug & Alcohol Dependence. 2004;75:301–308. doi: 10.1016/j.drugalcdep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Mott DD, Lewis DV, Swartzwelder HS, Wilson WA. GABAB-receptor mediated inhibition of the N-methyl-D-aspartate component of synaptic transmission in the rat hippocampus. J Neurosci. 1991;11:203–209. doi: 10.1523/JNEUROSCI.11-01-00203.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J. The NR2B subtype of NMDA receptor: a potential target for the treatment of alcohol dependence. Curr Drug Targets CNS Neurol Disord. 2004;3:169–179. doi: 10.2174/1568007043337409. [DOI] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I. Characterization of synaptically elicited GABAB responses using patch-clamp recordings in rat hippocampal slices. J Physiol. 1993;463:391–407. doi: 10.1113/jphysiol.1993.sp019600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Mody I. Gamma-hydroxybutyrate reduces mitogen-activated protein kinase phosphorylation via GABA B receptor activation in mouse frontal cortex and hippocampus. J Biol Chem. 2003;278:42006–42011. doi: 10.1074/jbc.M304238200. [DOI] [PubMed] [Google Scholar]
- Schwartz RH, Milteer R, LeBeau MA. Drug-facilitated sexual assault (‘date rape’) South Med J. 2000;93:558–561. [PubMed] [Google Scholar]
- Sircar R, Basak A. Adolescent gamma-hydroxybutyric acid exposure decreases cortical N-methyl-D-aspartate receptor and impairs spatial learning. Pharmacol Biochem Behav. 2004;79:701–708. doi: 10.1016/j.pbb.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Snead OC, III, Gibson KM. Gamma-hydroxybutyric acid. New England Journal of Medicine. 2005;352(26):2721–2732. doi: 10.1056/NEJMra044047. [DOI] [PubMed] [Google Scholar]
- Varela M, Nogue S, Oros M, Miro O. Gamma hydroxybutirate use for sexual assault. Emerg Med J. 2004;21:255–256. doi: 10.1136/emj.2002.002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Griffiths RR, Brown PR, Froestl W, Jakobs C, Gibson KM. Spontaneous and precipitated withdrawal after chronic intragastric administration of gamma-hydroxybutyrate (GHB) in baboons. Psychopharmacology. 2005;179:678–687. doi: 10.1007/s00213-004-2079-0. [DOI] [PubMed] [Google Scholar]
- Wong CG, Bottiglieri T, Snead OC., III GABA, gamma-hydroxybutyric acid, and neurological disease. Ann Neurol. 2003;54(Suppl 6):S3–12. doi: 10.1002/ana.10696. [DOI] [PubMed] [Google Scholar]
- Wong CG, Chan KF, Gibson KM, Snead OC. Gamma-hydroxybutyric acid: neurobiology and toxicology of a recreational drug. Toxicol Rev. 2004a;23:3–20. doi: 10.2165/00139709-200423010-00002. [DOI] [PubMed] [Google Scholar]
- Wong CG, Gibson KM, Snead OC., III From the street to the brain: neurobiology of the recreational drug gamma-hydroxybutyric acid. Trends Pharmacol Sci. 2004b;25:29–34. doi: 10.1016/j.tips.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wu YN, Shen KZ, Johnson SW. Presynaptic inhibition preferentially reduces in NMDA receptor-mediated component of transmission in rat midbrain dopamine neurons. Br J Pharmacol. 1999;127:1422–1430. doi: 10.1038/sj.bjp.0702680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Smart TG. γ-hydroxybutyrate depresses monosynaptic excitatory and inhibitory postsynaptic potentials in rat hippocampal slices. Eur J Pharmacol. 1992;223:193–196. doi: 10.1016/0014-2999(92)94839-n. [DOI] [PubMed] [Google Scholar]