Abstract
Understanding the regulation of cell surface expression of specific peptide–major histocompatibility complex (MHC) complexes is hindered by the lack of direct quantitative analyses of specific peptide–MHC complexes. We have developed a direct quantitative biochemical approach by engineering soluble divalent T cell receptor analogues (TCR–Ig) that have high affinity for their cognate peptide–MHC ligands. The generality of this approach was demonstrated by specific staining of peptide-pulsed cells with two different TCR–Ig complexes: one specific for the murine alloantigen 2C, and one specific for a viral peptide from human T lymphocyte virus–1 presented by human histocompatibility leukocyte antigens–A2. Further, using 2C TCR– Ig, a more detailed analysis of the interaction with cognate peptide–MHC complexes revealed several interesting findings. Soluble divalent 2C TCR–Ig detected significant changes in the level of specific antigenic–peptide MHC cell surface expression in cells treated with γ-interferon (γ-IFN). Interestingly, the effects of γ-IFN on expression of specific peptide–MHC complexes recognized by 2C TCR–Ig were distinct from its effects on total H-2 Ld expression; thus, lower doses of γ-IFN were required to increase expression of cell surface class I MHC complexes than were required for upregulation of expression of specific peptide–MHC complexes. Analysis of the binding of 2C TCR–Ig for specific peptide–MHC ligands unexpectedly revealed that the affinity of the 2C TCR–Ig for the naturally occurring alloreactive, putatively, negatively selecting, complex, dEV-8–H-2 Kbm3, is very low, weaker than 71 μM. The affinity of the 2C TCR for the other naturally occurring, negatively selecting, alloreactive complex, p2Ca–H-2 Ld, is ∼1000-fold higher. Thus, negatively selecting peptide–MHC complexes do not necessarily have intrinsically high affinity for cognate TCR. These results, uniquely revealed by this analysis, indicate the importance of using high affinity biologically relevant cognates, such as soluble divalent TCR, in furthering our understanding of immune responses.
Antigenic specificity of cellular immune responses is controlled by the recognition of a particular peptide– MHC complex by the clonotypic T cell receptor. Identification and quantitation of peptide–MHC complexes on the surface of APCs, both in vitro and in vivo, have been difficult. This difficulty is due to a variety issues: the low number of specific peptide–MHC complexes on individual APCs; the low intrinsic affinity of soluble probes for specific peptide–MHC complexes; and the nonquantitative, indirect nature of cellular readouts. Although cellular assays have greatly facilitated analysis of peptide–MHC complex expression, these assays, which involve readouts such as cytolysis, lymphokine secretion, or cell proliferation, are not exclusively dependent upon specific antigenic peptide–MHC complexes, but are also highly influenced by differences in costimulation (1, 2). The complexities associated with cellular-based assays have led investigators to develop biochemical analogues to directly analyze peptide–MHC expression.
Several approaches have been taken to study expression of specific peptide–MHC complexes using soluble ligands. A traditional soluble ligand-based approach is to generate mAbs specific for particular peptide–MHC complexes. Although theoretically this approach is appealing, practically, only a few such antibodies with high enough specificity for a particular peptide–MHC class I or class II complexes have been generated (3–7). Recently a new approach using a recombinant antibody phage display library was used to generate a relatively low affinity Fab antibody that was specific for a particular peptide–MHC complex (8). A second approach has entailed using soluble TCR analogues. A variety of different approaches have been used to develop soluble analogues of TCR (for review see reference 9). Similar to the natural peptide–MHC specificity of T cells, soluble versions of TCR have the requisite peptide specificity useful in probing specific antigenic peptide–MHC complexes. Although these reagents have been invaluable in defining the basic biochemistry involved in TCR interaction with peptide–MHC complexes (10–12), the intrinsic low affinity of these reagents limits their use in the direct identification and quantitation of peptide–MHC complexes.
The relationship between the affinity of a TCR for a specific peptide–MHC complex and the physiologic responses of T cells is poorly understood. In the 2C system, this is particularly relevant since, using CTL-based assays, several naturally occurring and synthetically derived cognate peptide–MHC ligands have been defined (13–17). However, the affinity of the 2C TCR for only one of the known naturally occurring cognate peptide–MHC ligands has been measured (12, 14). Specifically, the 2C TCR is alloreactive on two different MHC complexes (18–20), H-2 Ld and H-2 Kbm3. Both H-2 Ld and H-2 Kbm3 mediate negative selection of the 2C TCR in 2C transgenic mice (20). With regard to the alloresponse on H-2 Kbm3, a single HPLC peak containing one peptide, dEV-8, that can mediate lysis of H-2 Kbm3 target cells by 2C CTL has been defined (21, 22). This complex is the presumptive peptide– MHC complex involved in negative selection of the 2C TCR in H-2 Kbm3 mice and might be expected to be of high affinity. Soluble, high affinity probes for specific peptide–MHC complexes can be used to gain insights into the relationship between the affinity of a TCR for a specific peptide–MHC complex and the biological impact that same peptide– MHC complex has on T cells.
Soluble, high affinity probes for specific peptide–MHC complexes were developed, in part, to study the role of lymphokines such as γ-IFN on antigen expression. γ-IFN is known to have multiple sites of action that regulate expression of peptide–class I MHC complex expression (23–27). γ-IFN is known to impact on antigen presentation by increasing transcription of both class I heavy chain and TAP (transporter associated with antigen processing) genes (23). γ-IFN also regulates the proteasome by controlling expression of low molecular mass polypeptide (LMP)2, LMP7, and the proteasome regulator PA28α (25). Recent studies have shown that induction of the proteasome regulator PA28α influences both the amount and sequence of peptides generated by the proteasome (24, 28). It is not clear if the effects of γ-IFN on different parts of the antigen processing pathway are coordinated. Is the effect of γ-IFN on increased expression of class I heavy chain seen at the same dose of γ-IFN as are effects on increases in specific antigen–MHC complex expression? This is an important issue as discordant effects of γ-IFN may have implications for understanding the influence of γ-IFN immune responses. The development of a high affinity reagent could be used to analyze the impact γ-IFN on endogenous antigen–MHC complex expression.
We have developed a general system for expression of soluble high affinity TCR analogues. Using Ig as a molecular scaffold, soluble divalent TCR analogues were generated and used to analyze the interaction with cognate peptide– MHC complexes. The system utilized used the 2C TCR which is derived from alloreactive 2C CTL (13, 29) and the A6 TCR, which is specific for a viral peptide derived from the HTLV-1 tax protein (30, 31). The divalent nature of the TCR analogue significantly increased the affinity for peptide–MHC complexes while retaining the peptide specificity of the native TCR. (Since TCR–Ig was designed as a probe for peptide–MHC complexes its effective affinity/ avidity for such complexes is more relevant than the intrinsic affinity of each TCR combining site for specific peptide– MHC complexes. For this reason, we refer to the effective affinity/avidity of 2C TCR–Ig for peptide–MHC complexes simply as affinity throughout the text.) Due to this increased affinity, TCR–Ig chimeras were useful in direct flow cytometry experiments to detect specific peptide– MHC complexes. Using the 2C TCR–Ig, we have analyzed the correlation between affinity of the TCR for specific peptide–MHC complexes and lysis of target cells expressing the same peptide–MHC complexes. Furthermore, using 2C TCR–Ig, we were also able to directly analyze the modulatory effects of γ-IFN on expression of endogenous 2C TCR reactive peptide–MHC complexes.
Materials and Methods
Cells and Culture Conditions
RMA-S, RMA-S Ld, T2, T2 Kb, T2 Kbm3, T2 Kbm11, and RENCA cells were maintained by 1:10 passage three times weekly in RPMI-1640 supplemented with 2 mM glutamine, nonessential amino acids, 50 μg/ml of gentamicin, 5 × 10−5 M 2-mercaptoethanol, and 10% fetal calf serum.
Expression of Soluble Divalent TCR Analogues
The details of construction, expression, purification, and characterization of soluble divalent 2C TCR–Ig and A6 TCR–Ig were carried out as described elsewhere (O'Herrin, S.M., M.S. Lebowitz, and J.P. Schneck, manuscript in preparation). In brief, to generate the soluble divalent TCR, cDNAs encoding the TCR α and β chains were genetically linked via glycine/serine spacer to cDNAs encoding IgG1 heavy chains and kappa light chains, respectively (see Fig. 1 for protein schematic). Soluble monovalent 2C TCR was made and purified as previously described (12).
Figure 1.
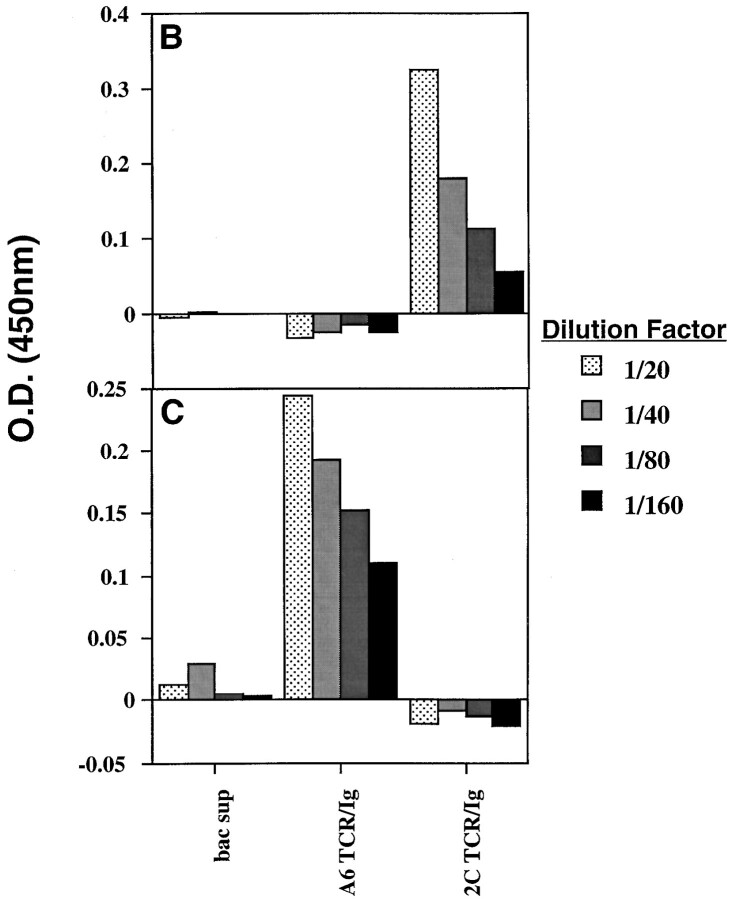
Proposed structure and biochemical characterization of soluble divalent TCR–Ig chimeras. A schematic of the chimeric protein showing the TCR-α polypeptide (heavily shaded) linked to IgG1 heavy chain and TCR-β polypeptide (lightly shaded) linked to Ig kappa light chain is shown in A. The linkers between the chimeric chains consist of glycine/serine spacers. Presumptive binding sites of mAb 1B2, an anticlonotypic 2C TCR-specific mAb, and anti–human Vβ13.1-specific mAb on the putative TCR–Ig structures are also noted. Detection of chimeras in baculovirus supernatants (Fig. 1, B and C). Plates were coated with goat anti–mouse Fc. For detection of TCR–Ig, the secondary antibody was biotinylated and consisted of either the anti 2C mAb, 1B2 (B) or the anti– human Vβ13.1 TCR-specific mAb H131.21Y (C).
Biochemical Analysis of Soluble TCR–Ig
The chimeric TCR–IgG proteins were detected by ELISA assays specific for the chimeric protein. The primary antibody used was specific for murine IgG1 Fc and a biotinylated second antibody was either mAb 1B2 (29, 32), a murine mAb specific for a clonotypic epitope expressed on 2C TCR, or the anti–human Vβ13.1, H131.21Y (see Fig. 1 for the proposed mAb binding sites). Wells were incubated with the primary antibody, 10 μg/ ml, for 1 h at room temperature, and then blocked with a 2% BSA solution before use. After three washes with PBS containing 0.05% Tween 20 and 1% FCS, culture supernatants (100 μl) from baculovirus infected cells containing the soluble divalent TCR–Ig were incubated for 1 h at room temperature. Plates were then washed extensively and then incubated with the biotinylated second antibody. When using biotinylated second antibody, wells were incubated with 100 μl 10% mouse serum for an additional hour to reduce background reactivity. After 1 h incubation with the biotinylated antibody, the plates were washed and incubated with horseradish peroxidase–conjugated strepavidin (100 μl of a 1:10,000 dilution) for 1 h, washed, and developed with 3,3′,5,5′-tetramethylbenzidine dihydrochloride (TMB) substrate for 3–5 min. The reaction was stopped by the addition of 1 M H2SO4 and optical density was measured at 450 nm.
Peptide Loading of Cells
RMA-S and T2 cell lines are defective in antigen processing and express functionally “empty” class I MHC on their cell surface (33, 34). These empty MHC molecules may be loaded with peptides as described (35, 36). In brief, cells (RMA-S, RMA-S Ld, T2, T2 Ld, T2 Kb, T2 Kbm3 or T2 Kbm11) were cultured at 27°C overnight. Subsequently, cells were incubated in the presence or absence of various antigenic peptides (100 μM final concentration) for an additional 1.5 h at 27°C and then for 1 h at 37°C.
RENCA cells were loaded with peptides by incubation with peptides (100 μM final concentration) for >2 h at 37°C. Cells were then harvested and processed for FACS® (Becton Dickinson, San Jose, CA) analysis as described.
All peptides were made by the Johns Hopkins University (Baltimore, MD) biopolymer laboratory peptide synthesis facility by F-MOC chemical synthesis and then purified by preparative HPLC. Sequences were confirmed by amino acid analysis and protein sequencing. Sequences of the peptides and the known affinities of monovalent 2C TCR for specific peptide–H-2 Ld complexes are shown in Table 1. The HLA-A2–restricted peptides used were: the HTLV-1–derived peptide tax (37), LLFGYPVYV, and the influenza-derived peptide, M1, GILGFVFTL.
Table 1.
Peptides Used in this Study: Their Reported Effectiveness in 2C CTL Assays and Affinities of 2C TCR for Peptide–MHC Complexes
| Peptide name | Peptide sequence | MHC restriction | 2C-mediated lysis | Kd | ||||
|---|---|---|---|---|---|---|---|---|
| μM | ||||||||
| p2Ca | LSPFPFDL | H-2 Ld | +++ | 0.5-0.1 | ||||
| QL9 | QLSPFPFDL | H-2 Ld | ++++ | 0.066 | ||||
| SL9 | LSPFPFDLL | H-2 Ld | +/− | 71 | ||||
| tum− | TQNHRALDL | H-2 Ld | na | na | ||||
| pMCMV | YPHFMPTNL | H-2 Ld | − | nd | ||||
| gp 70 | SPSYVYHQF | H-2 Ld | na | na | ||||
| dEV-8 | EQYKFYSV | H-2 Kb | − | Unknown | ||||
| dEV-8 | – | H-2 Kbm3 | +++ | Unknown | ||||
| SIY | SIYRYYGL | H-2 Kb | +++ | Unknown | ||||
| SIY | – | H-2 Kbm3 | Unknown | Unknown | ||||
| pVSV NP(52–59) | RGYVYQGL | H-2 Kb | − | nd |
na, not available; nd, none detected (the affinity was below the sensitivity of the assay used). Peptides listed are a collection of H-2 Ld– and H-2 Kb–binding peptides used in analysis of the reactivity of the soluble divalent 2C TCR–Ig. Lysis and affinity data are summarized from their primary references (12, 14, 15, 17, 22, 57–59).
Direct Flow Microfluorimetry
For analysis of 2C TCR–Ig specificity, ∼3 × 105 peptide-loaded or control cells were incubated for 60 min at 4°C with either ∼50 μg/ml mAb 30.5.7 culture supernatants in a 30–50 μl volume, 50 μl of 2C TCR–Ig culture supernatants (10 μg/ml final concentration), or 25–50 μg/ml purified 2C TCR–Ig in a 30 μl volume. For analysis of A6 TCR–Ig specificity, ∼3 × 105 peptide-loaded or control cells were incubated for 60 min on ice with either ∼50 μg/ml of the HLA-A2–reactive mAb BB7.2, or 50 μl of A6 TCR–Ig culture supernatants (1–10 μg/ml final concentration). Cells were then washed once in 1× PBS, 1% FBS, and 0.02% Na-azide (wash buffer), and then incubated for an additional 60 min at 4°C in 20 μl of a 1/40 dilution goat anti– mouse IgG–red algae phycoerythrin (Southern Biotechnology Assoc., Inc., Birmingham, AL). Cells were subsequently washed once with wash buffer, resuspended in 250 μl wash buffer and analyzed on a FACScan® flow cytometer.
Measurement of the Affinity of Soluble 2C TCR for H-2 Ld Molecules
Affinities of soluble 2C TCR analogues for peptide loaded cells were determined in a competition assay with FITC-30.5.7 Fab similar to one previously described (38). 30.5.7 is a mAb that recognizes an epitope near the peptide-exposed face of H-2Ld; thus 30.5.7 and 2C TCR compete for binding to the peptide exposed face of H-2Ld. Kd of 30.5.7 Fab for peptide-loaded RMA-S Ld cells were determined as follows. Cells (0.3 × 106/10 μl) were loaded with peptide as described above. Subsequently, peptide-loaded or control cells were incubated with varying concentrations of FITC-30.5.7 Fab for 1 h at 4°C, and then diluted 1:6 with wash buffer immediately before analysis by flow cytometry. Kd were estimated from a plot of 1/(mean channel fluorescence) versus 1/[FITC-30.5.7 Fab].
Affinities of 2C TCR analogues were determined by competition with constant concentrations of FITC-30.5.7 Fab. Cells were loaded with peptide, and subsequently incubated with a constant concentration of FITC-30.5.7 Fab and varying concentrations of 2C TCR analogues for 1 h at 4°C. Cells were diluted 1:6 with wash buffer immediately before analysis by flow cytometry. Maximal inhibition of FITC-30.5.7 Fab binding was determined by incubation in the presence of 30.5.7 mAb (75 μg). Kapp was determined from a plot of 1/(percent maximal inhibition) versus [2C TCR analogue]. Kapp was corrected for the affinity of FITC-30.5.7 Fab for peptide loaded cells according to the equation Kd,TCR = Kapp/(1 + ([FITC 30.5.7 Fab]/Kd,30.5.7)) (38).
CTL Experiments
Generation of CTLs.
Splenocytes from 2C TCR transgenic mice (18) were resuspended at 1.25 × 106/ml and stimulated with 1.75 × 106 BALB/c splenocytes that had been exposed to 3,000 cGy radiation. On day 7, the 2C T cell–enriched cultures were restimulated at 5 × 105/ml with 2.5 × 106/ml BALB/c splenocytes. Experiments were performed on this and subsequent stimulation on day 4. All subsequent stimulation was performed with 3.75 × 105/ml 2C splenocytes and 2.5 × 106/ml BALB/c cells in the presence of IL-2 (5 U/ml).
CTL assays.
Assays were performed in triplicate according to established CTL protocols. In brief, target cells (2–4 × 106) were incubated with 100 μCi 51Cr at 37°C for 1 h. After three washes, cells were added to V-bottomed 96-well plates (3 × 103/100 μl) and incubated (25°C for 1.5 h) with peptides at the indicated concentrations. 2C T cells (3 × 104/100 μl) were added to targets and plates were incubated at 37°C for 4.5 h. Maximum release was achieved by incubating targets with 5% Triton-X 100. Percent specific lysis was calculated from raw data using ([experimental release − spontaneous release]/[maximum release − spontaneous release]) × 100.
Results
Model Systems Used for Generating Soluble Divalent TCR.
The well-characterized 2C TCR was chosen as a model system for constructing soluble divalent TCR. 2C is an alloreactive, peptide-specific CTL clone derived from H-2b–expressing mice (29). This clone is specific for a naturally processed endogenous peptide, p2Ca, derived from α-ketoglutarate dehydrogenase bound by the murine class I molecule H-2 Ld (13). The importance of this response can be seen by the fact that p2Ca-like peptides are known to dominate certain murine alloreactive responses in strains expressing Vβ8+ TCR (39). Both higher affinity, peptide QL9, and lower affinity, peptide SL9, variants of p2Ca reactive with 2C cells also have been generated (Table 1; references 14, 15). In addition, to peptide/H-2 Ld ligands for 2C TCR, two peptides that sensitize either H-2 Kb or H-2 Kbm3 targets for lysis by 2C CTLs have also been defined (17, 22). dEV-8 is a peptide isolated from H-2 Kbm8 target cells that induces effective lysis of H-2 Kbm3 targets by 2C CTL (22). The deduced sequence of dEV-8 matches that of an endogenous peptide derived from the mitochondrial MLRQ protein. Another peptide, SIYRYYGL (SIY), was isolated from a random peptide library on the basis of its ability to mediate strong lysis of H-2 Kb target cells by 2C-CTL (17). This series of peptides facilitates the study of soluble divalent 2C TCR–Ig chimeras on a variety of different peptide–MHC ligands representing a wide range of affinities for the 2C TCR.
The TCR derived from the HTLV-specific CTL clone A6 was chosen as another model system. HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a paralytic disease associated with HTLV-1 infection (40, 41). In patients with HAM/TSP, an extremely high frequency of HTLV-1–specific T cells has been seen, up to 40–280-fold higher than in asymptomatic patients (37, 41). A dominant peptide antigen derived from the HTLV-1 tax protein, recognized by virus specific CTLs, has been defined in a subset of the patients, those who express HLA-A2 (31, 42). Although CTL responses to the dominant tax-derived peptide and variants thereof have been identified, no affinity measurements for the interaction of the A6 TCR with cognate peptide–HLA-A2 complexes have been reported (30, 43). Thus, this system also represents an interesting one for analysis using soluble high affinity divalent TCR–Ig complexes.
To generate soluble divalent TCRs, cDNAs encoding the TCR α and β chains were genetically linked to cDNAs encoding IgG1 heavy chains and kappa light chains, respectively (see Fig. 1 A for protein schematic and O'Herrin, S.M., M.S. Lebowitz, and J.P. Schneck, manuscript in preparation, for details of construction). For expression, Trichoplusia ni. cells were infected with a baculovirus expression vector encoding the soluble divalent 2C TCR–Ig constructs. Chimeric 2C TCR–Ig and A6 TCR–Ig could be easily purified from culture supernatants and retained epitopes associated with Ig portions of the molecule (O'Herrin, S.M., M.S. Lebowitz, and J.P. Schneck, manuscript in preparation).
The chimeric TCR–Ig proteins are conformationally intact (Fig. 1 B and C). The soluble divalent 2C TCR–Ig was reactive with 1B2, the anti-clonotypic mAb determinant specific for the 2C TCR (Fig. 1 B) as well as with H57, a mAb specific for a conformational epitope expressed on murine TcR Cβ chains (data not shown). Soluble divalent A6 TCR– Ig was reactive only with the Vβ13.1-specific mAb, H131.21Y (Fig. 1 C) and, as expected, not reactive with mAb 1B2 (Fig. 1 B). Control baculovirus infected supernatants were not reactive with either of the mAb (Fig. 1, B and C).
Binding Specificity of Soluble Divalent TCR Chimeras to Peptide-loaded MHC Molecules.
Based on the divalent nature of the soluble TCR–Ig complexes, we postulated that the molecules would have a high affinity for cognate peptide–MHC complexes. The high affinity of the soluble divalent TCR analogues could make them useful in analysis of peptide–MHC complexes by routine direct flow cytometry-based assays. Because of their intrinsic low affinity, soluble monovalent TCR analogues have routinely not been useful in direct flow cytometry–based assays. To study peptide specificity of TCR–Ig, we compared reactivity of 2C TCR–Ig with that of H-2 Ld reactive mAb 30.5.7, and A6 TCR–Ig with that of BB7.2, in direct flow cytometry– based assays.
The temperature-dependent reactivity of RMA-S Ld with 2C TCR–Ig was significantly different than the reactivity of RMA-S Ld with mAb 30.5.7. As expected (44, 45), RMA-S Ld cells, cells that express empty MHC molecules, expressed more serologically reactive H-2 Ld molecules recognized by mAb 30.5.7 on cells cultured at 27°C than when cells were cultured at 37°C (Fig. 2 A); mean channel fluorescence (MCF)1 increased approximately fivefold. Thus, the epitope on H-2 Ld molecules recognized by mAb 30.5.7 can be stabilized by incubating cells at low temperatures. In contrast, RMA-S Ld cells expressed very low amounts of H-2 Ld molecules recognized by 2C TCR–Ig on cells cultured at either 27 or at 37°C (Fig. 2, E). This finding is consistent with the expected peptide-dependent reactivity of 2C TCR–Ig which should not recognize MHCs that have not been pulsed with peptides even when conformationally stabilized by decreased temperature.
Figure 2.
Soluble divalent 2C TCR–Ig selectively reacts with H-2 Ld molecules loaded with peptides known to interact with 2C T cells. After overnight incubation of RMA-S Ld cells at 27°C, cells were cultured in the presence or absence of various H-2 Ld binding peptides: no peptide cells maintained at 27°C (A and E), tum− (B and F), p2Ca (C and G), and QL9 (D and H) were added to the cultures and incubated as described in Materials and Methods. Cells were then harvested and processed for flow cytometry analysis. Cells were stained with either purified mAb 30.5.7 (A–D), or 2C TCR–Ig culture supernatants (E–H) diluted to 20–40 μg/ml final concentration. In each panel, the histogram of treated cells (solid line) is contrasted with that of cells not treated with any peptide and cultured for 1 h at 37°C (broken line). Histograms shown are from one representative experiment that has been repeated at least three times.
2C TCR–Ig reactivity showed exquisite peptide specificity. Peptides (see Table 1 for sequences) were loaded into empty H-2 Ld molecules on RMA-S Ld cells. As expected, all H-2 Ld–binding peptides stabilized expression of the epitope recognized by mAb 30.5.7 (Fig. 2, B–D and Fig. 3). Only H-2 Ld molecules loaded with 2C-reactive peptides, peptides p2Ca, QL9, and SL9, expressed peptide/H-2Ld epitopes that reacted with 2C TCR–Ig (Fig. 2, F–H and Fig. 3). MCF increased ∼10–200-fold, from an MCF of 10 for either unloaded cells or cells loaded with an irrelevant H-2 Ld–binding peptide, to as high as 2,200 for RMA-S Ld cells loaded with peptide QL9 (Fig. 3). The pattern of reactivity mimicked the known affinities of monovalent 2C TCR for peptide–H-2 Ld complexes (see Table 1 for affinities). RMA-S Ld cells loaded with peptide QL9, p2Ca, or SL9 had MCF values of 2,200, 550, and 100, respectively, when stained with 2C TCR–Ig. Thus, soluble divalent 2C TCR–Ig chimeras reacted strongly with QL9–H-2 Ld complexes, modestly with p2Ca–H-2 Ld complexes, and weakly with SL9–H-2 Ld complexes. The fact that 2C TCR–Ig bound to SL9-loaded H-2 Ld molecules indicates that even in a direct flow cytometry assay, soluble divalent 2C TCR–Ig chimeras could be used to detect specific peptide–MHC complexes that have affinities as weak as 71 μM for monovalent 2C TCR.
Figure 3.
Soluble divalent 2C TCR–Ig retains the relative reactivity of 2C T cells towards specific peptide-stabilized H-2 Ld molecules. RMA-S Ld cells were incubated under various conditions as described in Fig. 2. Peptide stabilized H-2 Ld molecules were analyzed by flow cytometry as described above. To facilitate comparison of peptide-stabilized H-2 Ld molecules, data are presented as mean channel fluorescence.
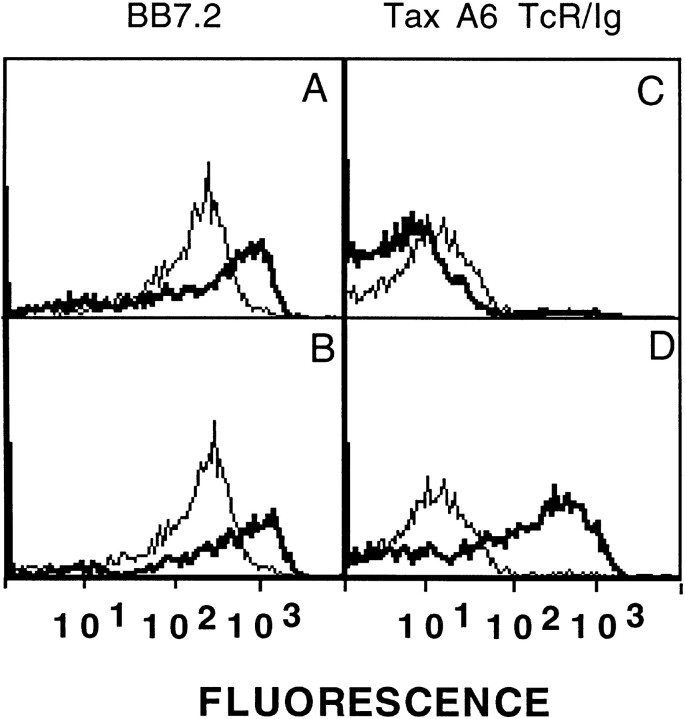
A6 TCR–Ig also demonstrated peptide specificity. This was studied by peptide-loading T2 cells, a human HLA-A2 positive cell line that expresses empty MHC molecules and can be readily loaded with specific peptides of interest (46, 47). When T2 cells were loaded with different peptides, only cells loaded the A6-reactive peptide derived from tax 11–19, LLFGYPVYV, reacted with A6 TCR–Ig (Fig. 4, C and D). MCF increased ∼20-fold, from an MCF of 21 for either unloaded cells or cells loaded with an irrelevant HLA-A2–binding peptide, M1, to 357 for T2 cells loaded with the tax peptide. As expected, all HLA-A2–binding peptides stabilized expression of the epitope recognized by mAb BB7.2 (Fig. 4, A and B).
Figure 4.
Divalent A6 TCR–IgG selectively reacts with HLA-A2 molecules loaded with tax 11–19 peptides. T2 cells (106/ml) were incubated overnight at 37°C in the presence (thick lines) or absence (thin lines) of peptides in a 96-well plate. Cells were then pelleted and coincubated on ice with 20 μl of A6 TCR–Ig protein, with a final concentration of ∼10 μg/ml, for 1 h. The peptides used in this experiment were the HLA-A2– binding peptides derived from HTLV-1 tax protein (11–19) LLFGYPVYV (B and D), and an HLA-A2–binding peptide derived from influenza M1, GILGFVFTL (A and C). Since peptides are resuspended in 50% DMSO, DMSO alone serves as a nonpeptide control. After the hour incubation with A6 TCR–Ig, cells were then washed and incubated with goat anti–mouse PE for an additional 1 h on ice, washed again, and resuspended in 250 μl of wash buffer. The experiments have been repeated at least twice. Comparable data have been obtained using either culture supernatants containing A6 TCR–Ig or purified A6 TCR–Ig. Representative histograms are shown.
The peptide specificity of these two disparate TCR–Ig chimeras indicates that the approach is a general one applicable to multiple different TCRs. Soluble divalent TCR– Ig chimeras have sufficiently high affinity to allow staining of cognate peptide–MHC ligands in direct flow cytometry–based assays.
Affinity Measurements of Soluble Divalent TCR Interaction with Peptide–MHC Complexes.
To directly analyze the impact of the divalent nature of TCR–Ig on affinity, a competitive inhibition assay was developed to measure the affinity of soluble 2C TCR analogues for peptide–MHC complexes. This assay, similar to one previously used to determine the affinity of soluble monovalent 2C TCR for peptide–MHC complexes (38), is based on mAb 30.5.7 binding to a region of the α2 helix of H-2 Ld that overlaps with TCR receptor binding (44, 45). Hence, the affinity of soluble TCR analogues can be measured in terms of their inhibition of 30.5.7 binding.
To determine the affinity of the soluble 2C TCR analogues, one has to first determine the Kd of 30.5.7 Fab fragments for peptide-loaded H-2 Ld molecules. This measurement was determined by direct saturation analysis of 30.5.7-FITC Fab binding to H-2 Ld molecules on the surface of RMA-S Ld cells. RMA-S cells were chosen since these cells express empty MHC molecules that can be readily loaded with specific peptides of interest (36, 48). The affinity of 30.5.7 for H-2 Ld molecules is dependent on the peptide loaded into H-2 Ld (Table 2). The affinity of the 30.5.7 for QL9-loaded H-2 Ld molecules is 12.2 nM, whereas the affinities for p2Ca, pMCMV (Table 2), and SL9 (data not shown) loaded H-2 Ld molecules range between 4.8–6.4 nM. These small, peptide-dependent, differences in affinity are reproducible and variations in affinity were accounted for in the competitive binding assays. These values are in good agreement with the previously measured affinities of 125I–30.5.7 Fab for the same peptide– H-2 Ld complexes (8.8–16 nM; reference 38).
Table 2.
Measured Affinities of TCR Analogues for Peptide-loaded RMA-S Ld Cells
| Peptide–MHC complex | 30.5.7 Fab | 2C TCR–Ig | 2C-sm TCR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kd | Kapp | Kd | Kapp | Kd | ||||||
| nM | nM | nM | nM | nM | ||||||
| QL9 | 12.2 | 18.3 | 13.3 | 953.4 | 613.6 | |||||
| p2Ca | 5.8 | 107.7 | 90.5 | >2000* | >2000* | |||||
| pMCMV | 4.8 | ndc‡ | ndc‡ | ND§ | ND§ | |||||
Competition was detected at the highest concentration of 2C-soluble monovalent (sm)TCR used, but an accurate measure of the Kd could not be determined.
ndc, no detectable competition with 30.5.7 Fab fragments.
not determined. Affinities of 30.5.7 Fab fragments for RMA-S Ld cells were determined by direct saturation analysis of 30.5.7 Fab binding to cells analyzed by flow cytometry. Cells were incubated with increasing amounts of FITC-labeled 30.5.7 Fab, and Kd s were estimated from a plot of 1/MCF versus 1/[30.5.7 Fab]. Affinities of 2C TCR analogues were determined by competition of the 2C TCR analogue with a constant amount of FITC-labeled 30.5.7 Fab fragments for RMA-S Ld cells as described in Materials and Methods. Kapp was calculated from a plot of 1/(percent maximal 30.5.7 Fab binding) versus [2C TCR analogue]. The Kapp was corrected for the affinity of 30.5.7 Fab for RMA-S Ld cells according to the equation Kd,TCR = Kapp/(1 + [30.5.7 Fab]/Kd,30.5.7) (38). The values reported in the table are from one representative experiment that has been repeated at least three times. Each data point used in determination of the Kd is the average of duplicate points.
2C TCR–Ig inhibited binding of 30.5.7 Fab to H-2 Ld molecules loaded with either QL9 or p2Ca peptides, but did not inhibit 30.5.7 Fab binding to pMCMV-loaded H-2 Ld molecules (Fig. 5). The affinity of soluble divalent 2C TCR–Ig for QL9 loaded molecules is 13.3 nM (Fig. 5 and Table 2). As expected, the affinity of 2C TCR–Ig for p2Ca-loaded molecules, 90 nM, is lower than that for QL9-loaded H-2 Ld. Although a small amount of competitive inhibition was seen with SL9 loaded cells, the affinity of the soluble divalent 2C TCR–Ig chimeras for SL9-loaded molecules is too low to be accurately measured under the conditions tested (data not shown).
Figure 5.
The affinity of soluble divalent 2C TCR–Ig for peptide–H-2 Ld complexes is higher than that of soluble monovalent 2C TCR. RMA S-Ld cells were loaded with peptides (QL9, □, and  ; p2Ca, ⋄; or pMCMV, ▵) and subsequently incubated with a constant amount of FITC-labeled 30.5.7 Fab and varying concentrations of either 2C TCR–Ig (solid lines) or soluble monovalent 2C TCR (sm2C TCR; dashed line). Binding of FITC–30.5.7 Fab was determined by flow cytometry. Plotted as the percent maximal (no 2C TCR analogue) 30.5.7 binding versus the concentration of 2C TCR analogue. Apparent affinities were determined from a replot of 1/(percent maximal 30.5.7 binding) versus [TCR analogue] (see text and Table 2 for further discussion). Data shown are from one representative experiment that has been repeated at least three times. Each data point is the average of duplicates.
; p2Ca, ⋄; or pMCMV, ▵) and subsequently incubated with a constant amount of FITC-labeled 30.5.7 Fab and varying concentrations of either 2C TCR–Ig (solid lines) or soluble monovalent 2C TCR (sm2C TCR; dashed line). Binding of FITC–30.5.7 Fab was determined by flow cytometry. Plotted as the percent maximal (no 2C TCR analogue) 30.5.7 binding versus the concentration of 2C TCR analogue. Apparent affinities were determined from a replot of 1/(percent maximal 30.5.7 binding) versus [TCR analogue] (see text and Table 2 for further discussion). Data shown are from one representative experiment that has been repeated at least three times. Each data point is the average of duplicates.
In all cases analyzed, the affinity of the soluble divalent 2C TCR–Ig was significantly higher than the affinity of the soluble monovalent 2C TCR for its cognate ligand (Fig. 5 and Table 2). The affinity of soluble divalent 2C TCR–Ig was 50-fold higher for QL9-loaded H-2 Ld and at least 20-fold higher for p2Ca-loaded H-2 Ld molecules than that of soluble monovalent 2C TCR for the same peptide–MHC complexes (Table 2). Thus, the divalent nature of soluble 2C TCR–Ig chimeras significantly increased the affinity of the TCR analogue for its cognate ligands.
Binding of Soluble Divalent 2C TCR–Ig to Cognate Peptide– MHC Ligands.
As discussed above, in addition to recognizing the alloreactive peptide–H-2 Ld ligands, additional cognate ligands for 2C TCR have been defined. The 2C TCR is also alloreactive on H-2 Kbm3. Both alloreactive MHC molecules, H-2 Ld and H-2 Kbm3, mediate negative selection of the 2C TCR in 2C transgenic mice. With regard to the alloresponse on H-2 Kbm3, a single peptide– MHC complex, dEV-8/H-2 Kbm3, has been defined (see Table 1 for sequences) and is known to be a target for 2C CTLs and the presumptive negatively selecting peptide– MHC complex. In addition to alloreactive complexes for 2C CTL, an artificially derived peptide-dependent syngeneic target has also been defined, SIY/H-2 Kb (see Table 1 for sequences). To analyze the ability of 2C TCR–Ig to bind to these alternate 2C-reactive complexes, the binding of 2C TCR–Ig to peptide-loaded transfected T2 cells was studied. Since T2 cells are derived from a human cell line, T2 cells do not naturally express H-2 Kb as do RMA-S cells. Thus, to study the binding of 2C TCR–Ig to peptide-loaded H-2 Kb or various H-2 Kbm mutant molecules, the T2 system was chosen since it is not complicated by the expression of MHC molecules from the parental cell line. Similar to RMA-S Ld cells, T2 cells also express empty MHC molecules that can be readily loaded with different peptides. For these studies, T2 cells transfected with: H-2 Kb, T2 Kb; H-2 Kbm3, T2 Kbm3; and H-2 Kbm11, T2 Kbm11 (21, 22) were loaded with a variety of different MHC-binding peptides.
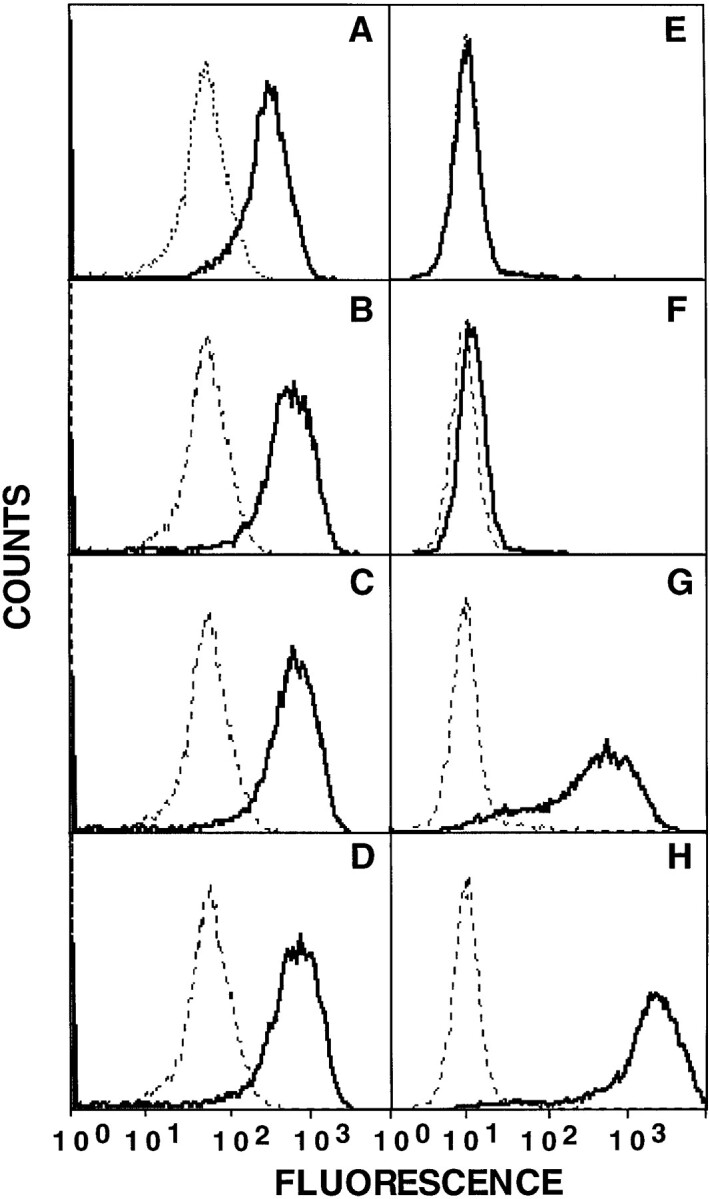
Peptide SIY-loaded T2 Kb, T2 Kbm3, or T2 Kbm11 cells all expressed epitopes recognized by 2C TCR–Ig (Fig. 6, A–C). MCF of cells incubated with 2C TCR–Ig increased ∼20-fold, from 14 for pVSV (H-2 Kb binding peptide isolated from vesicular stomatitis virus NP residues) -loaded to 276 for SIY-loaded T2 Kb and from 16 for pVSV-loaded to 250 for SIY-loaded T2 Kbm11. SIY-loaded T2 Kbm3 cells showed a much weaker but still significant interaction with 2C TCR–Ig (Fig. 6 B); compare SIY-loaded (solid lines; MCF of 36) to pVSV-loaded (dotted lines; MCF of 12) T2 Kbm3 cells. The 2C TCR–Ig-binding data to SIY–MHC complexes was consistent with 2C CTL-mediated lysis on various SIY–MHC targets (Fig. 7). 2C CTL-mediated efficient lysis of SIY-loaded T2 Kb and T2 Kbm11 cells (Fig. 7 B and data not shown, 50% lethal dose (LD50) of ∼10 ng/ml for SIY T2 Kb). 2C CTL-mediated lysis of SIY-loaded T2 Kbm3 cells was significantly less efficient (Fig. 7 C, LD50 ∼100 ng/ml).
Figure 6.

Soluble divalent 2C TCR–Ig detects SIY–MHC complexes, but not dEV-8–MHC complexes. T2 cells transfected with either H-2 Kb, H-2 Kbm3, or H-2 Kbm11 were incubated overnight at 27°C and loaded with peptides dEV-8 (broken line), SIY (solid line), or pVSV (dotted line) as described in Materials and Methods. Cells were stained with purified 2C TCR– Ig (∼50 μg/ml) and GAM-IgG-RPE as described in Materials and Methods, and analyzed by FACS®. Resultant histograms are shown; (A), T2-Kb cells, (B) T2-Kbm3, (C), T2-Kbm11. In the histograms presented 2C TCR–Ig reactivity with either dEV-8 (broken line) or pVSV (dotted line) was virtually identical leading to difficulty in discriminating between these two histograms.
Figure 7.
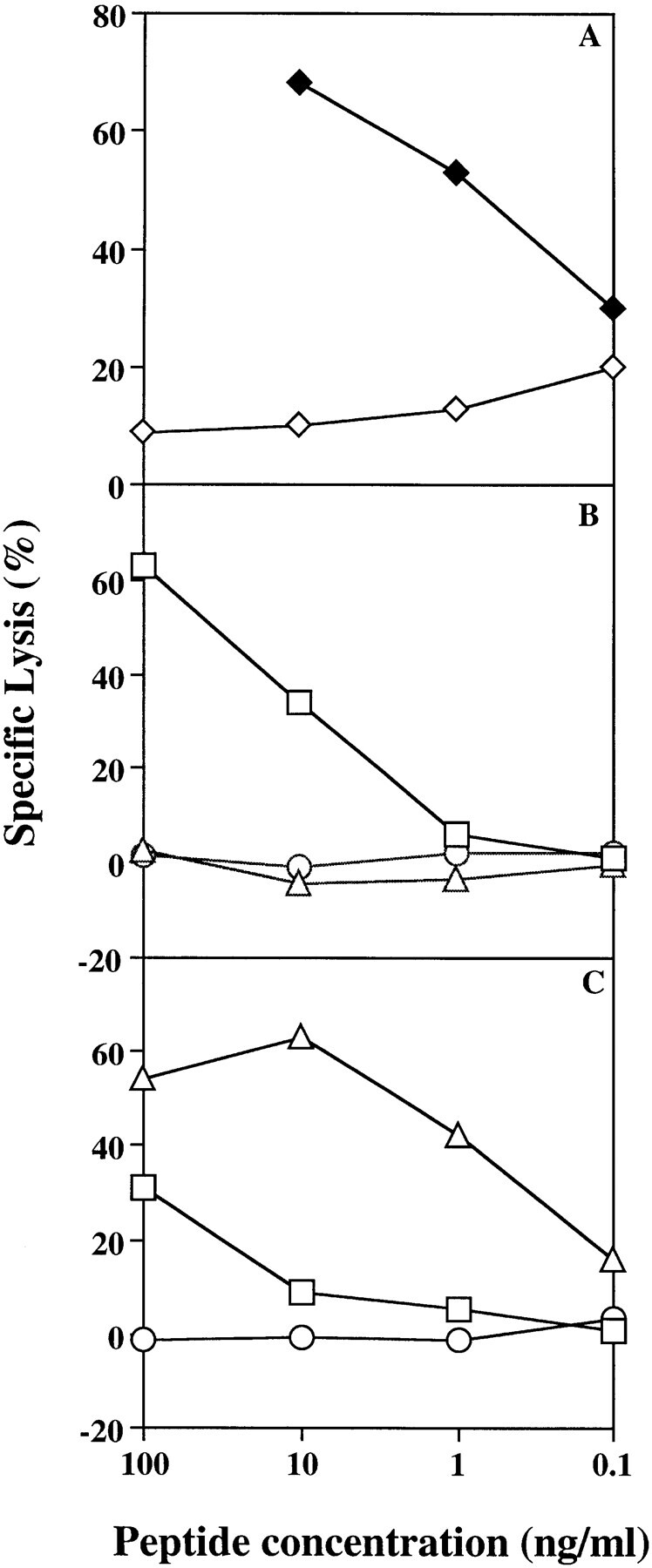
2C CTL-mediated lysis on various peptide–MHC targets. T2 cells transfected with either H-2 Ld (A), H-2 Kb (B), or H-2 Kbm3 (C) were chromium labeled as described and then loaded with peptides by incubating at 25°C for 1.5 h in the presence of variable amounts of peptides: p2Ca (♦) and pMCMV (⋄) (A); and dEV-8 (▵), SIY (□), or pVSV (○) (B and C). Peptide-loaded target cells were then incubated at an effector to target ratio of 10:1 and specific lysis calculated as described in Materials and Methods. Data shown are representative of three separate experiments.
The binding of 2C TCR–Ig to dEV-8–loaded cells revealed a striking difference between the affinity of 2C TCR–Ig for dEV-8–MHC complexes and the ability of that same peptide–MHC complex to mediate lysis by 2C CTLs. As expected, dEV-8–loaded T2 Kb cells were neither lysed by 2C CTL (Fig. 7 B), nor were they recognized by 2C TCR–Ig in flow cytometry assays (Fig. 6 A). Unexpectedly, no significant binding of 2C TCR–Ig could be found to dEV-8–loaded T2 Kbm3 cells (Fig. 6 B). MCF of cells stained with 2C TCR–Ig was similar whether cells were loaded with dEV-8 or control peptide pVSV (Fig. 6 B; compare dotted lines to dashed lines). This is most surprising in that, consistent with previous reports (22), dEV-8– loaded T2 Kbm3 cells were efficiently lysed by 2C CTL (Fig. 7 C). In fact, dEV-8–loaded T2 Kbm3 cells were much better target cells (LD50 of ∼0.5–1.0 ng/ml) than SIY-loaded T2 Kbm3 cells (LD50 of ∼100 ng/ml), where a significant binding of 2C TCR–Ig was seen (Fig. 6 B). The efficiency of lysis by 2C CTL of dEV-8–loaded T2 Kbm3 cells was on the same order of magnitude as that of p2Ca-loaded T2 Ld cells (Fig. 7 A, LD50 of ∼0.5 ng/ml) which was also efficiently recognized in the 2C TCR–Ig-binding assay (Fig. 2 G). These findings were not due to poor binding of dEV-8 to H-2 Kbm3 molecules since peptide-loading experiments showed that H-2 Kbm3 molecules were stabilized equally well by both SIY and dEV-8 peptides (data not shown). A similar, although less dramatic, lack of correlation between cytolysis and 2C TCR–Ig binding was seen for dEV-8–loaded T2 Kbm11 cells. dEV-8–loaded T2 Kbm11 cells are relatively poor targets for 2C CTL (reference 22 and data not shown), but were also not reactive with 2C TCR–Ig in flow cytometry assays (Fig. 6 C). Thus, a significant discrepancy exists between the affinity of the 2C TCR for a naturally occurring alloreactive peptide–MHC complex and the ability of that same complex to mediate lysis of target cells exists.
Analysis of the Effects of γ-IFN on Expression of Endogenous 2C-specific Peptide–MHC Complexes.
The specificity and affinity of 2C TCR–Ig for peptide–MHC complexes suggested that one might be able to use this reagent to probe the influence of lymphokines on endogenous, cell surface, peptide–MHC complexes. To analyze this possibility and follow the expression of endogenous 2C-reactive peptide– H-2 Ld complexes within a heterogeneous peptide–MHC environment, the influence of γ-IFN on the H-2 Ld–expressing murine cell line RENCA was studied. RENCA cells were cultured in the presence of variable amounts of γ-IFN to induce upregulation of naturally loaded peptide–MHC complexes. 2C TCR–Ig binding to RENCA cells increased as a function of γ-IFN induction (Fig. 8, A–D, solid lines). The effect of γ-IFN was dose dependent with a maximal two- to threefold increase seen on cells treated with 10 U/ml of γ-IFN. Since γ-IFN is known to have a direct effect on class I expression (Fig. 8, E–H and reference 23), it is necessary to normalize for any nonspecific 2C TCR–Ig binding secondary to increased expression of H-2 Ld. This was accomplished by incubating RENCA cells with a control irrelevant H-2 Ld–binding peptide from pp89 of murine cytomegalovirus, pMCMV. Since p2Ca is known to have a weak affinity for H-2 Ld (14, 15) exchange with a higher affinity H-2 Ld–binding peptide like pMCMV (14) should be very efficient. Therefore, background reactivity of 2C TCR–Ig could be determined by the efficient displacement of endogenous p2Ca or p2Ca-like peptides by incubating the cells with saturating amounts of the control pMCMV peptide. In all cases, 2C TCR–Ig binding could be blocked by earlier incubation of cells with the control H-2 Ld–binding pMCMV (Fig. 8, A–D, dotted lines). Earlier incubation of RENCA cells with a 2C-specific peptide, QL9, induced a dramatic increase in 2C TCR–Ig binding (data not shown). The results of these experiments indicate that 2C TCR–Ig could be used to analyze the impact of cytokines on cell surface expression of endogenous 2C-reactive peptide–MHC complexes.
Figure 8.
Modulation of endogenous 2C-specific peptide–H-2 Ld complexes on the surface of RENCA cells by γ-IFN. RENCA cells were cultured for 48 h with 0 (A and E), 5 (B and F), 10 (C and G), or 50 (D and H), U/ml γ-IFN. As described in Results, γ-IFN is known to have a direct effect on class I expression, making it necessary to establish background binding of 2C TCR–Ig to γ-IFN–treated cells. This was accomplished by incubating RENCA cells with saturating amounts of the H-2 Ld–binding peptide, MCMV, which efficiently displaced the endogenous H-2 Ld–bound peptides, including any 2C-reactive peptides. Cells were harvested, stained with 2C TCR–Ig (75 μg/ml; A–D) or the mAb 30.5.7 (45 μg/ml; E–H) as described in Materials and Methods. Cells were subsequently stained with GAM-IgG-RPE and analyzed by FACS®. Resultant histograms are shown. Solid lines represent histograms of cultures with no added peptide, whereas dotted lines represent histograms from cultures incubated with pMCMV. All experiments were done in duplicate and repeated at least three times. Note the differences in the extents of fluorescence (see the scales on the histograms) upon staining with 2C-TCR–Ig versus staining with 30.5.7.
The effect of γ-IFN on 2C TCR–Ig reactivity was distinct from its effects on 30.5.7 reactivity. At all concentrations analyzed, 5–50 U/ml, γ-IFN induced a five to sixfold increase in serologically reactive H-2 Ld, as recognized by mAb 30.5.7 (Fig. 8, E–H). MCF of unstimulated RENCA cells was 500, whereas the MCF of γ-IFN–stimulated cells was between 2,666 and 3,038. The maximal effect of γ-IFN was seen at the lowest dose used, in the experiment presented, 5 U/ml, and in other experiments was seen even at dose of γ-IFN as low as 1 U/ml (data not shown). Interestingly, the dose response curve of γ-IFN on 2C TCR–Ig reactivity was shifted. γ-IFN at 5 U/ml had a relatively small, but significant effect on 2C TCR–Ig reactivity. Maximal effects of γ-IFN on 2C TCR–Ig reactivity required γ-IFN treatment at 10 U/ml, ∼10-fold more than needed for maximal effects of γ-IFN on 30.5.7 reactivity. These results indicate a differential effect of γ-IFN on MHC heavy chain expression than that of γ-IFN on specific peptide antigen–MHC complex expression.
Discussion
Interest in understanding T cell–mediated immune responses led us to develop a general system for expression of high affinity soluble analogues of TCR. This was accomplished using Ig as a molecular scaffold to generate soluble divalent TCR analogues. The proof of the efficacy of this approach was shown by the development of two different TCR–Ig molecules, one derived from a TCR recognizing a murine alloantigen, and the other from a TCR recognizing a viral peptide presented by human HLA-A2. The divalent nature of soluble TCR–Ig increased the affinity ∼50-fold, as proven by measuring the values of both the monovalent and divalent 2C TCR in a single system. The divalent construct had an affinity for QL9-loaded H-2 Ld molecules ∼50-fold higher than soluble monovalent 2C TCR. Divalent 2C TCR–Ig also had an affinity for p2Ca-loaded H-2 Ld molecules at least 20-fold higher than soluble monovalent 2C TCRs. Although 2C TCR–Ig interacted with SL9–H-2 Ld complexes, its affinity was too low to accurately estimate under the conditions tested, yet it was significantly higher than that of soluble monovalent 2C TCR. Thus, for all ligands analyzed, significant increases in affinity of the soluble divalent 2C TCR–Ig over soluble monovalent versions of 2C TCR were found. This increase in affinity enhanced our ability to use this reagent to study peptide–MHC interactions.
Soluble divalent TCR–Ig used in flow cytometry assays proved to be a sensitive way of analyzing TCR interaction with peptide–MHC complexes. In our model system, mean channel fluorescence of peptide-loaded RMA-S Ld cells was concordant with the reported affinities of 2C TCR for various peptide–MHC ligands. Using this relatively simple assay we were able to detect interactions of the 2C TCR with three different peptide–H-2 Ld complexes. These peptide–MHC complexes reflect an affinity range of over three orders of magnitude, from 0.066 to 71 μM for monovalent 2C TCR (14). This range of affinities is thought to reflect most of the range of known class I-specific TCR interactions with either peptide agonists–MHC or peptide antagonist–MHC complexes (49–52). Although soluble monovalent 2C TCR was useful in the direct flow cytometry assay only with the highest affinity QL9/H-2 Ld MHC complexes (data not shown), no reactivity could be seen between the soluble monovalent 2C TCR and one of the natural ligands, p2Ca, or the low affinity peptide, SL9 (data not shown). We were unable to detect the very weak interactions occurring between 2C TCR and p2Ca/H-2 Kb molecules (data not shown and reference 14). The affinity of this interaction has been measured as 333 μM (14) suggesting a lower limit of detection of peptide–MHC complexes using soluble 2C TCR–Ig in a flow cytometry based assay.
The affinities measured for soluble monovalent 2C TCR in our system are about an order of magnitude different than previously measured affinities for soluble monovalent 2C TCR (12, 14, 15). Using a competitive 1B2 binding assay, investigators estimated an affinity of 66 nM for the interaction of 2C TCR with QL9-loaded H-2 Ld molecules and 500 nM for its interaction with p2Ca-loaded H-2 Ld molecules (14). Differences in assay systems, including involvement of the CD8 coreceptor probably account for the order of magnitude weaker affinity we measured for soluble monovalent 2C TCR. Recent work supporting this view has shown that CD8 enhances the affinity of TCR for certain peptide–MHC complexes ∼10-fold (53, 54).
Using 2C TCR–Ig, an unexpected finding was revealed in analysis of the affinity of 2C TCR binding for cognate peptide–MHC complexes. Binding of 2C TCR–Ig to peptide-loaded syngeneic MHC complexes displayed a strict correlation with the CTL data. 2C TCR–Ig binding to dEV-8–H-2 Kb was undetectable, whereas binding to SIY– H-2 Kb was quite strong. Surprisingly, binding of 2C TCR–Ig did not correlate with efficacy of lysis for the allogeneic MHC, H-2 Kbm3. No binding of 2C TCR–Ig could be detected to the naturally occurring allogeneic peptide dEV-8–H-2 Kbm3 complex. The lack of 2C TCR–Ig binding occurred despite the fact that dEV-8–H-2 Kbm3 is more efficient in mediating lysis by 2C CTL than SIY–H-2 Kb and that 2C TCR–Ig binding to SIY–H-2 Kbm3 could easily be detected. Our experiments using 2C TCR–Ig demonstrate that the affinity of the 2C TCR for the dEV-8–H-2 Kbm3 complex is weaker than 71 μM. This is based on the fact that although 2C TCR–Ig recognized SL9–H-2 Ld complexes (Fig. 3), an interaction known to have an affinity of 71 μM (14), 2C TCR–Ig did not recognize dEV-8–H-2 Kbm3. Therefore, the affinity of 2C TCR for dEV-8–H-2 Kbm3 must be weaker than 71 μM. This is particularly important in view of the fact that allogeneic MHC targets are thought to be relatively high affinity ligands, affinities of 1–0.1 μM for cognate TCR interactions (14, 15). Since in normal allogeneic responses, the T cell repertoire has not been negatively selected, some investigators have proposed that the TCR interaction with allogeneic MHC will routinely be of higher affinity than TCR interactions with peptide–MHC complexes that have passed through negative selection (15). Based on the previously estimated affinities of negatively selecting peptide–MHC complexes, one would have expected that the 2C TCR would not be negatively selected on H-2 Kbm3. However, in transgenic 2C TCR animals, the allogeneic H-2 Kbm3 MHC molecule serves as a mediator of negative selection in the thymus (20). Thus, assuming dEV8–H-2 Kbm3 is a negatively selecting complex, the intrinsic affinity of peptide–MHC complexes required to mediate negative selection is significantly lower than previously predicted.
Previously, a lack of a correlation between TCR affinity and efficiency of lysis by CTLs was noted with certain variant p2Ca peptides, not naturally occurring endogenous peptides, on H-2 Ld (16). From that work, investigators concluded that other factors such as CD8, which are not part of soluble TCR analogues, could be influencing the affinity of the TCR for its peptide–MHC ligand, thereby, impacting on the lysis of targets pulsed with these peptides. As mentioned above, this hypothesis is supported by recent work showing the enhancement of the interaction between TCR and peptide–MHC complexes by CD8 (54). Our analysis of the 2C TCR–Ig reactivity, which was unable to detect certain 2C-reactive complexes, dEV-8–H-2 Kbm3 complexes, may also reflect a differential importance of CD8 engagement in facilitating lysis of certain peptide– MHC targets over others.
By analyzing the expression of endogenous cell surface peptide–MHC complexes, we found that the effect of γ-IFN on regulation of class I heavy chain is distinct from its effect on regulation of the peptide–MHC complexes. This could reflect different target genes for the two activities of γ-IFN. Presumably the effects of γ-IFN on class I heavy chain expression is due the γ-IFN–responsive element in the class I MHC promoter. The effects on expression of the epitope recognized by 2C TCR–Ig could be due to the effects of γ-IFN on peptide processing, as recently found to relate to induction of the proteosome component PA28 (24, 25, 28), or at other potentially more complex γ-IFN–controlled pathways involved in regulation of antigenic peptide delivery. Of interest when the effect of the proteasome regulator PA28α on antigen presentation was studied using cells transfected with PA28α, investigators saw an approximate threefold increase in alloreactive C57BL/6 anti-BALB/c responses. This effect is similar in magnitude to the effect of γ-IFN on 2C TCR–Ig binding.
In addition to using soluble divalent high affinity TCR as a general approach to study expression of peptide–MHC complexes, mAbs that differentiate between MHC molecules on the basis of peptides resident in the groove are also useful in studying expression of specific peptide–MHC complexes. As previously discussed, using both conventional approaches and a recombinant antibody phage display library, a few such antibodies have been produced (3– 8). The mAb made using a recombinant antibody phage display library (8) has affinities generally comparable to the affinity of the soluble 2C TCR–Ig for high affinity peptide–MHC ligands. A distinct advantage of soluble high affinity TCR–Ig chimeras is that even in the absence of any a priori knowledge about their ligands, they may be useful in defining the specific ligands recognized by poorly defined TCR such as γ/δ TCR, undefined tumor-specific T cells, and T cells involved in autoimmune responses. The generation of mAbs specific for a particular peptide–MHC complex requires the prior identification of the peptide ligand for development and obviously cannot be used to help define the TCR ligands or the peptide–MHC complex. Furthermore, T cell activation requires cross-linking of multiple TCRs. Since the mode of interaction of TCR–Ig chimeras with peptide–MHC complexes is similar to that of the natural TCR, TCR–Ig will mimic the interaction of a T cell with APCs and may help facilitate elucidation of biochemical interactions involved in TCR recognition of peptide–MHC complexes. Although mAbs with high affinity for specific peptide–MHC complexes will cross-link TCR, there is no guarantee that they will mimic the interaction of TCR with peptide–MHC ligands. Thus, one can expect that use of high affinity biologically relevant cognates such as TCR–Ig molecules is likely to reveal details of TCR interactions with peptide–MHC molecules that peptide-specific mAbs may miss. Advantages offered by both systems will facilitate the analysis of expression of specific peptide– MHC complexes in both normal and aberrant immune responses.
Here we presented a general approach for producing soluble divalent versions of heterodimeric proteins such as T cell receptors. This is the first time that soluble high affinity and specificity analogues of TCR proteins have been made. This approach is not only of relevance for TCR, but also for other immunoregulatory proteins such as class II MHC molecules (O'Herrin, S.M., M.S. Lebowitz, and J.P. Schneck, manuscript in preparation), and potentially useful in other cell biological systems involving heterodimeric integral membrane proteins. The experimental system described here outlines a general approach of using divalent high affinity ligands to study cell–cell interactions, driven by multivalent ligand–receptor interactions.
Acknowledgments
This work was supported by the National Institutes of Health, American Cancer Society, and National Multiple Sclerosis Society. J. Schneck holds a Harry Weaver Neuroscience Scholars' Award from the National Multiple Sclerosis Foundation.
Footnotes
RMA-S, RMA-S Ld, T2, T2 Ld were gifts from Ted Hansen (Washington University, St. Louis, MO), T2 Kb was a gift from P. Cresswell (Yale University, New Haven, CT), and T2 Kbm3 and T2 Kbm11 were gifts from L. Pease (Mayo Foundation, Rochester, MN). cDNA encoding the murine IgG1 arsonate-specific heavy chain, 93G7, and kappa light chain, 91A3, were gifts from D. Capra and C. Haseman (Southwestern University, Dallas, TX). The modified pAcUW51 expression vector used was a gift from John Kappler (University of Colorado Health Science Center, Denver, CO). 2C TCR transgenic mice were obtained from Ted Hansen (Washington University, St. Louis, MO) with the consent of Dennis Loh. We also thank Drs. M. Edidin, H. Levitsky, D. Pardoll, S. Sadegh-Nasseri, J. Slansky, and R. Silicano for their critical reviews of the manuscript.
Address correspondence and reprint requests to Dr. Michael S. Lebowitz, Johns Hopkins Medical Institutions, Department of Pathology, 720 Rutland Ave., 664 G Ross Bldg., Baltimore, MD 21205. Phone: 410-614-4589; FAX: 410-614-3548. E-mail: mslebo@welchlink.welch.jhu.edu
Note added in proof. During review of this manuscript two papers reporting new peptide-specific mAbs were published (55, 56).
S.M. O'Herrin and M.S. Lebowitz contributed equally to this work.
References
- 1.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 2.Seder RA, Paul WE, Davis MM, Fazekus de St B, Groth The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Froscher BG, Klinman NR. Immunization with SV40-transformed cells yields mainly MHC-restricted monoclonal antibodies. J Exp Med. 1986;164:196–210. doi: 10.1084/jem.164.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wylie DE, Sherman LA, Klinman NR. Participation of the major histocompatibility complex in antibody recognition of viral antigens expressed on infected cells. J Exp Med. 1982;155:403–414. doi: 10.1084/jem.155.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leeuwen AV, Goulmy E, Rood JJV. Major histocompatibility complex–restricted antibody reactivity mainly, but not exclusively, directed against cells from male donors. J Exp Med. 1979;150:1075–1083. doi: 10.1084/jem.150.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy DB, Rath S, Pizzo E, Rudensky AY, George A, Larson JK, Janeway CA., Jr Monoclonal antibody detection of a major self peptide: MHC class II complex. J Immunol. 1992;148:3483–3492. [PubMed] [Google Scholar]
- 7.Duĉ HT, Rucay P, Righenzi S, Halle-Pannenko O, Kourilsky P. Monoclonal antibodies directed against T cell epitopes presented by class I MHC antigens. Int Immunol. 1993;5:427–431. doi: 10.1093/intimm/5.4.427. [DOI] [PubMed] [Google Scholar]
- 8.Andersen PS, Stryhn A, Hansen BE, Fugger L, Engberg J, Buus S. A recombinant antibody with the antigen-specific, major histocompatibility complex–restricted specificity of T cells. Proc Natl Acad Sci USA. 1996;193:1820–1824. doi: 10.1073/pnas.93.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fremont DH, Rees WA, Kozono H. Biophysical studies of T-cell receptors and their ligands. Curr Opin Immunol. 1996;8:93–100. doi: 10.1016/s0952-7915(96)80111-7. [DOI] [PubMed] [Google Scholar]
- 10.Matsui K, Boniface JB, Steffner P, Reay PA, Davis MM. Kinetics of T-cell receptor binding to peptide/I-Ekcomplexes: Correlation of the dissociation rate with T-cell responsiveness. Proc Natl Acad Sci USA. 1994;91:12862–12866. doi: 10.1073/pnas.91.26.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabinowitz JD, Beeson C, Wulfing C, Tate K, Allen PM, Davis MM, McConnell HM. Altered T cell receptor ligands trigger a subset of early T cell signals. Immunity. 1996;5:125–135. doi: 10.1016/s1074-7613(00)80489-6. [DOI] [PubMed] [Google Scholar]
- 12.Corr M, Slanetz AE, Boyd LF, Jelonek MT, Khiko S, Al-Ramadi BK, Kim YS, Maher SE, Bothwell ALM, Margulies DH. T cell receptor–MHC class I peptide interactions: affinity, kinetics and specificity. Science (Wash DC) 1994;265:946–949. doi: 10.1126/science.8052850. [DOI] [PubMed] [Google Scholar]
- 13.Udaka K, Tsomides TJ, Eisen HN. A naturally occurring peptide recognized by alloreactive CD8+cytotoxic T lymphocytes in association with a class I MHC protein. Cell. 1992;69:989–998. doi: 10.1016/0092-8674(92)90617-l. [DOI] [PubMed] [Google Scholar]
- 14.Sykulev Y, Brunmark A, Jackson M, Cohen RJ, Peterson PA, Eisen HN. Kinetics and affinity of reactions between an antigen-specific T cell receptor and peptide–MHC complexes. Immunity. 1994;1:15–22. doi: 10.1016/1074-7613(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 15.Sykulev T, Brunmark A, Tsomides TJ, Kageyama S, Jackson M, Peterson PA, Eisen HN. High-affinity reactions between antigen-specific T-cell receptors and peptides associated with allogeneic and syngeneic major histocompatibility complex class I proteins. Proc Natl Acad Sci USA. 1994;91:11487–11491. doi: 10.1073/pnas.91.24.11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Ramadi BK, Jelonek MT, Boyd LF, Margulies DH, Bothwell ALM. Lack of strict correlation of functional sensitization with the apparent affinity of MHC– peptide complexes for the TCR. J Immunol. 1995;155:662–673. [PubMed] [Google Scholar]
- 17.Udaka K, Wiesmuller K-H, Kienle S, Jung G, Walden P. Self-MHC–restricted peptides recognized by an alloreactive T lymphocyte clone. J Immunol. 1996;157:670–678. [PubMed] [Google Scholar]
- 18.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature (Lond) 1988;336:73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- 19.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature (Lond) 1988;335:271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 20.Sha WC, Nelson CA, Newberry RD, Pullen JK, Pease LR, Russell JH, Loh DY. Positive selection of transgenic receptor-bearing thymocytes by Kb antigen is altered by Kbmutations that involve peptide binding. Proc Natl Acad Sci USA. 1990;87:6186–6190. doi: 10.1073/pnas.87.16.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tallquist MD, Pease LR. Alloreactive 2C T cells recognize a self peptide in the context of the mutant Kbm3molecule. J Immunol. 1995;155:2419–2426. [PubMed] [Google Scholar]
- 22.Tallquist MD, Yun TJ, Pease LR. A single T cell receptor recognizes structurally distinct MHC/peptide complexes with high specificity. J Exp Med. 1996;184:1017–1026. doi: 10.1084/jem.184.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hengel H, Lucin P, Jonjic S, Ruppert T, Koszinowski UH. Restoration of cytomegalovirus antigen presentation by γ-interferon combats viral escape. J Virol. 1994;68:289–297. doi: 10.1128/jvi.68.1.289-297.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groettrup M, Soza A, Eggers M, Kuehn L, Dick TP, Schild H, Rammensee H-G, Koszinowski UH, Kloetzel P-M. A role for the proteasome regulator PA28α in antigen presentation. Nature (Lond) 1996;381:166–168. doi: 10.1038/381166a0. [DOI] [PubMed] [Google Scholar]
- 25.Groettrup M, Ruppert T, Kuehn L, Seeger M, Standera S, Koszinowski U, Kloetzel PM. The interferon-γ–inducible 11 S regulator (PA28) and the LMP2/ LMP7 subunits govern the peptide production by the 20 S proteasome in vitro. . J Biol Chem. 1995;270:23808–23815. doi: 10.1074/jbc.270.40.23808. [DOI] [PubMed] [Google Scholar]
- 26.Boes B, Hengel H, Ruppert T, Multhaup G, Koszinowski UH, Kloetzel P-M. Interferon γ stimulation modulates the proteolytic activity and cleavage site preference of 20S mouse proteasomes. J Exp Med. 1994;179:901–909. doi: 10.1084/jem.179.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bikoff EK, Jaffe L, Ribaudo RK, Otten ER, Germain RN, Robertson EJ. MHC class I surface expression in embryo-derived cell lines inducible with peptide or interferon. Nature (Lond) 1991;354:235–238. doi: 10.1038/354235a0. [DOI] [PubMed] [Google Scholar]
- 28.Dick TP, Ruppert T, Groettrup M, Kloetzel PM, Kuehn L, Koszinowski UH, Stevanovic S, Schild H, Rammensee H-G. Coordinated dual cleavages induced by the proteasome regulator PA 28 lead to dominant MHC ligands. Cell. 1996;86:253–262. doi: 10.1016/s0092-8674(00)80097-5. [DOI] [PubMed] [Google Scholar]
- 29.Kranz DM, Sherman DH, Sitkovsky MV, Pasternack MS, Eisen HN. Immunoprecipitation of cell surface structures of cloned cytotoxic T lymphocytes by clone-specific antisera. Proc Natl Acad Sci USA. 1984;81:573–577. doi: 10.1073/pnas.81.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garboczi DN, Utz U, Ghosh P, Seth A, Kim J, VanTienhoven EAE, Biddison WE, Wiley DC. Assembly, specific binding, and crystallization of a human TCR-αβ with an antigen tax peptide from human T lymphotropic virus type 1 and class I MHC molecule HLA-A2. J Immunol. 1996;157:5403–5410. [PubMed] [Google Scholar]
- 31.Utz U, Banks D, Jacobson S, Biddison WE. Analysis of the T-cell receptor repertoire of human T-cell leukemia virus type 1 (HTLV-1) tax-specific CD 8+cytotoxic T lymphocytes from patients with HTLV-1–associated disease: evidence for oligoclonal expansion. J Virol. 1996;70:843–851. doi: 10.1128/jvi.70.2.843-851.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brodnicki TC, Holman PO, Kranz DM. Reactivity and epitope mapping of single-chain T cell receptors with monoclonal antibodies. Mol Immunol. 1996;33:253–263. doi: 10.1016/0161-5890(95)00142-5. [DOI] [PubMed] [Google Scholar]
- 33.Townsend A, Ohlen C, Bastin J, Ljunggren H-G, Foster L, Karre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature (Lond) 1989;340:443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- 34.Spies T, Cerundolo V, Colonna M, Cresswell P, Townsend A, De Mars R. Presentation of viral antigen by MHC class I molecules is dependent on a putative peptide transporter heterodimer. Nature (Lond) 1992;355:644–646. doi: 10.1038/355644a0. [DOI] [PubMed] [Google Scholar]
- 35.Townsend A, Ohlen C, Bastin J, Ljunggren H-G, Foster L, Karre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature (Lond) 1989;340:443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- 36.Catipovic B, Dal J, Porto, Mage M, Johansen TE, Schneck JP. Major histocompatibility complex conformational epitopes are peptide specific. J Exp Med. 1992;176:1611–1618. doi: 10.1084/jem.176.6.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koenig S, Woods RM, Brewah YA, Newell AJ, Jones GM, Boone E, Adelsberger JW, Baseler MW, Robinson SM, Jacobson S. Characterization of MHC class I restricted cytotoxic T cell responses to tax in HTLV-1 infected patients with neurologic disease. J Immunol. 1993;156:3874–3883. [PubMed] [Google Scholar]
- 38.Schlueter CJ, Schodin BA, Tetin GY, Kranz DM. Specificity and binding properties of a single-chain T cell receptor. J Mol Biol. 1996;256:859–569. doi: 10.1006/jmbi.1996.0132. [DOI] [PubMed] [Google Scholar]
- 39.Connolly JM. The peptide p2Ca is immunodominant in allorecognition of Ld by β chain variable region Vβ8+ but not Vβ8−strains. Proc Natl Acad Sci USA. 1994;91:11482–11486. doi: 10.1073/pnas.91.24.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobson, S., M. Levin, U. Utz, and P. Drew. 1997. Infectious immune disorders: HTLV-1. In Neuroimmunology. J.A. Antel, G. Birnbaum and H.-P. Hartung, editors. Blackwell Science, Cambridge. In press.
- 41.Elovaara I, Koenig S, Brewah AY, Woods RM, Lehky T, Jacobson S. High human T cell lymphotropic virus type 1 (HTLV-1)–specific precursor cytotoxic T lymphocyte frequencies in patients with HTLV-1–associated neurological disease. J Exp Med. 1993;177:1567–1573. doi: 10.1084/jem.177.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Utz U, Koenig S, Coligan JE, Biddison WE. Presentation of three different viral peptides. HTLV-1 tax, HCMV gB, and influenza virus M1, is determined by common structural features of the HLA-A2.1 molecule. J Immunol. 1992;149:214–221. [PubMed] [Google Scholar]
- 43.Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC. Structure of the complex between human T-cell receptor, viral peptide, and HLA-A2. Nature (Lond) 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 44.Solheim JC, Carreno BM, Smith JD, Gorka J, Myers NB, Wen Z, Martinko JM, Lee DR, Hansen TH. Binding of peptides lacking consensus anchor residue alters serological recognition of H-2Ld . J Immunol. 1993;151:5387–5397. [PubMed] [Google Scholar]
- 45.Solheim JC, Carreno BM, Myers NB, Lee DR, Hansen TH. Peptide-induced rescue of serologic epitopes on class I MHC molecules. J Immunol. 1995;154:1188–1197. [PubMed] [Google Scholar]
- 46.Riberdy JM, Cresswell P. The antigen-processing mutant T2 suggests a role for MHC-linked genes in class II antigen presentation. J Immunol. 1992;148:2586–2590. [PubMed] [Google Scholar]
- 47.Wei ML, Cresswell P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides . Nature (Lond) 1992;356:443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- 48.Townsend, A., C. Ohlen, L. Foster, J. Bastin, H.G. Ljunggren, and K. Karre. 1989. A mutant cell in which association of class I heavy and light chains is induced by viral peptides. Cold Spring Harbor Symposia on Quantitative Biology, Cold Spring Harbor, NY. [DOI] [PubMed]
- 49.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 50.Jameson SC, Hogquist KA, Bevan MJ. Specificity and flexibility in thymic selection. Nature (Lond) 1994;369:750–752. doi: 10.1038/369750a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jameson SC, Carbone FR, Bevan MJ. Clone-specific T cell receptor antagonists of major histocompatibility complex class I–restricted cytotoxic T cells. J Exp Med. 1993;177:1541–1550. doi: 10.1084/jem.177.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NRJ. T-cell receptor affinity and thymocyte positive selection. Nature (Lond) 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 53.Luescher IF, Vivier E, Layer A, Mahiou J, Godeau F, Malissen B, Romero P. CD8 modulation of T-cell antigen receptor–ligand interactions on living cytotoxic T lymphocytes. Nature (Lond) 1995;373:353–356. doi: 10.1038/373353a0. [DOI] [PubMed] [Google Scholar]
- 54.Garcia KC, Scott CA, Brunmark A, Carbone FR, Peterson PA, Wilson IA, Teytom L. CD8 enhances formation of stable T-cell receptor/MHC class I molecule complexes. Nature (Lond) 1996;384:577–581. doi: 10.1038/384577a0. [DOI] [PubMed] [Google Scholar]
- 55.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide–MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 56.Dadaglio G, Nelson CA, Deck MB, Petzold SJ, Unanue ER. Characterization and quantitation of peptide–MHC complexes produced from hen egg lysozyme using a monoclonal antibody. Immunity. 1997;6:727–738. doi: 10.1016/s1074-7613(00)80448-3. [DOI] [PubMed] [Google Scholar]
- 57.Solheim JC, Alexander-Miller MA, Martinko JM, Connolly JM. Biased T cell receptor usage by Ld-restricted, Tum−peptide-specific cytotoxic T lymphocyte clones. J Immunol. 1993;150:800–811. [PubMed] [Google Scholar]
- 58.Huang AYC, Gulden PH, Woods AS, Thomas MC, Tong CD, Wang W, Engelhard VH, Pasternack G, Cotter R, Hunt D, et al. The immunodominant major histocompatibility complex class I–restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci USA. 1996;93:9730–9735. doi: 10.1073/pnas.93.18.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Bleek GM, Nathenson SG. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kbmolecule. Nature (Lond) 1990;348:213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]