Abstract
The zebrafish has become a major model system for biomedical research and is an emerging model for the study of behaviour. While adult zebrafish express a visually mediated shoaling preference, the onset of shoaling behaviour and of this preference is unknown. To assess the onset of these behaviours, we first manipulated the early social environment of larval zebrafish subjects, giving them three model shoaling partners of the same pigment phenotype. We then assayed the subjects’ preferences using binary preference tests in which we presented subjects with two shoals, one shoal of fish exhibiting the same pigment pattern phenotype as their models and another shoal with a radically different pigment pattern. To determine whether or not the visually mediated preference could be altered once it was established, we further manipulated the social environment of a number of subjects, rearing them with one model shoal and testing them, then changing their social consorts and retesting them. Our results demonstrate that larval zebrafish shoal early in their development, but do not exhibit a shoaling preference until they are juveniles. Moreover, we find that the shoaling preference is stable, as changing the social environment of fish after they had acquired a preference did not change their preference. These data will facilitate investigations into the mechanisms underlying social behaviour in this vertebrate model system.
Understanding the interplay of development and early experience is critical for an understanding of behaviour (Schneirla, 1957). Some behaviours are variable across ontogenetic stages. Adults of many organisms engage in elaborate courtship displays, for example, while younger individuals do not. Knowing the onset and ontogeny of a behaviour, we can determine critical periods for behavioural development and assess the environmental and genetic factors that give rise to the behavioural phenotype (Hultsch & Todt, 2004).
Many fishes engage in a social behaviour called shoaling, which plays a key role in foraging, predator avoidance, and mating (Pitcher & Parrish, 1993). While many fish species shoal, they exhibit tremendous ontogenetic variation in their timing and tendency to aggregate (Bowen, 1931; Bowen, 1932; Baerends & Roon, 1950; Shaw, 1960). A useful species for investigating behavioural mechanisms underlying shoaling is the zebrafish, Danio rerio. Zebrafish are an established model system for biomedical research and developmental genetics, and researchers are increasingly studying a broad range of behaviours exhibited by this species (Darrow & Harris, 2004; Orger & Baier, 2005; Rosenthal & Ryan, 2005)
Zebrafish are members of the family Cyprinidae and their range includes much of Northern India, Bangladesh, and parts of Southern Nepal. Diurnal micro-predators of aquatic invertebrates, they reproduce via external fertilization in shallow silt bottomed pools, rice paddies, and seasonal steams, and show no parental care of their eggs or larvae. This lack of parental care makes zebrafish an interesting system for studying the intrinsic and extrinsic factors leading to social behaviours as larvae are unable to imprint on their parents or use them as models for appropriate social consorts. Nevertheless, zebrafish form shoals in the wild and presumably must distinguish between conspecifics and other co-occurring minnows, such as members of the genera Barilius, Danio, Devario, and Puntius (Engeszer et al., in press).
Previous work on shoaling in the zebrafish, Danio rerio, has focused on the propensity of individuals to shoal (Wright et al., 2003) and the choice of shoal mates (Mann et al., 2003). We showed that zebrafish express a visually mediated shoaling preference and that early environment determines the preferred phenotype (Engeszer et al., 2004): wild-type zebrafish preferred either other wild-type zebrafish, or a stripeless mutant, nacre, depending on which phenotype they experienced during development (Fig. 1a and b).
Figure 1. Adult and embryonic phenotypes.

Adult on the left (a), exhibits the wild-type pigment pattern and on the right (b), the nacre mutant pattern. The embryo on the left (c), exhibits the wild-type embryonic pigment phenotype and that on the right (d), exhibits the nacre mutant phenotype.
In this study, we ask three critical questions for understanding shoaling in wild-type zebrafish. When do zebrafish begin shoaling? When is the visual preference first exhibited? And does this preference change over the fish’s life as it experiences other phenotypes, or, is it immutable once established? We find that shoaling begins relatively soon after hatching. By contrast, a visual preference for shoal-mates is not exhibited until the later, juvenile stages. Furthermore, this preference does not change even in response to prolonged exposure to alternative visual phenotypes.
METHODS
Overview
We raised small groups of wild-type fishes (Fig. 1a) in visual isolation from other fishes in the lab. When these wild-type fish reached a particular developmental stage, we assayed their propensity to shoal and their shoaling preference using a binary preference test. One subject was chosen at random from the group, placed in a test tank (Fig. 2) and presented with a shoal of wild-type fish and a shoal of nacre fish that lack melanophore stripes (Lister et al., 1999) (Fig. 1b). The subject was separated from each stimulus shoal by a transparent barrier. We recorded each subject’s time in association with each shoal. We tested fish at several development stages ranging from freshly hatched larvae to adults
Figure 2. Test Tank.
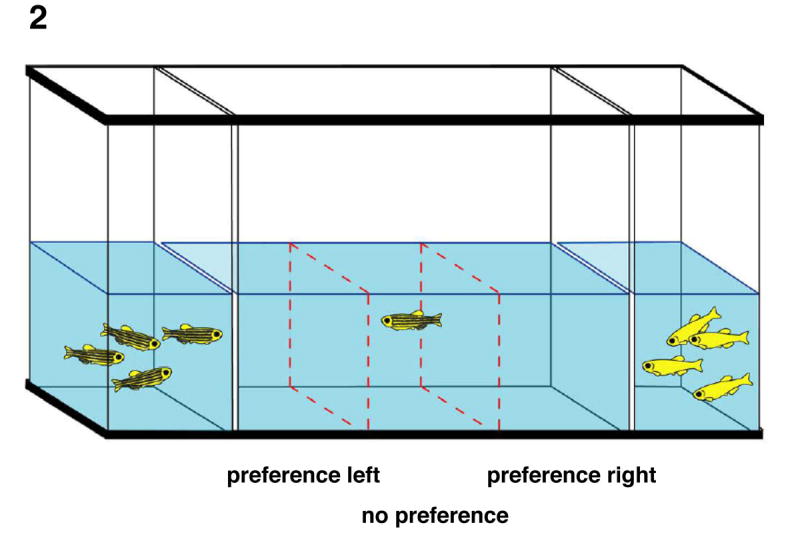
The schematic above represents the test tank. The dashed lines mark the interior boundaries of the preference areas. The double lines show both the position of double panes of Plexiglas and the outer boundaries of the preference areas. Stimulus shoals are shown in each of the outermost compartments and the subject is in the central “no preference” area.
To assess the stability of shoaling preference, we assayed the visual preference of juvenile wild-type zebrafish. Each subject had been raised with three wild-type siblings. We tested them in the same tank and using the same protocol described above. We then manipulated the social environment of these subjects for an additional 30 days, housing half with wild-type shoal-mates and half with nacre shoal-mates. We then retested the subjects to see if their preference had changed.
Fish stocks and rearing conditions
Stocks
We backcrossed nacrew2 heterozygous fish in the ABwp background to nacre homozygotes to obtain sibships that contained half wild-type individuals and half nacre mutant individuals (Fig. 1a and b) (for zebrafish stock naming conventions, see: http://zfin.org). nacrew2 is a recessive, single locus mutant phenotype arising from a mutation in microphthalmia-a, which encodes a transcription factor essential for melanophore development (Lister et al., 1999). Shoaling behaviours exhibited by nacre mutant fish are qualitatively and quantitatively indistinguishable from wild-type (Engeszer et al., 2004; personal observations).
Onset of shoaling and preference
Fish from these nacre backcrosses were then sorted into either subject treatments, from which we later chose individuals as subjects for analyzing shoaling behaviour, or stimulus treatments, from which we chose fish to act as members of stimulus shoals. As nacre mutants have an embryonic pigment pattern phenotype different from the wild-type (Fig. 1c and d), we were able to sort individuals into treatments prior to their hatching. Wild-type fish were raised in groups of four with one fish from each group chosen haphazardly as a subject for analysis. Stimulus fish were raised in groups of four of which two were wild-type and two were nacre mutants, we chose fish from these treatments for use in the stimulus shoals. Rearing the stimulus fish under the same conditions as the subject fish controlled for environmental effects that might cause them to grow at a different rate than their subject siblings, while rearing them as mixed wild-type and nacre groups eliminates any shoaling bias they might otherwise express (Engeszer et al. 2004). We raised these groups in opaque cups in which they were visually isolated from any fish not in their treatment until they reached one of the following stages (Snyder and Muth 2004) in chronological sequence:
Preflexion larvae are approximately 3.5 mm standard length (SL) and are characterized by a continuous fin fold, straight notochord and no fin rays.
Early flexion larvae are approximately 6 mm SL and are characterized by the upturn of the posterior notochord (urostyle) and the appearance of the first caudal fin rays.
Postflexion larvae are approximately 7 mm SL and have completed flexion, posses well developed hypurals, and a bilobate swim bladder (rather than the single lobed swimbladder seen at earlier stages).
Metalarvae are approximately 8.5 mm SL and have nearly complete median fins and pelvic fin buds.
Juveniles are approximately 10 mm in SL, are not yet reproductively active and, exhibit complete fins, complete squamation and a nearly complete adult pigment pattern.
Adults are over 15 mm SL and have developed gonads.
We tested 20 subject fish at each stage; individual fish were tested only once.
Plasticity of preference
Forty wild-type juveniles that had each been raised with three wild-type siblings (as described for subjects above) were tested for preference (see Test protocol below). We then placed individuals at random into one of two social environments, control or cross-rear. We placed each of the 20 control fish in a 2.8-l Aquaneering flow-through tank with three other wild-type zebrafish as social models. Model and subject fish were siblings matched in size and age. We covered the sides of each tank with translucent white plastic to obscure the view of fish in any nearby tanks. We placed each of the cross-rear subjects in identical tanks, containing three nacre siblings as social models. We kept subjects in their treatment tanks for 30 days and then retested them. While subjects and model fish were siblings, they had no prior experience of one another. The fish used as shoaling stimuli in the experiments were also siblings of the subjects, but the test subjects had not previously seen any of the stimulus fish.
Test tanks
Adult fish examined for both onset and plasticity of shoaling preference were tested in a 122 cm long, 55 cm high, and 32 cm deep all-glass 245 L aquarium that was divided into three compartments. The two 25 cm flanking regions were separated from the center by a double pane of UV-transmittant Rhöm Plexiglas GS2458 that was sealed with silicon adhesive to prevent the flow of any water between the panes. The 15 mm airspace between the two Plexiglas panes would block all chemical communication between the compartments and greatly diminish the transmission of auditory cues. The aquarium was lit with a double lamp, 125 cm long, fluorescent fixture (lamped with one 40 W cool blue tube and one 40 W Reptical tube). The tank was covered on sides and back with neutral gray photographic paper. Washed gravel was used as a substrate covering the bottom of all three compartments. The aquarium was filled with water to the 25 cm level. The water temperature was maintained at 29°C with a submersible Ebo-Jager 100 W heater that was removed during testing. The two 25 cm flanking areas of the inner compartment were marked on the exterior of the glass with a black grease pencil to demarcate the left and right preference areas (Fig. 2).
The preflexion, early flexion, post flexion, and metalarval stages were tested in a tank 22.5 cm × 9.5 cm × 5.5 cm made of 2 mm thick UV transparent Plexiglas and filled to a depth of 4.5 cm. The exterior sides were covered with neutral grey photographic paper. The tank was divided into stimulus and preference areas that were the same proportions as the adult tank. Thinner Plexiglas was used in place of the Rhöm Plexiglass to separate the preference areas from the stimulus areas. The tank was otherwise similar to the tank for adults.
The juvenile stage subjects were tested in a 40-l all glass aquarium (40.5 cm × 21 cm × 24 cm) filled to a depth of 18 cm. The sides were covered with neutral gray photographic paper and a finer sand substrate was used. The tank was divided into stimulus and preference areas that were the same proportions as the adult tank. The tank was otherwise similar to the tank for adults.
Test protocol
Fish were kept in their treatments until they reached one of the stages described above, at which time we measured shoaling preference as follows. Fish were then chosen at random from the available subjects and used in the preference assay. Opaque plastic barriers were placed at either end of the central portion of the test tank. A shoal of four wild-type fish were placed in one stimulus compartment (side determined by coin toss). A shoal of four nacre fish were placed in the other stimulus compartment. All stimulus fish were chosen from stimulus treatments and matched in size and stage to the subject. We used four zebrafish in the stimulus shoals because groups of this size exhibit shoaling behaviour indistinguishable from that of larger groups (Breder & Halpern, 1946). The subject fish was placed in the central compartment. When the subjects were adults the stimulus shoals included two female and two male fish, at earlier stages we were unable to sex the stimulus fish.
The fish were allowed 10 min to acclimate to the tank. The barriers were then removed and the subject fish was given the next 15 min to recognize both stimulus shoals. Recognition was defined as parallel swimming with a member of the stimulus shoal. The time needed for the fish to recognize both stimuli was noted as the latency. If the subject did not recognize both shoals in 15 min, the test was aborted. During the following 5 min, the time spent by the subject in either preference area was noted. The barriers were then replaced, the stimulus shoals were exchanged to control for side bias, and the above steps were repeated. The association times noted in these two 5 min intervals were combined in the analysis. We carried out tests from late morning through the afternoon and time of day had no effect on the analysis (data not shown).
Analysis
Onset of shoaling and preference
We compared the time spent in the “preference” areas across stages to ascertain the onset of shoaling. Fish that do not shoal should be spending less time in the “preference” areas than fish that are shoaling with the stimulus fish. We performed an arcsine transform on the proportion of time the subjects spent in the “preference” areas to the total test time (600 sec) to assess the onset of shoaling behaviour. The results were then compared between stages using a one-way ANOVA and the means compared using Tukey-Kramer HSD.
We then compared the time spent in association with either stimulus to determine if a preference was exhibited by the subjects. We compared the time spent with the reared stimulus with the time spent with the non-reared stimulus using Student’s t-test for each stage.
Plasticity of preference
To assay any change in shoaling preference between the first time the fish were tested and the second, we compared the proportion of time spent with the wild-type stimulus in each test. We used the arcsine transform of this proportion in a paired Student’s t-test to determine any significant difference between the amounts of time spent associating with wild-type shoals.
RESULTS
Onset of shoaling and preference
Since fish vary tremendously in the ontogenetic timing of shoaling behaviour (see references above), we asked if zebrafish shoal immediately upon hatching or if this behaviour arises during later development. The two hypotheses that we considered were: (i) fish shoal immediately upon hatching; or (ii) fish only begin shoaling at some point during post-hatching development.
Our analyses reveal a clear ontogeny for shoaling behaviour, from relatively sessile, early stage preflexion larvae to later stage, postflexion larvae whose propensity to shoal is indistinguishable from that of adults. Preflexion larvae did not respond in the protocol, remaining immobile during the test and so are not included in the analysis. The time individuals spent shoaling differed significantly among stages (ANOVA: F1,4 = 5.23, P < 0.001; Fig. 3). Nevertheless, shoaling times for postflexion, metalarvae, juveniles and adults did not differ significantly from one another and all of these except metalarvae differed significantly from early flexion larvae (Tukey-Kramer HSD: q* = 2.79, α = 0.05). Thus shoaling behaviour arises during larval development and zebrafish continue to shoal from this point onwards.
Figure 3. Onset of shoaling.
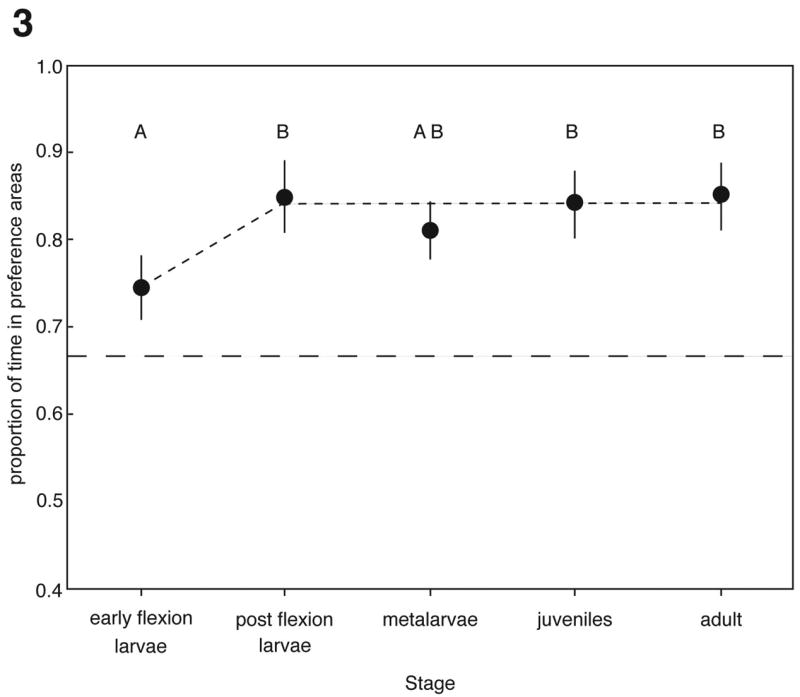
The proportion of time spent in the preference areas to the total observation time ((time left + time right)/600sec) is shown for each stage. The dashed line represents the null expectation of 66% of the time spent in the preference areas. The circles represent the means for each stage (back transformed from the data used for the ANOVA), and the bars represent the 95% confidence intervals. Sample sizes are: early flexion larvae, N = 19; post flexion, N = 20; metalarvae, N = 16; juvenile, N = 20; and adults, N = 20. The analysis showed a significant effect of stage on time spent shoaling (ANOVA: F1,4 = 5.23, P < 0.001). The letters A and B designate groups that are significantly different (Tukey-Kramer q*=2.79, α=0.05). The increase in the time spent in the preference areas between early flexion stage and the post flexion stage suggests the onset of shoaling behaviour.
Having identified the onset of shoaling behaviour, we asked when visual preferences are first expressed. We considered the following hypotheses: (i) fish exhibit a visual shoaling preference as soon as they begin shoaling; or (ii) fish express a visual shoaling preference only beginning at some later developmental stage. To distinguish between these hypotheses, we raised fish with their wild-type siblings to various stages (see Methods for stage descriptions) and gave them a choice between associating with either wild-type or nacre shoals.
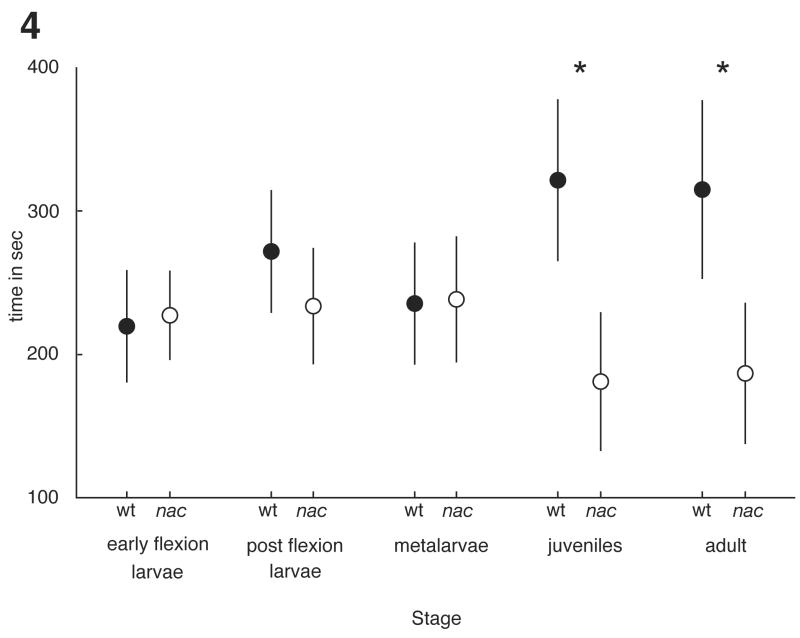
Our data show that although larval zebrafish exhibit shoaling behaviour, they do not discriminate between the stimulus shoals on the basis of visual signals. Juveniles, however, exhibit a robust, visually mediated preference. The juvenile and adult stages showed a significant difference in the amount of time spent with the reared stimulus (two-tailed paired Student’s t-tests: juvenile, N = 20, t19 = −2.83, P = 0.01; adults, N = 20, t19 = −2.4, P < 0.05) while none of the other stages did (two-tailed paired Student’s t-tests: early flexion larvae, N = 19, t18 = 0.253, P = 0.8; post flexion, N = 20, t19 = −0.98, P = 0.3; metalarvae, N = 16, t15 = 0.065, P > 0.9; Fig. 4). The onset of shoaling and the first expression of the visually mediated preference are, therefore, decoupled with the preference being exhibited long after shoaling begins.
Figure 4. Onset of preference.
For each stage the mean time in association with both wild-type (wt, filled circles) and nacre (nac, open circles) shoals is shown. Error bars represent the 95% confidence intervals. Early flexion larvae (two-tailed paired Student’s t-test: N = 18, t17 = 0.253, P = 0.80), post flexion (two-tailed paired Student’s t-test: N = 20, t19 = −0.977, P = 0.34) and metalarvae (two-tailed paired Student’s t-test: N = 16, t15 = 0.065, P > 0.9) showed no preference. While juveniles (two-tailed paired Student’s t-test: N = 20, t19 = −2.83, P = 0.010) and adults (two-tailed paired Student’s t-test: N = 20, t19 = −2.44, P = 0.025) showed a significant preference for the wild-type shoals. Asterisks denote stages exhibiting a significant preference.
Plasticity of preference
We know that early life experience plays a key role in the acquisition of shoaling preference (Engeszer et al., 2004), but we did not know if changes in the subsequent social environment might cause changes in this shoaling preference. Does the preference remain labile throughout the life of the fish, or is the preference immutable once it has been established? After rearing zebrafish with wild-type shoal-mates and testing their preference, we then manipulated their social environment, housing half with wild-type shoals (controls) and half with nacre shoals (cross-rears) for one month.
Even with prolonged exposure to other fish exhibiting a dramatically different pigment pattern, the preference of subject fish for wild-type shoals did not change. Neither control nor cross-reared subjects showed a significant change in the proportion of time they spent with the wild-type stimulus shoal (two-tailed paired Student’s t-tests: controls, N = 18, t17 = 0.051, P > 0.5; cross-rears, N = 15, t14 = 1.650, P > 0.1). Thus, the visually mediated shoaling preference was stable in this assay and may be resistant to change during later life.
DISCUSSION
This work shows that zebrafish shoal relatively early in post-embryonic development, they exhibit a visual preference for certain shoal-mates much later in development, and this preference appears immutable once it has been established. At very early stages, zebrafish do not shoal as seen in the failure of preflexion larvae to respond to the protocol. This lack of response is hardly surprising. Preflexion larvae spend their time adhering to nearby surfaces via their adhesive organs and swim only when startled. Early flexion larvae, while far more mobile than preflexion larvae, spent significantly more time in the “no preference” area than all of the later stage larvae with the exception of the metalarvae. These data do not exclude the possibility that early flexion larvae are shoaling, but they do show that shoaling occurs very early in post embryonic development and, by the postflexion stage, zebrafish are clearly shoaling.
The relatively late appearance of the visually mediated preference is interesting given the relatively early onset of shoaling behaviour. Though juveniles and adults exhibit a robust preference for the reared stimulus phenotype, earlier stages do not. Zebrafish posses a functional visual system at the preflexion stage (Schmitt & Dowling, 1999). Preflexion larvae can recognize food (Kimmel et al., 1995) and respond to shadows passing over them (Easter & Nicola, 1996), but the full complement of photoreceptors is not expressed until approximately 12 days post fertilization (Branchek & Bremiller, 1984). This timing roughly coincides with the transition from preflexion larva to postflexion larva (Engeszer, unpub. data). At this point in development all of the receptor types are present and functioning in the retinal mosaic, which continues to grow in size as the zebrafish grows to adulthood (Branchek & Bremiller, 1984). Ontogenetic changes in the visual system during the larval stages and through metamorphosis could, therefore, explain the late onset of the visually mediated preference. Alternatively changes in the higher processing of visual signals in the optic pathways of the brain may account for the ontogenetic variation in the preference.
The appearance of the preference also coincides with the emergence of the post-metamorphic, or adult, pigment pattern (Parichy & Turner, 2003). The larval pigment pattern of three melanophore stripes, one dorsal, one medial and one ventral, is conserved throughout the close relatives of D. rerio (Quigley et al., 2004; Parichy, 2006). This larval pigment pattern is further conserved throughout the cyprinids and is even found in the related Catostomidae (Snyder & Muth, 2004). Zebrafish inhabit waters with a number of other cyprinids and one, Puntius shalynius, spawns in rice paddies in which zebrafish larvae and juveniles are found (Engeszer et al., in press). In situations where larval cyprinids co-occur visual cues would be a poor indicator of conspecific shoal-mates. Unlike their larvae, adult danios and, more broadly, adult cyprinids, exhibit dramatically different pigment patterns (Quigley et al., 2005). The late onset of the visually mediated shoaling preference, thus, co-occurs with the stage at which zebrafish would be able to visually distinguish between conspecific and heterospecific fishes.
Further observations regarding zebrafish life history lend insight into possible selective advantages of a late onset shoaling preference. Larval and juvenile zebrafish inhabit rice paddies and seasonal waters that may provide refuge from predators (Engeszer et al., in press) and the selective pressures on these fish to shoal only with fish of a similar phenotype may be greatly reduced. The onset of the preference coincides with the stage at which fish are leaving these refugia and coming into contact with piscine predators. At this point the oddity effect (Landeau & Terborgh, 1986) implies a significant survival cost to individuals for shoaling with fish of a different phenotype, as individuals that do so will be preferentially preyed upon. Changing selective regimes as these fish move from one area to another may account for the late appearance of the visual preference.
Given the importance of the early social environment in the determination of the visual preference, larval zebrafish must either be using a different aspect of the visual signal, such as overall size of the shoaling fish, or an alternative sensory modality to assess shoal-mates. Strong evidence exists for the use of olfactory cues in fishes in general and zebrafish in particular in the identification of conspecifics (McLennan & Ryan, 1997) and even relatives (Mann et al., 2003). If larval zebrafish identify conspecifics using a different sensory modality, they would associate with conspecifics and be exposed to their pigment pattern during preference acquisition.
Finally, our data suggest the visual preference of zebrafish may be immutable once it is established. Individuals raised with wild-type fish and later cross-reared with nacre fish did not lose their preference for wild-type shoals. Since the adult pigment pattern develops rather late, the subject fish had less than a month of exposure to it before they were tested and shifted into their new social environment. Yet even spending a month with extremely different social consorts did not significantly change the preference exhibited. This result, coupled with early cross-rearing experiments, strongly suggests that there exists a critical period during which the visually mediated shoaling preference is acquired, after which time it is unchangeable. This situation is reminiscent of the process of song learning in birds (Marler & Tamura 1964). Social behaviour in zebrafish could provide a fascinating parallel system for the study of this type of learning and the arsenal of tools available to investigate changes at the neurological and molecular level in zebrafish could make the investigation of the mechanisms underlying the establishment of this visual preference particularly exciting.
Acknowledgments
R.E.E. was supported by an NSF Predoctoral Fellowship and University of Washington set-up funds to D.M.P. Other support for this research came from NSF IOB-0541733 and NIH R01 GM62182 to D.M.P. We thank C. Lee and other members of the Parichy lab for discussions and assistance with fish rearing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baerends GP, Roon JMB-v. An introduction to the study of the ethology of cichlid fishes. Behaviour. 1950;(suppl 1):1–242. [Google Scholar]
- Bowen ES. The role of the sense organs in aggregations of Ameiurus melas. Ecological Monographs. 1931;1:1–35. [Google Scholar]
- Bowen ES. Further studies of the aggregating behavior of Ameiurus melas. Biological Bulletin. 1932;63:242–270. [Google Scholar]
- Branchek T, Bremiller R. The development of the photoreceptors in the zebrafish, Brachydanio rerio. I. Structure. Journal of Comparative Neurology. 1984;224:107–115. doi: 10.1002/cne.902240109. [DOI] [PubMed] [Google Scholar]
- Breder CM, Halpern F. Innate and acquired behavior affecting the aggregation of fishes. Physiological Zoology. 1946;19:154–190. doi: 10.1086/physzool.19.2.30151891. [DOI] [PubMed] [Google Scholar]
- Darrow KO, Harris WA. Characterization and development of courtship in zebrafish, Danio rerio. Zebrafish. 2004;1:40–45. doi: 10.1089/154585404774101662. [DOI] [PubMed] [Google Scholar]
- Easter SS, Nicola GN. The development of vision in the zebrafish (Danio rerio) Developmental Biology. 1996;180:646–663. doi: 10.1006/dbio.1996.0335. [DOI] [PubMed] [Google Scholar]
- Engeszer RE, Ryan MJ, Parichy DM. Learned social preference in zebrafish. Current Biology. 2004;14:881–884. doi: 10.1016/j.cub.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Engeszer RE, Patterson LB, Parichy DM. Zebrafish in the wild: a review of natural history and new notes from the field. 2006 doi: 10.1089/zeb.2006.9997. submitted. [DOI] [PubMed] [Google Scholar]
- Hultsch H, Todt D. Learning to sing. In: Marler P, Slabbekoorn H, editors. Nature’s Music: The Science of Birdsong. San Diego: Elsevier Academic Press; 2004. pp. 80–107. [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Developmental Dynamics. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Landeau L, Terborgh J. Oddity and the confusion effect in predation. Animal Behaviour. 1986;38:1372–1380. [Google Scholar]
- Lister JA, Robertson CP, Lepage T, Johnson SL, Raible DW. nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development. 1999;126:3757–67. doi: 10.1242/dev.126.17.3757. [DOI] [PubMed] [Google Scholar]
- Mann KD, Turnell ER, Atema J, Gerlach G. Kin recognition in juvenile zebrafish (Danio rerio) based on olfactory cues. Biological Bulletin. 2003;205:224–5. doi: 10.2307/1543264. [DOI] [PubMed] [Google Scholar]
- Marler P, Tamura M. Culturally transmitted patterns of vocal behavior in sparrows. Science. 1964;146:1483–1486. doi: 10.1126/science.146.3650.1483. [DOI] [PubMed] [Google Scholar]
- McLennan DA, Ryan MJ. Responses to conspecific and heterospecific olfactory cures in the swordtail Xiphophorus cortezi. Animal Behaviour. 1997;54:1077–1088. doi: 10.1006/anbe.1997.0504. [DOI] [PubMed] [Google Scholar]
- Orger MB, Baier H. Channeling of red and green cone inputs to the zebrafish optomotor response. Visual Neuroscience. 2005;22:275–281. doi: 10.1017/S0952523805223039. [DOI] [PubMed] [Google Scholar]
- Parichy DM. Evolution of danio pigment pattern development. Heredity. 2006;97:200–210. doi: 10.1038/sj.hdy.6800867. [DOI] [PubMed] [Google Scholar]
- Parichy DM, Turner JM. Zebrafish puma mutant decouples pigment pattern and somatic metamorphosis. Developmental Biology. 2003;256:242–57. doi: 10.1016/s0012-1606(03)00015-0. [DOI] [PubMed] [Google Scholar]
- Pitcher TJ, Parrish JK. Functions of shoaling behaviour in teleosts. In: Pitcher TJ, editor. Behaviour of teleost fishes. London: Chapman & Hall; 1993. pp. 363–439. [Google Scholar]
- Quigley IK, Manuel JL, Roberts RA, Nuckels RJ, Herrington ER, MacDonald EL, Parichy DM. Evolutionary diversification of pigment pattern in Danio fishes: differential fms dependence and stripe loss in D. albolineatus. Development. 2005;132:89–104. doi: 10.1242/dev.01547. [DOI] [PubMed] [Google Scholar]
- Quigley IK, Turner JM, Nuckels RJ, Manuel JL, Budi EH, MacDonald EL, Parichy DM. Pigment pattern evolution by differential deployment of neural crest and post-embryonic melanophore lineages in Danio fishes. Development. 2004;131:6053–6069. doi: 10.1242/dev.01526. [DOI] [PubMed] [Google Scholar]
- Rosenthal GG, Ryan MJ. Assortative preferences for stripes in danios. Animal Behaviour. 2005;70:1063–1066. [Google Scholar]
- Schmitt EA, Dowling JE. Early retinal development in the zebrafish, Danio rerio: Light and electron microscopic analyses. Journal of Comparative Neurology. 1999;404:515–536. [PubMed] [Google Scholar]
- Schneirla TC. The concept of development in comparative psychology. In: Harris DB, editor. The concept of development. Minneapolis: University of Minnesota Press; 1957. [Google Scholar]
- Shaw E. The development of schooling behavior in fishes. Physiological Zoology. 1960;33:79–86. [Google Scholar]
- Snyder DE, Muth RT. Colorado Divison of Wildlife Technical Publication. Vol. 42 2004. Catostomid fish larvae and early juveniles of the Upper Colorado River Basin---morphological descriptions, comparisons, and computer-interactive key. [Google Scholar]
- Wright D, Rimmer LB, Pritchard VL, Krause J, Butlin RK. Inter and intra-population variation in shoaling and boldness in the zebrafish (Danio rerio) Naturwissenschaften. 2003;90:374–377. doi: 10.1007/s00114-003-0443-2. [DOI] [PubMed] [Google Scholar]