Abstract
DNA bases carrying an exocyclic amino group, namely adenine (A), guanine (G) and cytosine (C), encounter deamination under nitrosative stress. Oxanine (O), derived from deamination of guanine, is a cytotoxic and potentially mutagenic lesion and studies of its enzymatic repair are limited. Previously, we reported that the murine alkyladenine glycosylase (Aag) acts as an oxanine DNA glycosylase (JBC, (2004), 279: 38177). Here, we report our recent findings on additional oxanine DNA glycosylase (ODG) activities in Aag knockout mouse tissues and other mammalian tissues. Analysis of the partially purified proteins from the mammalian cell extracts indicated the existence of ODG enzymes in addition to Aag. Data obtained from oxanine DNA cleavage assays using purified human glycosylases demonstrated that two known glycosylases, hNEIL1 and hSMUG1, contained weak but detectable ODG activities. ODG activity was the highest in hAAG and lowest in hSMUG1.
Keywords: deamination, oxanine DNA glycosylase, AAG, NEIL1, SMUG1, mammals
1. Introduction
The DNA base guanine (G) is subject to deamination caused by endogenous and environmental agents [1-4]. Xanthine (X) and Oxanine (O) are the corresponding deamination products derived from guanine [5]. Oxanine is produced when deoxyguanosine or DNA is treated with nitrous acid, nitric oxide, or 1-nitrosoindole-3-acetonitrile. Oxanine is an intracyclic guanine deamination product in which the N1 nitrogen is substituted by an oxygen atom [5,6]. A chemical pathway leading to the formation of xanthine and oxanine from guanine by nitrous acid treatment has been proposed based on the isolation of a diazoate intermediate [7]. Oxanine in DNA is as stable as guanine by comparison of spontaneous hydrolysis of the N-glycosidic bond [8].
Oxanine is promutagenic as demonstrated by in vitro DNA polymerase studies. Deoxyoxanosine triphosphate (dOTP) can be incorporated to pair with both a C or T in DNA by the Klenow Fragment, thus generating G/C to A/T transitions [9]. Previously, we investigated the mutagenicity of oxanosine in mammalian systems using human DNA polymerase β [10]. While dOTP was preferentially incorporated to pair with a C-containing template, dCTP, dATP, dTTP, and to a much lesser extent dGTP, was incorporated to pair with an O-containing template. These results reveal the potential mutagenicity of oxanine in mammalian systems.
Mammalian alkyladenine DNA glycosylase (AAG in humans, Aag in mice) is a repair enzyme with broad substrate specificity [11]. Both alkylated and deaminated bases are substrates for this enzyme. It is well documented that hAAG acts on hypoxanthine, a deaminated product from adenine [12]. Recent studies also show that AAG can excise xanthine, a deaminated base from DNA [13,14]. Previously, we determined that mAag was a major oxanine DNA glycosylase in mammalian tissues through examination of ODG activity in spleen tissue of wild type and Aag knockout (KO, -/-) mice and through examination of purified human AAG protein. Here, we investigated ODG activity in various tissues of Aag (-/-) mice. In lung tissues, significant additional ODG activity was detected in Aag (-/-) mice. Biochemical fractionation followed by enzyme activity assays and Western blot analysis indicated that SMUG1, an enzyme previously identified as a uracil DNA glycosylase, contained ODG activity. Analysis of a panel of human glycosylases also reveals ODG activity from hNEIL1. hAAG possesses the highest ODG activity while hSMUG1 appears the least active.
2. Materials and Methods
2.1. Reagents, media and strains
All routine chemical reagents were purchased from Sigma Chemicals (St. Louis, MO), Fisher Scientific (Suwanee, GA) or VWR (Suwanee, GA). E. coli Klenow Fragment (3’ exo-) was purchased from New England Biolabs (Beverly, MA). BSA and dNTPs, were purchased from Promega (Madison, WI). Deoxyribooligonucleotides were ordered from Integrated DNA Technologies Inc. (Coralville, IA). 5-hydroxyuracil-containing Deoxyribooligonucleotide was ordered from The Midland Certified Reagent Company (Midland, TX). Protein assay kit, the horseradish peroxidase substrate Opti-4CN for western blot and PVDF membrane were purchased from Bio-Rad (Hercules, CA). Anti-His (C-term)-HRP antibody was purchased from Invitrogen (Carlsbad, CA). SMUG1 polyclonal antibody against the common peptide (GMNPGPFGMAQTG) in hSMUG1, mouse SMUG1 and pig SMUG1 was synthesized by Sigma-Genosys (The woodlands, TX). NEIL1 polyclonal antibody was prepared as previously described [15]. HiTrap Q columns were purchased from GE Healthcare Life Sciences (Piscataway, NJ). GeneScan Stop Buffer consisted of 80% formamide (Amresco, Solon, OH), 50 mM EDTA (pH 8.0), and 1% blue dextran (Sigma Chemicals).
2.2. Construction of oxanosine-containing oligodeoxyribonucleotide substrates
Deoxyoxanosine 3’-triphosphate (dOTP) was generated by treating deoxyguanosine 3’-triphosphate (dGTP, Sigma) with acidified sodium nitrite and purified by reverse-phase HPLC as previously described [10]. Single oxanosine (O)-containing oligonucleotide was constructed by primer extension with dOTP and regular dNTPs as previously described [10]. This 62-mer O-containing strand was annealed with a 66-mer complementary strand at a ratio of 1:1.5 to form C/O, T/O, A/O and G/O base pairs (Fig. 1A).
Figure 1. Oxanine DNA glycosylase activities in various tissues.
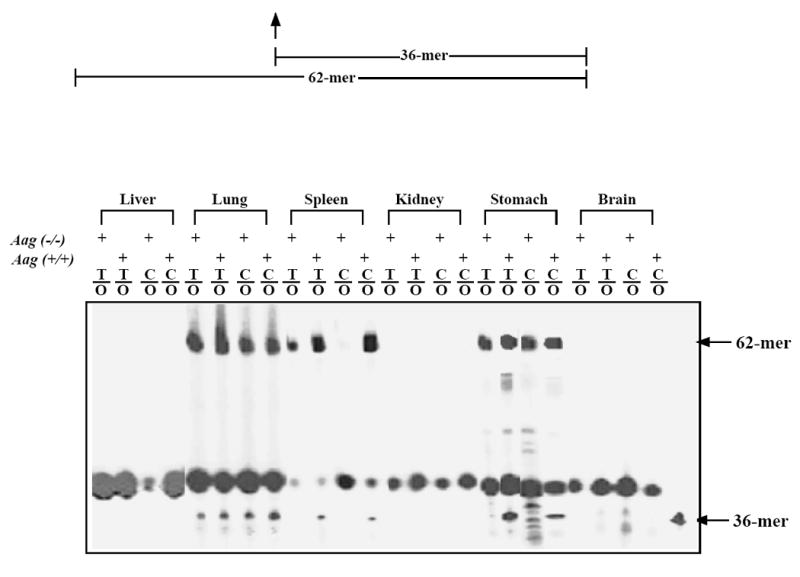
A. Sequence of O-containing duplex oligonucleotide. FAM, 6-carboxyfluorescein. B. ODG activities in cell extracts from wild type and Aag KO mice. The DNA glycosylase assays were carried out at 37°C for 60 min in a 10 μl volume containing 9 μl (about 100 μg proteins) of Aag (-/-) or matched Aag (+/+) cell extract and 1 μl of 100 nM substrate. M, 36-mer length marker.
2.3. Preparation of mammalian cell extracts
Pig lung tissues (2000 g) were homogenized in a Warring blender with 2L of extraction buffer containing 20 mM Tris-HCl (pH 8.0), 1 mM EDTA, 0.2 mM DTT, 0.1 mM PMSF (phenylmethylsulfonyl fluoride), 0.5 μg/ml pepstatin and 0.5 μg/ml leupeptin at 4°C. The extract was filtered through cheesecloth. Cell debris was removed by centrifugation at 15,000 g for 10 min in a Beckman Coulter Avanti J-25 centrifuge. Proteins were precipitated by adding ammonium sulfate to 80% saturation. After dialysis using Spectrum Spectra/Por 10 kD membrane against the dialysis buffer containing 20 mM Tris-HCl (pH 8.0), 1 mM EDTA and 0.2 mM DTT, the supernatant was precipitated by step-wise ammonium sulfate saturation from 20% to 80% with 10% increments.
Aag (-/-) and matched Aag (+/+) mouse tissues were thawed on ice in 2.5 volumes of glycosylase assay buffer containing 20 mM Tris-HCl (pH 7.2), 100 mM KCl, 5 mM EDTA, 5 mM 2-mercaptoethanol as previously described [16]. Tissues were homogenized on ice in a 7-ml Dounce homogenizer (Wheaton). Cell debris was removed by centrifugation at 18,000 g for 30 min in a refrigerated microcentrifuge. The supernatants were stored at −70°C prior to glycosylase activity assays.
2.4. DNA glycosylase activity assays
DNA glycosylase cleavage assays were performed at 37°C for 60 min in a 10 μl reaction mixture containing 10 nM double-stranded or single-stranded oligonucleotide substrate, indicated amount of glycosylase or cell extracts, 20 mM Tris-HCl (pH 7.2), 100 mM KCl, 5 mM EDTA and 2 mM 2-mercaptoethanol as previously described [16]. The resulting abasic sites were cleaved by incubating at 95°C for 5 min after adding 0.5 μl of 1 N NaOH. Reactions were quenched by addition of an equal volume of GeneScan stop buffer. Samples (3.5 μl) were loaded onto a 7 M urea-10% denaturing polyacrylamide gel. Electrophoresis was conducted at 1500 voltage for 1.6 hr using an ABI 377 sequencer (Applied Biosystems). Cleavage products and remaining substrates were quantified using GeneScan analysis software.
2.5. Western blot analysis
Western Blot analysis was carried out using SMUG1 and NEIL1 antibodies. The protein samples were first separated on 12.5% SDS-PAGE, and then transferred onto a PVDF membrane by electro-blotting at 100 V for 1 h using a Bio-Rad Mini Trans-Blot apparatus. The membrane was probed with SMUG1 antibody (1:250 dilution) or NEIL1 antibody (1:5000 dilution), respectively; Horseradish peroxidase conjugated mouse anti-rabbit IgG (Santa Cruz Biotechnology) was used as secondary antibody. The color reaction was developed using Opti-4CN as a substrate.
3. Results and Discussion
3.1. ODG activities in Aag KO tissues
Oxanine DNA glycosylase activities have been detected in mammalian extracts and the major activity in mouse spleen tissues was attributed to the murine alkyladenine DNA glycosylase, Aag [10]. To further understand deaminated base repair in mammalian systems, we examined oxanine DNA glycosylase activities in various other tissues from the Aag knockout (KO) mice and matched wild type mice. Protein extracts were prepared from liver, lung, spleen, kidney, stomach, and brain tissues. The initial glycosylase assay was performed using T/O and C/O substrates (Fig. 1A). Oxanine DNA glycosylase activity was not detected in liver, kidney and brain due to nonspecific degradation of the oligonucleotide substrates (Fig. 1B). ODG activity was found in the spleen tissue of the wt mice but not in the spleen tissue of Aag KO mice. Consistent with previous studies, these results indicate that Aag is the primary ODG activity in this tissue [10]. Aag also appeared to be the major source of ODG activity in stomach, although the existence of additional ODG could not be ruled out (Fig. 1B). ODG activity was detected in extracts from lung in both the wt and Aag KO mice (Fig. 1B). The intensities of the cleavage bands were higher in the wt tissue, suggesting that the ODG activities in lung might come from Aag plus other glycosylases (Fig. 1B).
To more thoroughly investigate the ODG activities in lung, we assayed ODG activities using all five O-containing substrates, C/O, T/O, G/O, A/O and single-stranded O (Fig. 2). As expected, the wt tissue showed strong activities against all O-containing substrates. This is in keeping with our previous finding that mammalian Aag is active towards both double-stranded and single-stranded O-containing substrates [10]. Interestingly, all five O-containing substrates were also cleaved in the Aag KO tissue, indicating that the additional ODG can act on both double-stranded and single-stranded DNA (Fig. 2).
Figure 2. ODG activities in lung cell extracts from wild type and Aag KO mice.
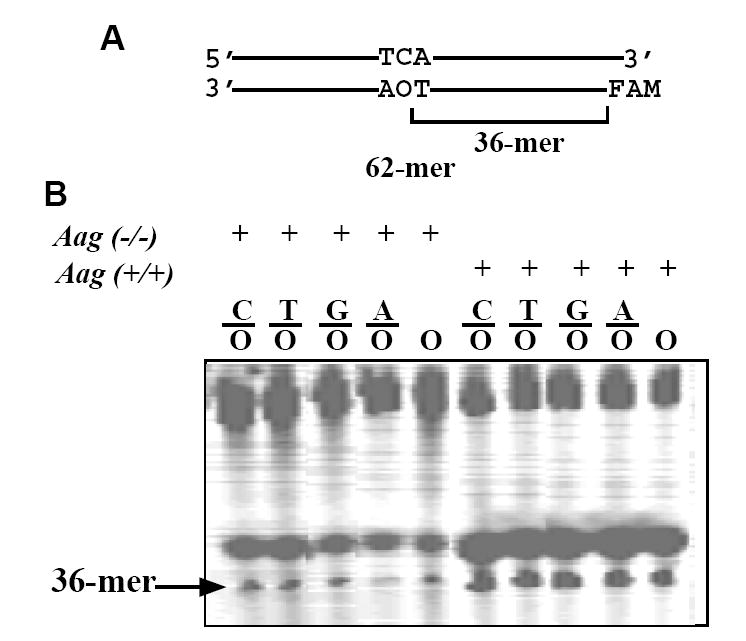
The DNA glycosylase assays were carried out at 37°C for 60 min in a 10 μl volume containing 9 μl of Aag (-/-) or matched Aag (+/+) lung cell extract and 1 μl of 100 nM substrate.
3.2. Separation of ODG activities in pig lung tissues
To identify the additional ODG enzymes in lung tissues, we set out to separate ODG activities in large animal tissues. Crude protein extracts were prepared using lung tissues from pigs and fractionated by stepwise ammonium sulfate precipitation. Since Aag is a hypoxanthine DNA glycosylase (HDG), we assayed for the activity using a T/I substrate (Fig. 3A). The HDG activity was found mainly in 30-50% ammonium sulfate saturation (Fig. 3A). On the other hand, ODG activities as represented by cleavage of C/O substrate was detected in all fractions. Thus, whereas the ODG activities in 30-50% cuts may primarily come from Aag, ODG activities in other fractions may represent previously unknown activities. We then pooled 60-80% fractions together and pursued further separation by Q column ion exchange chromatography. ODG activity was found in fraction 44 (Fig. 3B). To verify the source of this additional ODG activity, we performed hypoxanthine DNA glycosylase assay in all fractions after Q column chromatography. HDG activity was not found in any of the fractions, indicating that Aag was not the source of the ODG activity (data not shown).
Figure 3. ODG activities in fractionated samples.
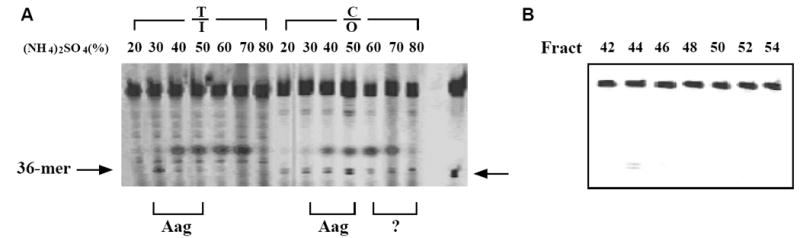
Pos: positive control lane in which the C/O substrate was treated with hAAG. A. Ammonium sulfate fractions of crude extracts from pig lungs. Crude extracts prepared from pig lung tissues as described in Materials and Methods were precipitated by step-wise ammonium sulfate saturation. I: inosine or hypoxanthine. The inosine-containing strand of the T/I substrate was labeled with FAM fluorophore [34]. The oxanine DNA glycosylase assays were conducted in a reaction mixture (10 μl) containing 10 nM fluorescently labeled oligonucleotide substrate (T/I, C/O), 2 μl of ammonium sulfate-fractionated cell extract, 20 mM Tris-HCl (pH 7.2), 100 mM KCl, 5 mM EDTA and 2 mM 2-mercaptoethanol. After incubating at 37°C for 60 min, the reaction mixtures were supplemented with 0.5 μl of 1 N NaOH and incubated at 95°C for 5 min to cleave the resulting AP sites from glycosylase action. B. Q column separation of ODG activities. Ammonium sulfate fractions (60-80%) of pig lung tissues were applied to a 100 ml Q column and eluted by a salt gradient (0-1M NaCl). The elution volume was 500 ml and collected as 10 ml per fraction. The ODG activity assay was conducted in a reaction mixture (10 μl) containing 10nM fluorescently labeled oligonucleotide substrate (C/O), 2 μl of fraction and other components as detailed in Materials and Methods.
3.3. Identification of ODG activities in NEIL1 and SMUG1
To determine whether any known mammalian DNA glycosylase was accountable for the additional ODG activity, we performed ODG activity assay on all known human DNA glycosylases. Among the nine enzymes tested, we observed weak ODG activities in hNEIL1 and hSMUG1 (Fig. 4A). ODG activities from both hNEIL1 and hSMUG1 were active on both double-stranded and single-stranded substrates (Fig. 4A).
Figure 4. Examination of ODG activity of purified human DNA glycosylases.
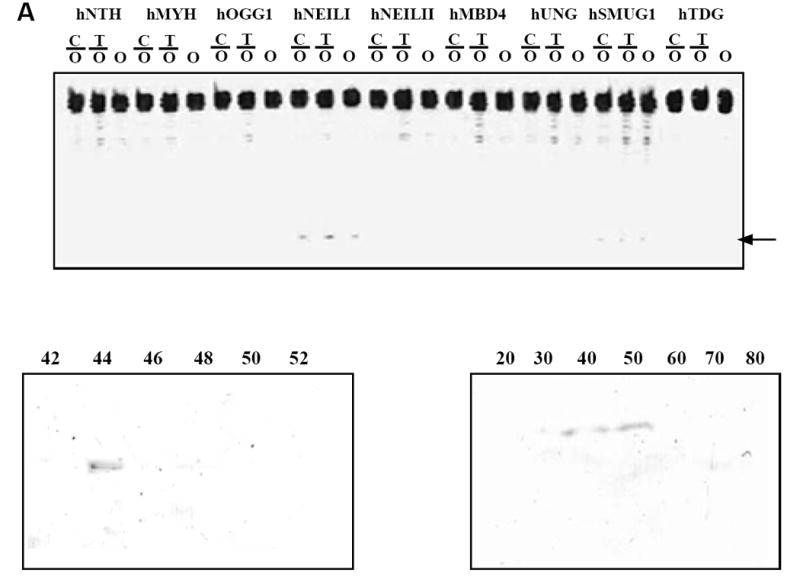
A. ODG activity of purified human DNA glycosylases. Glycosylase assays were performed as described in Materials and Methods with 100 nM human glycosylase. B. Western blot analysis using anti-SMUG1 antibody on Q column fractions. C. Western blot analysis using anti-NEIL1 antibody on fractions from ammonium sulfate precipitation.
To determine whether the ODG activity observed in fraction 44 was attributable to NEIL1 or SMUG1, we performed a Western blot analysis using NEIL1 and SMUG1 antibodies. While the NEIL1 antibody showed negative results against all Q column fractions, the SMUG1 antibody was positive in fraction 44. These results suggested that the additional ODG activity can be attributed to SMUG1 enzyme (Fig. 4B).
To determine the fractions that contained NEIL1 protein, we performed Western blot analysis using all fractions from ammonium sulfate precipitation. NEIL1 was found in 40-50% fractions (Fig. 4C), suggesting that NEIL1 was not separated from Aag by ammonium sulfate precipitation.
Since hAAG, hNEIL1 and hSMUG1 all contained ODG activities, we compare their activities using all five O-containing substrates and purified enzymes (Fig. 5). hAAG possessed the highest ODG activity against all five substrates. After one-hr incubation, around 35% of substrates were cleaved by hAAG, 8% by hNEIL1 and 3% by hSMUG1. All three enzymes were equally active on both double-stranded and single-stranded DNA. The low glycosylase activities of hNEIL1 and hSMUG1 against oxanine appear to be intrinsic to the enzymes. Assayed under the same reaction conditions, hNEIL1 and hSMUG1 showed about 39% cleavage of 5-hydroxyuracil (OHU)-containing substrate (G/OHU) and about 42% cleavage of G/U substrate, respectively (Fig. 5A). These levels of glycosylase activities are comparable to the values previously reported [17-19]. The ODG activity from hAAG observed in this work is also similar to an earlier study performed in our laboratory although they are different batches of hAAG protein [10].
Figure 5. Cleavage of O-containing substrates by hAAG, hNEIL1 and hSMUG1.
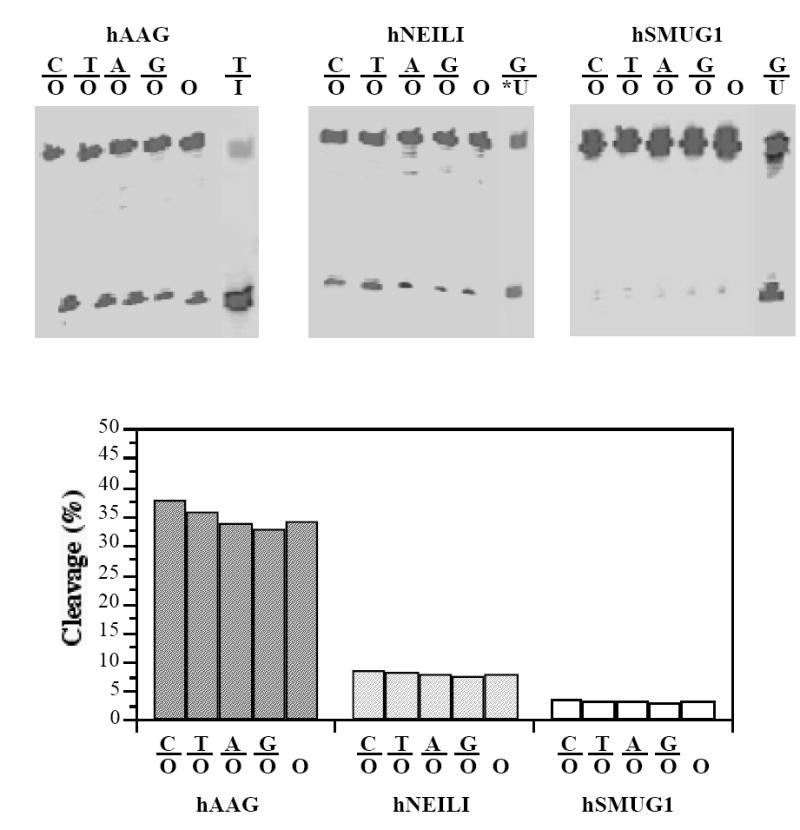
The ODG assays were conducted as described in Materials and Methods in a reaction mixture containing 10 nM fluorescently labeled oligonucleotide substrate (C/O, T/O, A/O, G/O and single-stranded O) and 100 nM of purified human glycosylase. A. Representative Genescan gel picture of ODG activity. I, hypoxanthine; *U, 5-hydroxyuracil; U, uracil. I-containing strand is a 60-mer (FAM-CCCGCAACAAGACTAGAGGATCAAACTATGA CAACTIACGCAACACCGCAGACGCTGGGG-3’). *U-containing strand is identical to the 62-mer shown in Fig. 1A except that O is replaced by 5-hydroxyuracil (Midland). U-containing strand is a 60-mer identical to the I-containing strand except that hypoxanthine (I) is replaced by uracil. B. Quantification of ODG activity. Cleavage products and remaining substrates were quantified using GeneScan analysis software.
3.4. Mammalian oxanine DNA glycosylases
Oxanine is a deaminated product of guanine generated by substitution of N1-nitrogen with oxygen [7]. The mutagenic nature of this DNA lesion has been studied using E. coli and human DNA polymerases [9,10,20,21]. Bacterial base excision repair (BER) enzymes that can act on oxanine have been investigated using purified proteins from E. coli. It was reported that E. coli AlkA, endo VIII and Fpg contained weak ODG activities [22,23]. In addition to enzymes in BER, bacterial endonuclease V can also cleave both double-stranded and single-stranded O-containing DNA [21]. However, the mode of action is different from DNA glycosylases since endonuclease V is an authentic endonuclease.
In mammalian systems, Aag appears to be the primary source of ODG in some tissues [10]. Using Aag KO mice, this study reveals additional sources of ODG activities in some tissues such as lung. The ODG activity in the 20% ammounium sulfate fraction may represent a novel unknown ODG activity. Further experiments are needed to uncover the nature of this repair activity. The detection of ODG activity from NEIL1 by this investigation and a previous study indicates mammalian NEIL1 is a broad substrate DNA glycosylase [23]. This is consistent with the previous observation that human NEIL1 is active towards a variety of oxidatively damaged base lesions [17,24]. NEIL2 is another homolog of E. coli endonuclease VIII found in mammalian systems. In keeping with its narrow substrate specificity, we did not find ODG activity in NEIL2. These results suggest that mammalian NEIL1 and NEIL2 have adopted a different path in evolving their substrate specificities.
Another significant finding from this study is detection of ODG activity from SMUG1. SMUG1 was initially identified as a single-stranded uracil DNA glycosylase but later found active on double-stranded uracil-containing DNA and some uracil derivatives [19,25-28]. Based on the crystal structure of SMUG1-DNA complex, it was proposed that the base recognition pocket of SMUG1 resembles the chimeric active site of UNG and MUG/TDG [29,30]. Although human TDG was considered a T/G and U/G DNA glycosylase, it showed weak activity against some hypoxanthine-containing DNA ([31] and H. Lee and W. Cao, unpublished data). In addition to the ODG activity reported here, we also detected xanthine DNA glycosylase activity in hSMUG1 (L. Dong and W. Cao, unpublished data). Taken together, these data indicate that the active sites of SMUG1 and MUG/TDG family proteins are more plastic than previously recognized, which allow them to excise both pyrimidine and purine deaminated base damages. Despite of its weak catalytic activity (Fig. 5), the fact that we were able to detect ODG in column-separated fractions is consistent with high level of uracil DNA glycosylase activity from SMUG1 in lung tissues [25]. It is well known that reactive nitrogen species cause DNA base deamination [3,32,33]. Since lungs are the largest gas exchange tissue that are exposed to reactive nitrogen species, SMUG1 may play a significant role in repair of deaminated base damage in lung.
Acknowledgments
This project was supported in part by CSREES/USDA (SC-1700274, technical contribution No. 5321), DOD-Army Research Office (W911NF-05-1-0335) and the Concern Foundation. LDS is an American Cancer Society Research Professor and supported by NIH Grants ES02109 and CA75576. We thank Dr. Yoshihiro Matsumoto for providing MYH; Dr. Tim O’Connor for full-length AAG and hMBD4; Dr. Rabindra Roy for hNTH1; Dr. Geir Sluphaug for hUNG and hSMUG1; Dr. Primo Schär for hTDG; and Jonathan Campbell at Clemson University Meat Laboratory for mammalian tissues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 2.Spencer JP, Whiteman M, Jenner A, Halliwell B. Nitrite-induced deamination and hypochlorite-induced oxidation of DNA in intact human respiratory tract epithelial cells. Free Radic Biol Med. 2000;28:1039–1050. doi: 10.1016/s0891-5849(00)00190-8. [DOI] [PubMed] [Google Scholar]
- 3.Burney S, Caulfield JL, Niles JC, Wishnok JS, Tannenbaum SR. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res. 1999;424:37–49. doi: 10.1016/s0027-5107(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro R. Damage to DNA caused by hydrolysis. In: Seeberg E, Kleppe K, editors. Chromosome Damage and Repair. Plenum Press; New York: 1981. pp. 3–18. [Google Scholar]
- 5.Suzuki T, Yamaoka R, Nishi M, Ide H, Makino K. Isolation and characterization of a novel product, 2’-deoxyoxanosine, from 2’-deoxyguanosine, oligodeoxynucleotide and calf thymus DNA treated by nitrous-acid and nitric-oxide. J Am Chem Soc. 1996;118:2515–2516. [Google Scholar]
- 6.Lucas LT, Gatehouse D, Shuker DE. Efficient nitroso group transfer from N-nitrosoindoles to nucleotides and 2’-deoxyguanosine at physiological pH. A new pathway for N- nitrosocompounds to exert genotoxicity. J Biol Chem. 1999;274:18319–18326. doi: 10.1074/jbc.274.26.18319. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki T, Ide H, Yamada M, Endo N, Kanaori K, Tajima K, Morii T, Makino K. Formation of 2’-deoxyoxanosine from 2’-deoxyguanosine and nitrous acid: mechanism and intermediates. Nucleic Acids Res. 2000;28:544–551. doi: 10.1093/nar/28.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki T, Matsumura Y, Ide H, Kanaori K, Tajima K, Makino K. Deglycosylation susceptibility and base-pairing stability of 2’-deoxyoxanosine in oligodeoxynucleotide. Biochemistry. 1997;36:8013–8019. doi: 10.1021/bi970166l. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki T, Yoshida M, Yamada M, Ide H, Kobayashi M, Kanaori K, Tajima K, Makino K. Misincorporation of 2’-deoxyoxanosine 5’-triphosphate by DNA polymerases and its implication for mutagenesis. Biochemistry. 1998;37:11592–11598. doi: 10.1021/bi980971f. [DOI] [PubMed] [Google Scholar]
- 10.Hitchcock TM, Dong L, Connor EE, Meira LB, Samson LD, Wyatt MD, Cao W. Oxanine DNA glycosylase activity from Mammalian alkyladenine glycosylase. J Biol Chem. 2004;279:38177–38183. doi: 10.1074/jbc.M405882200. [DOI] [PubMed] [Google Scholar]
- 11.Wyatt MD, Allan JM, Lau AY, Ellenberger TE, Samson LD. 3-methyladenine DNA glycosylases: structure, function, and biological importance. Bioessays. 1999;21:668–676. doi: 10.1002/(SICI)1521-1878(199908)21:8<668::AID-BIES6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Saparbaev M, Laval J. Excision of hypoxanthine from DNA containing dIMP residues by the Escherichia coli, yeast, rat, and human alkylpurine DNA glycosylases. Proc Natl Acad Sci U S A. 1994;91:5873–5877. doi: 10.1073/pnas.91.13.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wuenschell GE, O’Connor TR, Termini J. Stability, miscoding potential, and repair of 2’-deoxyxanthosine in DNA: implications for nitric oxide-induced mutagenesis. Biochemistry. 2003;42:3608–3616. doi: 10.1021/bi0205597. [DOI] [PubMed] [Google Scholar]
- 14.Dong M, Vongchampa V, Gingipalli L, Cloutier JF, Kow YW, O’Connor T, Dedon PC. Development of enzymatic probes of oxidative and nitrosative DNA damage caused by reactive nitrogen species. Mutat Res. 2006;594:120–134. doi: 10.1016/j.mrfmmm.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Mokkapati SK, Wiederhold L, Hazra TK, Mitra S. Stimulation of DNA glycosylase activity of OGG1 by NEIL1: functional collaboration between two human DNA glycosylases. Biochemistry. 2004;43:11596–11604. doi: 10.1021/bi049097i. [DOI] [PubMed] [Google Scholar]
- 16.Engelward BP, Weeda G, Wyatt MD, Broekhof JL, de Wit J, Donker I, Allan JM, Gold B, Hoeijmakers JH, Samson LD. Base excision repair deficient mice lacking the Aag alkyladenine DNA glycosylase. Proc Natl Acad Sci U S A. 1997;94:13087–13092. doi: 10.1073/pnas.94.24.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandaru V, Sunkara S, Wallace SS, Bond JP. A novel human DNA glycosylase that removes oxidative DNA damage and is homologous to Escherichia coli endonuclease VIII. DNA Repair (Amst) 2002;1:517–529. doi: 10.1016/s1568-7864(02)00036-8. [DOI] [PubMed] [Google Scholar]
- 18.Hazra TK, Izumi T, Boldogh I, Imhoff B, Kow YW, Jaruga P, Dizdaroglu M, Mitra S. Identification and characterization of a human DNA glycosylase for repair of modified bases in oxidatively damaged DNA. Proc Natl Acad Sci U S A. 2002;99:3523–3528. doi: 10.1073/pnas.062053799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haushalter KA, Todd Stukenberg MW, Kirschner MW, Verdine GL. Identification of a new uracil-DNA glycosylase family by expression cloning using synthetic inhibitors. Curr Biol. 1999;9:174–185. doi: 10.1016/s0960-9822(99)80087-6. [DOI] [PubMed] [Google Scholar]
- 20.Nakano T, Asagoshi K, Terato H, Suzuki T, Ide H. Assessment of the genotoxic potential of nitric oxide-induced guanine lesions by in vitro reactions with Escherichia coli DNA polymerase I. Mutagenesis. 2005;20:209–216. doi: 10.1093/mutage/gei027. [DOI] [PubMed] [Google Scholar]
- 21.Hitchcock TM, Gao H, Cao W. Cleavage of deoxyoxanosine-containing oligodeoxyribonucleotides by bacterial endonuclease V. Nucleic Acids Res. 2004;32:4071–4080. doi: 10.1093/nar/gkh747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terato H, Masaoka A, Asagoshi K, Honsho A, Ohyama Y, Suzuki T, Yamada M, Makino K, Yamamoto K, Ide H. Novel repair activities of AlkA (3-methyladenine DNA glycosylase II) and endonuclease VIII for xanthine and oxanine, guanine lesions induced by nitric oxide and nitrous acid. Nucleic Acids Res. 2002;30:4975–4984. doi: 10.1093/nar/gkf630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano T, Katafuchi A, Shimizu R, Terato H, Suzuki T, Tauchi H, Makino K, Skorvaga M, Van Houten B, Ide H. Repair activity of base and nucleotide excision repair enzymes for guanine lesions induced by nitrosative stress. Nucleic Acids Res. 2005;33:2181–2191. doi: 10.1093/nar/gki513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazra TK, Izumi T, Kow YW, Mitra S. The discovery of a new family of mammalian enzymes for repair of oxidatively damaged DNA, and its physiological implications. Carcinogenesis. 2003;24:155–157. doi: 10.1093/carcin/24.2.155. [DOI] [PubMed] [Google Scholar]
- 25.Nilsen H, Haushalter KA, Robins P, Barnes DE, Verdine GL, Lindahl T. Excision of deaminated cytosine from the vertebrate genome: role of the SMUG1 uracil-DNA glycosylase. Embo J. 2001;20:4278–4286. doi: 10.1093/emboj/20.15.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masaoka A, Matsubara M, Hasegawa R, Tanaka T, Kurisu S, Terato H, Ohyama Y, Karino N, Matsuda A, Ide H. Mammalian 5-formyluracil-DNA glycosylase. 2. Role of SMUG1 uracil-DNA glycosylase in repair of 5-formyluracil and other oxidized and deaminated base lesions. Biochemistry. 2003;42:5003–5012. doi: 10.1021/bi0273213. [DOI] [PubMed] [Google Scholar]
- 27.Matsubara M, Masaoka A, Tanaka T, Miyano T, Kato N, Terato H, Ohyama Y, Iwai S, Ide H. Mammalian 5-formyluracil-DNA glycosylase. 1. Identification and characterization of a novel activity that releases 5-formyluracil from DNA. Biochemistry. 2003;42:4993–5002. doi: 10.1021/bi027322v. [DOI] [PubMed] [Google Scholar]
- 28.Boorstein RJ, Cummings A, Jr, Marenstein DR, Chan MK, Ma Y, Neubert TA, Brown SM, Teebor GW. Definitive identification of mammalian 5-hydroxymethyluracil DNA N-glycosylase activity as SMUG1. J Biol Chem. 2001;276:41991–41997. doi: 10.1074/jbc.M106953200. [DOI] [PubMed] [Google Scholar]
- 29.Wibley JE, Waters TR, Haushalter K, Verdine GL, Pearl LH. Structure and specificity of the vertebrate anti-mutator uracil-DNA glycosylase SMUG1. Mol Cell. 2003;11:1647–1659. doi: 10.1016/s1097-2765(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 30.Matsubara M, Tanaka T, Terato H, Ohmae E, Izumi S, Katayanagi K, Ide H. Mutational analysis of the damage-recognition and catalytic mechanism of human SMUG1 DNA glycosylase. Nucleic Acids Res. 2004;32:5291–5302. doi: 10.1093/nar/gkh859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sartori AA, Fitz-Gibbon S, Yang H, Miller JH, Jiricny J. A novel uracil-DNA glycosylase with broad substrate specificity and an unusual active site. EMBO J. 2002;21:3182–3191. doi: 10.1093/emboj/cdf309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wink DA, Kasprzak KS, Maragos CM, Elespuru RK, Misra M, Dunams TM, Cebula TA, Koch WH, Andrews AW, Allen JS, et al. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science. 1991;254:1001–1003. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- 33.Dedon PC, Tannenbaum SR. Reactive nitrogen species in the chemical biology of inflammation. Arch Biochem Biophys. 2004;423:12–22. doi: 10.1016/j.abb.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Feng H, Klutz AM, Cao W. Active site plasticity of endonuclease V from Salmonella typhimurium. Biochemistry. 2005;44:675–683. doi: 10.1021/bi048752j. [DOI] [PubMed] [Google Scholar]


