Abstract
Purpose
Adenovirus serotype 5 (Ad5) has been used for gene therapy with limited success due to insufficient infectivity in cells with low expression of the primary receptor, the coxsackie and adenovirus receptor (CAR). Evidence that adenovirus serotype receptors other than CAR may be of use was presented in previous studies that showed that the Ad3 receptor is expressed at high levels in ovarian cancer cells. We hypothesized that combined use of unique chimeric fibers in the context of novel mosaic adenovirus vectors would enhance infectivity via non-CAR pathways in ovarian cancer cells.
Experimental Design
We constructed and characterized Ad5 vectors that use Ad3 knob and reovirus fibers to generate a mosaic fiber virion. Serotype 3 Dearing reovirus uses a fiber-like σ1 protein to infect cells expressing sialic acid and junction adhesion molecule 1. We therefore constructed a mosaic fiber Ad5 vector, designated Ad5/3-σ1, encoding two fibers: a σ1 chimeric fiber and the chimeric Ad5/3 fiber composed of an Ad3 knob.
Results
Functionally, Ad5/3-σ1 used sialic acid, junction adhesion molecule 1, and Ad3 receptor for cell transduction and achieved maximum infectivity enhancement in ovarian cancer cells with low CAR expression. Furthermore, Ad5/3-σ1 achieved infectivity enhancement in primary tissue slices of human ovarian tumor.
Conclusions
We have developed a new type of Ad5 vector with the novel tropism, possessing fibers from Ad3 and reovirus, which exhibits enhanced infectivity via CAR-independent pathway (s). In addition, the flexible genetic platform of vector allows different combination of fiber variants that can be incorporated within the same particle.
Cancer gene therapy has been widely investigated in the last decade as one of the new approaches for ovarian cancer. Adenoviral vectors, particularly vectors based on human serotype 5 (Ad5), have shown a great applicability in preclinical evaluations (1). Despite exciting preclinical data, adenoviral gene therapy approaches have yet to display significant clinical benefit. In general, poor therapeutic results have been attributed in large part to insufficient transduction of tumor cells. Human tumor cells frequently express little to none of the primary adenovirus receptor coxsackie and adenovirus receptor (CAR; refs. 2, 3). This CAR deficiency renders many tumor cells resistant to adenovirus infection, undermining cancer gene therapy strategies that require efficient tumor cell transduction. To address this issue, strategies are being developed that aim at modifying the adenovirus vector tropism to achieve CAR-independent transduction.
Genetic capsid modification has rationally focused on the fiber knob domain, which is the primary determinant of adenovirus tropism, to achieve CAR-independent cell entry. We previously showed that a mosaic fiber Ad5 vector provided viral entry via two different pathways, resulting in an additive gain in infectivity in a variety of transformed cells, including ovarian cancer cells (4). We derived a mosaic fiber Ad5 vector that incorporates two distinct fibers: the Ad5 fiber and a reovirus chimeric fiber (4). The reovirus chimeric fiber includes the fiber-like σ1 protein, which is a receptor-binding molecule of serotype 3 Dearing reovirus. The σ1 protein has been reported to use the coreceptors junction adhesion molecule 1 (JAM1) and sialic acid for cellular binding (5, 6). These receptors are clearly distinct from the Ad5 receptor and together determine serotype 3 Dearing reovirus tropism. Of note, this mosaic fiber modification greatly enhanced vector infectivity in cancer cells compared with the wild-type Ad5 fiber. To further increase infectivity of the mosaic fiber Ad5 vector, and to gain more specific infectivity for ovarian cancer, we replaced the Ad5 wild-type fiber gene in a tandem fiber cassette with the gene for a chimeric Ad5/3 fiber. The Ad5/3 fiber contains the tail and shaft domains of Ad5 and the knob domain of serotype 3. It has been suggested that the Ad3 receptor is expressed at high levels in ovarian cancer cells. Ad5 vectors that express a chimeric fiber consisting of the adenovirus serotype 3 knob domain (Ad5/3) have been shown to significantly increase infectivity in ovarian cancer cells. Thus, we engaged in the construction and characterization of Ad5 vectors that were mosaics of Ad3 knob and reovirus fibers. The goal is to developed mosaic fiber adenovirus vector, which could bind to ovarian cancer cells using the Ad3 receptor JAM1 and sialic acid cell receptors, thus establishing a novel strategy to achieve infectivity enhancement based on a CAR-independent tropism.
A noteworthy aspect of our mosaic fiber strategy is the flexibility whereby different combinations of fiber variants can be incorporated within the same particle. On this basis, mosaic fiber virions can be proposed, which embody ever greater potential for enhancement of vector infectivity.
To evaluate the efficacy of the newly established adenovirus vector, we introduced a thin-slice tumor model technique that has been evaluated in our group. In this regard, Kirby et al. (7) has clarified that the thin-slice tumor model system represents a stringent method of ex vivo evaluation of novel adenoviral vectors. For this technique, the Krumdieck tissue slicer is a novel instrument, which was introduced in 1980s to cut precise tissue slices from an organ of interest (8). Tissue slices thus obtained, including those of human derivation, are capable of maintaining their original in vivo structure and composition. This study provides a means for rigorous preclinical analysis of adenovirus-based transduction of patient tumors. We explored the infectivity efficacy of our newly developed mosaic fiber adenovirus vector using this established technique for preclinical evaluation, thus providing a powerful tool to determine the therapeutic index for clinical translation.
Materials and Methods
Cell lines
The 293 cells were purchased from Microbix. Human ovarian cancer cell lines ES-2 and OV-3 were obtained from the American Type Culture Collection. Human ovarian adenocarcinoma cell lines OV-4 and Hey were a kind gift from Dr. Timothy J. Eberlein (Harvard Medical School, Boston, MA) and Dr. Judy Wolf (M. D. Anderson Cancer Center, Houston, TX), respectively. L929 cells were maintained as described previously (6). All other cell lines were cultured in medium recommended by suppliers (Mediatech and Irvine Scientific). Fetal bovine serum was purchased from Hyclone. All cells were grown at 37°C, in a humidified atmosphere of 5% CO2.
Generation of the σ1 chimeric fiber construct
A schematic of the σ1 chimeric fiber structure is shown in Fig. 1A. To design the σ1 chimeric fiber, the fiber tail domain of Ad5 was fused in its whole length to the entire σ1 coding region in frame with a COOH-terminally encoded 6-histidine (6-His) sequence, resulting in F5S1H as described previously (4).
Fig. 1.
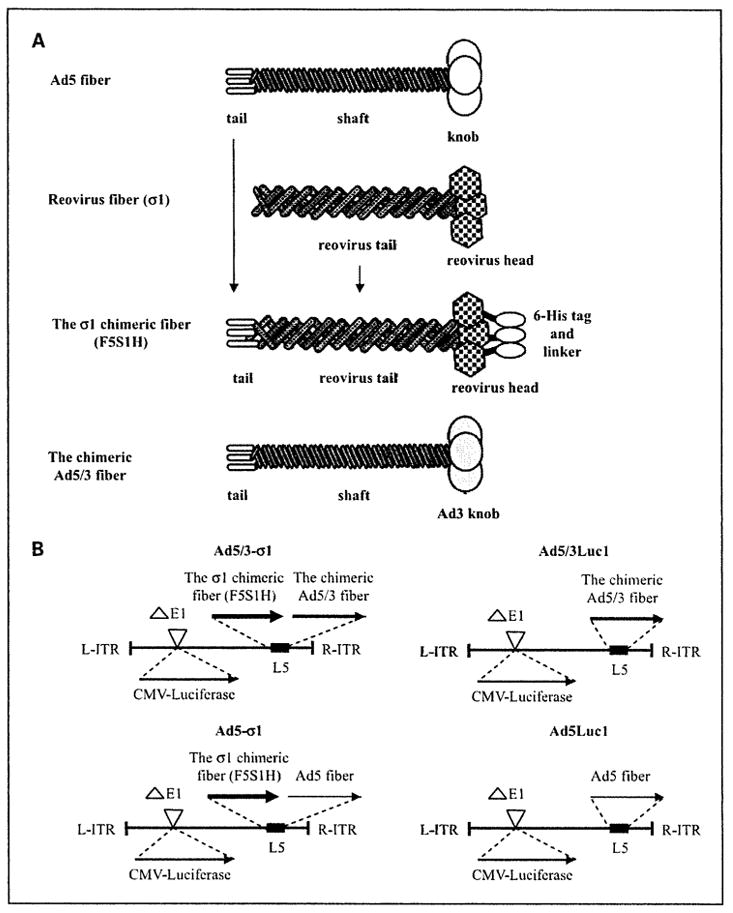
Schema of mosaic fiber Ad5 genomes. A, key components of the σ1 chimeric fiber. In the σ1 chimeric fiber, the tail of Ad5 fiber is fused to the reovirus fiber protein σ1 and a 6-His tag is fused to the COOH terminus of the σ1 chimeric fiber through a linker (designated F5S1H). B, map of Ad5 genomes with fiber modification. In all vectors, the E1 region is replaced by cytomegalovirus (CMV) promoter/luciferase transgene cassette. Ad5/3-σ1 is a mosaic fiber vector that carries the σ1 chimeric fiber with a COOH-terminal 6-His tag (F5S1H) as well as the chimeric Ad5/3 fiber. Ad5/3Luc1 is a control virus that carries the chimeric Ad5/3 fiber. Ad5-σ1 is a mosaic fiber vector that carries the σ1 chimeric fiber with a COOH-terminal 6-His tag (F5S1H) as well as the wild-typeAd5 fiber. Ad5Luc1 is a control virus that carries the wild-type Ad5 fiber.
Generation of shuttle plasmids for the mosaic fiber Ad5 genome
We previously created a shuttle vector, pNEB.PK.FSPF5S1/F5, which contained tandem fiber genes for the σ1 chimeric fiber F5S1H and the wild-type Ad5 fiber (4). The current shuttle vector was based on pNEB.PK.FSPF5S1/F5. We replaced the Ad5 knob sequence of the wild-type Ad5 fiber with the Ad3 knob coding sequence in frame, resulting in pNEBσ5/3. Technically, a fiber shuttle vector, pNEB.PK.F5/3 (9), containing an Ad5 tail, Ad5 shaft, and an Ad3 knob was used to extract the coding sequence of the Ad3 knob with NheI-MunI digestion. Another shuttle vector, pNEB.PK.FSPF5S1/F5, was digested with NheI and MunI for removal of the Ad5 knob sequence. A NheI/MunI fragment containing the coding sequence of the Ad3 knob from pNEB.PK.F5/3 was cloned into the NheI-MunI-digested pNEB.PK.FSPF5S1/F5, resulting in pNEBσ5/3, containing the σ1 chimeric fiber F5S1H and the chimeric Ad5/3 fiber genes in tandem.
Generation of recombinant adenovirus
A schematic of the viruses used in this study is shown in Fig. 1B. Recombinant Ad5 genomes containing the tandem fiber genes were derived by homologous recombination in Escherichia coli BJ5183 with SwaI-linearized rescue plasmid pVK700 and the PacI and KpnI fragment of pNEBσ5/3 containing tandem fibers essentially as described previously (10). The recombinant region of the genomic clones was sequenced before transfection into 293 cells. All vectors were propagated in 293 cells and purified using a standard protocol (11). The resultant mosaic fiber virus was Ad5/3-σ1. Viral particle (vp) concentration was determined by the A260 method as described by Maizel et al. (12). The infectious titer was determined according to the AdEasy Vector System (Qbiogene, Inc.).
PCR amplification of viral genome fragments
Viral DNA was amplified using the Taq PCR Core kit (Qiagen, Inc.). The sequences of the primers were as follows: Ad5tail-sense, 5′-ATGAAGCGCGCAA-GACCGTCTGAAGAT; Ad3knob-antisense, 5′-GTCATCTTCTCTAATA-TAGGAAAAGGTAAATGGGG; and σ1 head-antisense, 5′-ATTCTTGCGT-GAAACTACGCGG.
Protein electrophoresis and Western blotting
To detect the incorporation and the trimerization of fibers in vps, adenovirus vectors equal to 1.0 × 1010 to 1.0 × 1011 vp were resolved by SDS-PAGE and Western blotting as described previously (13).
Recombinant proteins
The fiber knob domains of Ad5 and Ad3 fibers were produced and purified as described previously (9). The protein concentrations in all experiments were determined by the Bradford method (Bio-Rad Laboratories).
Gene transfer assays
Cells were infected with viruses at 37°C for 1 h and unbound virus was washed away. A luciferase assay was done 24 h after infection (Promega) according to the manufacturer’s instructions. Tumor slices were cut, infected, and processed for luciferase assay after 24 h of incubation at 37°C as described previously (7, 14).
Competitive inhibition assay
Recombinant Ad3 fiber knob proteins or anti-JAM1 polyclonal antibody (c-15; Santa Cruz Biotechnology, Inc.) was incubated with the cells at 37°C for 15 min before infection. Alternatively, cells were treated with 333 milliunits/mL of Clostridium perfringens neuraminidase type X (Sigma-Aldrich Co.) at 37°C for 30 min to remove cell surface sialic acid followed by two washes with PBS. Cells were then exposed to viruses at 37°C for 1 h. Unbound virus and blocking agents were washed away. After 24 h of incubation at 37°C, the cells were processed for luciferase assay as described previously. Subsequent procedures were the same as described previously.
Precision-cut human ovarian tumor slices
Following institutional review board approval, primary human ovarian tumors were obtained from newly diagnosed ovarian cancer patients who underwent debulking surgery as primary treatment. Precision-cut ovarian tumor slices were prepared using a Krumdieck tissue slicer (Alabama Research and Development; ref. 7). The number of cells contained within each tissue slice was determined using the method of Kirby et al. using an estimation of 1 × 106 cells per slice based on a 10-cell thick slice (~ 250 μm) and 8-mm slice diameter (7).
Statistics
Data are presented as mean values ± SD. Student’s t test was used for pairwise comparison. The difference is deemed statistically significant if P < 0.05.
Results
Construction of mosaic fiber viruses
To create a chimeric fiber structurally compatible with Ad5 capsid incorporation, the σ1 chimeric fiber (F5S1H) was designed to comprise the NH2-terminal tail segment of the Ad5 fiber sequence genetically fused to the entire serotype 3 Dearing σ1 protein, and a COOH-terminal 6-His tag was included as a detection marker (Fig. 1A; ref. 4). Our previous mosaic fiber virus (Ad5-σ1) contained tandem fiber genes, wherein the F5S1H σ1 chimeric fiber was positioned in front of the Ad5 wild-type fiber gene. We replaced the Ad5 wild-type fiber gene of the tandem fiber cassette with the genome of the chimeric Ad5/3 fiber containing the tail and shaft domains of adenovirus serotype5 and the knob domain of serotype 3 (9). Our current tandem fiber cassette contained tandem fiber genes, wherein the F5S1H σ1 chimeric fiber was positioned in front of the chimeric Ad5/3 fiber gene in the L5 region of the Ad5 genome (Fig. 1B). In this configuration, each fiber was positioned before the untranslated sequences of the wild-type fiber to provide equal transcription, splicing, poly-adenylation, and regulation by the major late promoter. We constructed E1-deleted recombinant adenovirus genomes (Ad5/3-σ1) containing the σ1 chimeric fiber (F5S1H), the chimeric Ad5/3 fiber, and a firefly luciferase reporter gene controlled by the cytomegalovirus immediate early promoter/enhancer. Following virus rescue and large-scale propagation in 293 cells, we obtained Ad5/3-σ1 vector at concentrations of 6.48 ×1012 vp/mL. These concentrations compared favorably with that of Ad5Luc1 at 3.74 × 1012 vp/mL, Ad5/3Luc1 at 8.7 × 1011 vp/mL, andAd5-σ1 vector at 5.31 × 1012vp/mL. In addition, the vp per plaque-forming unit ratios determined for Ad5/3-σ1, Ad5Luc1, Ad5/3Luc1, and Ad5-σ1 was 13.8, 13.3, 2.76, and 22, respectively, indicating excellent virion integrity for both species. Of note, the control vectors used throughout this study, Ad5Luc1 and Ad5/3Luc1 each contained one fiber and are isogenic to Ad5/3-σ1 in all respects, except for the fiber locus.
Definition of fiber gene configurations for mosaic fiber adenovirus
We confirmed the fiber genotype of Ad5/3-σ1 via diagnostic PCR using the σ1 chimeric fiber or the chimeric Ad5/3 fiber primer pairs and genomes from purified virions as PCR templates (Fig. 2A). To confirm that Ad5/3-σ1 virions contained both trimerized fibers, we did SDS-PAGE followed by Western blot analysis on vps. The 4D2 anti-Ad5 fiber tail monoclonal antibody (NeoMarkers) was used and fiber bands were observed at ~180 kDa for unboiled samples of Ad5Luc1, Ad5/3Luc1, and Ad5/3-σ1 virions, corresponding to fiber trimers (Fig. 2B, lanes 1, 3, and 5), In boiled samples, the 4D2 antibody detected bands of apparent molecular mass of ~ 60 kDa, indicative of fiber monomers (Fig. 2B, lanes 2, 4, and 6). The mosaic fiber viruses were difficult to resolve by Western blotting due to the near-identical sizes of the σ1 chimeric and the chimeric Ad5/3 fiber proteins.
Fig. 2.
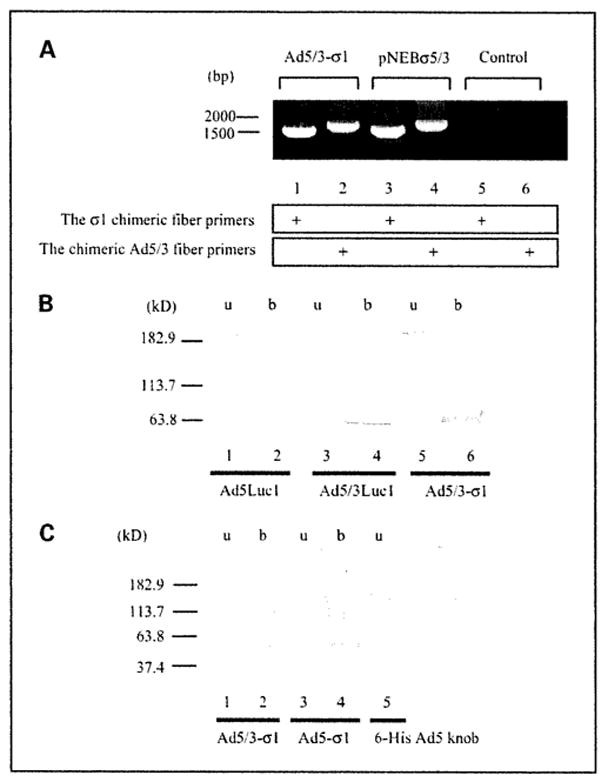
Analysis of fibers in rescued vps. A, detection of fiber genes in the adenovirus genome. Rescued vps were analyzed with PCR using pairs of the σ1 chimeric fiber primers or the chimeric Ad5/3 fiber primers. PNEBσ5/3 was used as a positive control for both fibers. Absence of a PCR template was designated as the “Control.” B and C, Western blot analysis of fiber proteins in purified virions. B, a total of 1.0 × 1010 vp per lane of Ad5Luc1 with the wild-type Ad5 fiber (lanes 1 and 2), Ad5/3Luc1 with the chimeric Ad5/3 fiber (lanes3 and 4), or Ad5/3-σ1 with dual fibers (lanes 5 and 6) was resuspended in Laemmli buffer before SDS-PAGE, electrotransferred, and detected with the 4D2 anti-Ad5 fiber tail antibody. The samples in lanes 2,4, and 6 were boiled (b), whereas lanes 1, 3, and 5 [unboiled (u)] contain proteins in their native trimeric configuration. C, a total of 1.0 × 1011 vp per lane of Ad5/3-σ1 (lanes 1 and 2) and Ad5-σ1 (lanes 3 and 4) with dual fibers was probed with an anti-6-His antibody. Lane 1, unboiled Ad5/3-σ1 virions; lane 2, boiled Ad5/3-σ1 virions; lane 3, unboiled Ad5-σ1 virions; lane 4, boiled Ad5-σ1 virions; lane 5, recombinant Ad5 knob with a 6-His tag as a positive antibody control. We consider the protein band appearing at~113 kDa to be the dimeric form of the σ1 chimeric fiber.
To confirm the presence of the σ1 chimeric fiber protein in virions, we used the anti-Penta His monoclonal antibody (Qiagen), which recognizes 6-His tags (Fig. 3C). Fiber bands corresponding to both trimeric and monomeric σ1 chimeric fiber protein were observed using the anti-Penta His antibody (Fig. 3C, lanes 1 and 2). These results confirm that the trimeric F5S1H σ1 chimeric fiber was incorporated into Ad5/3-σ1 virions.
Fig. 3.
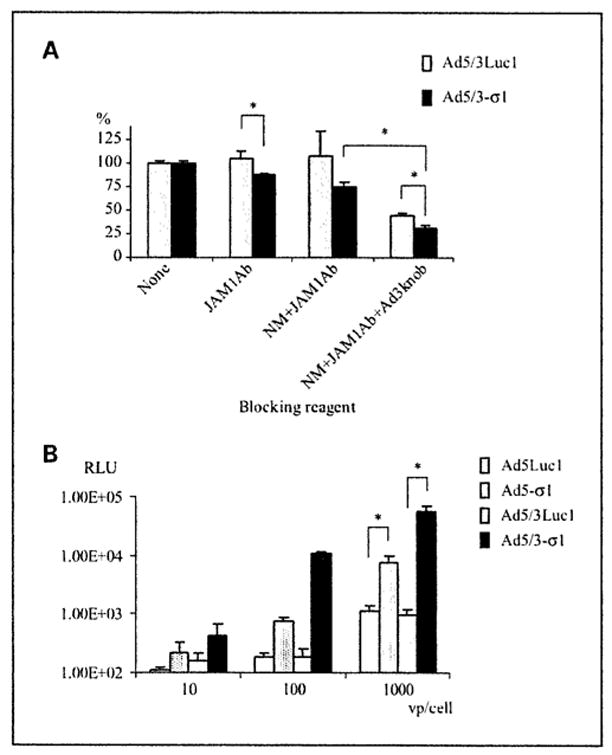
Evaluation of the efficacy and receptor specificity of Ad5/3-σ1 – mediated gene transfer. A, analysis of Ad5/3-σ1 receptor usage in Hey cells. C. perfringens neuraminidase (NM), an anti-JAM1 antibody (JAM1Ab). and recombinant Ad3 fiber knob protein (Ad3knob) were used to block Ad5/3-σ1 infection. Hey cells were either untreated or treated with 100 μg/mL anti-JAM1 antibody, both an anti-JAM1 and antibody 333 milliunits/mL neuraminidase, or combined reagents with neuraminidase, anti-JAM1 antibody, and 50 μg/mL recombinant Ad3 fiber knob protein. Cells were incubated with 100 vp/cell of Ad5/3Luc1 (gray column) or Ad5/3-σ1 (black column) and harvested 24 h later for luciferase activity. All luciferase values were normalized against the activity of controls receiving no blocking treatment valued at 100%. Similar results were obtained in three independent experiments. Columns, average of four replicates; bars, SD.***, P < 0.05, Student’s t test. B, mouse fibroblast cells (L929) were incubated with Ad5Luc1 (white column), Ad5-σ1 (clotted column), Ad5/3Luc1 (gray column), or Ad5/3-σ1 (black column) at 10,100, and 1,000 vp/cell. Luciferase activity was determined 24 h after infection and is expressed as relative light units (RLU). Columns, average of three replicates; bars, SD.***, P< 0.005, Student’s t test.
The Ad5/3-σ1 vector exhibits sialic acid–dependent, JAM1-dependent, and Ad3 receptor–dependent tropism
Our hypothesis was that inclusion of both the σ1 chimeric fiber (F5S1H) and the chimeric Ad5/3 fiber into an Ad5 vector would enhance infectivity of adenovirus-refractory cell types via expanding the vector tropism. To test the Ad5/3-σ1 tropism, we did neuraminidase treatment to remove cell surface sialic acid and competitive blocking experiments using an anti-JAM1 antibody or recombinant Ad3 knob protein. Ad5/3Luc1 was used as a positive control for the chimeric Ad5/3 fiber function. For this analysis, we used the low CAR-expressing human ovarian cancer cell line Hey due to its high sialic acid, JAM1, and Ad3 receptor expression (4). Transduction with Ad5/3-σ1 was inhibited by 12% using the anti-JAM1 antibody, which increased to 25% when combined with neuraminidase (Fig. 3A). Combined treatment with neuraminidase, anti-JAM1 antibody, and the Ad3 knob protein reduced transduction by 78% compared with controls receiving no blocking agent (Fig. 3A). Together, these data confirm that the Ad5/3-σ1 vector uses sialic acid, the JAM1-binding domain of the σ1 chimeric fiber (F5S1H), and the Ad3 receptor-binding domain of the chimeric Ad5/3 fiber for cell transduction. These findings indicate that Ad5/3-σ1 created sialic acid-dependent, JAM1-dependent, and Ad3 receptor-dependent tropism, confirming the functionality of the σ1 chimeric fiber (F5S1II) and the chimeric Ad5/3 fiber in our mosaic fiber Ad5.
Ad5/3-σ1 vector exhibits increased transduction of CAR-deficient cells
To determine the contribution of the σ1 chimeric fiber to the expanded adenovirus tropism, we evaluated Ad5/3-σ1 infectivity in L929 murine fibroblast cells that are commonly used for propagating reovirus. L929 cells express both the sialic acid and JAM1 σ1 receptors but no detectable CAR. As expected, Ad5/3-σ1 resulted in the maximum increase level of gene transfer (57-fold) relative to both Ad5Luc1 and Ad5/3Luc1 in L929 cells (Fig. 3B).
Ad5/3-σ1 vector exhibits increased transduction of low CAR ovarian cancer cells
In our previous study, we confirmed that the Hey, OV-4, ES-2, and OV-3 ovarian cancer cell lines were sialic acid/JAM1 positive but low CAR (4). Ad5/3-σ1 results in a 33-fold (OV-3) to 62-fold (Hey) increase in luciferase activity compared with Ad5Luc1. Luciferase activities were also enhanced in OV-4 (1.74-fold) and OV-3 (3.43-fold) cells using Ad5/3-σ1 compared with Ad5/3Luc1 (Fig. 4A). Many clinically relevant tissues are refractory to adenovirus infection, including ovarian cancer cells, due to negligible CAR levels (15). To more closely model the clinical situation with the most stringent substrate, we analyzed Ad5/3-σ1 transduction of primary human ovarian carcinoma cells. Importantly, Ad5/3-σ1 increased gene transfer to precision-cut ovarian cancer tissue slices from 1.7- to 59-fold versus Ad5Luc1 (Fig. 4B). Herein, we have outlined the construction, rescue, purification, and initial tropism characterization of a novel vector containing a nonadenovirus fiber molecule. Our results show that, in low CAR cells, Ad5/3-σ1 provides novel tropism and results in increased gene transfer rates compared with wild-type Ad5. This is accomplished using the reovirus coreceptors JAM1 and sialic acid in combination with the Ad3 receptor. The novel tropism of this vector represents a crucial attribute for adenovirus-based gene therapy vectors.
Fig. 4.
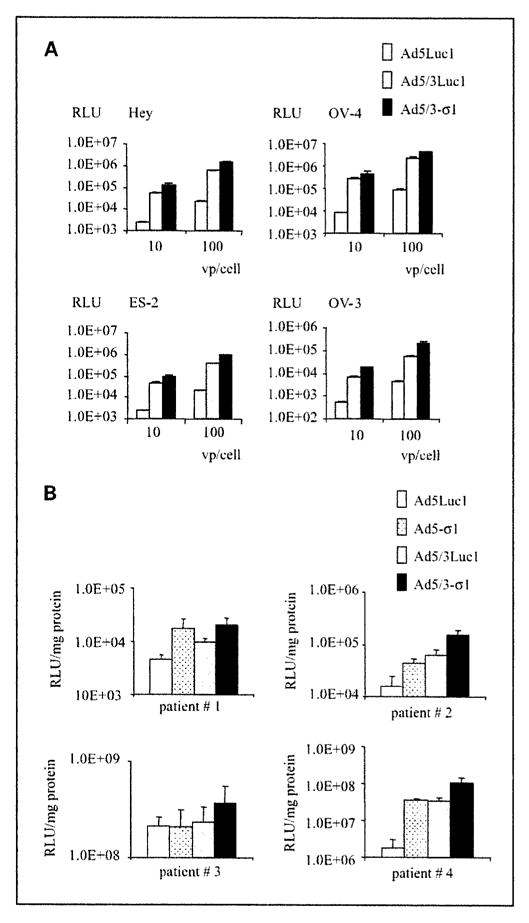
Infectivity profiles of Ad5/3-σ1. A, representative ovarian cancer cell lines Hey, OV-4, ES-2, and OV-3 were infected with Ad5/3Luc1 (gray column) and Ad5/3-σ1 (black column) at 10 and 100 vp/cell. Luciferase activity was measured 24 h after infection and is expressed as relative light units. Columns, mean of three experiments; bars, SD. B, human tumor slices of ovarian cancer patient were infected with Ad5Luc1 (white column), Ad5-σ1 (dotted column), Ad5/3Luc1 (gray column), orAd5/3-σ1 (black column) at 100 vp/cell. Luciferase activity was measured 24 h after infection and is expressed as relative light units/mg protein. Columns, mean of four experiments; bars, SD.
Discussion
One strategy to enhance the therapeutic potential of Ad5-based vectors has been to alter the native tropism toward non-CAR receptors that are abundant on the surface of primary tumor cells. Variable and/or low expression of CAR has been documented in many cancer cell types, including glioma, rhabdomyosarcoma, and ovarian cancer (16–18). We have reported that a mosaic fiber Ad5 vector provided viral entry via two different pathways with additive gains in infectivity in a variety of cell types (4). In this study, we evaluated the use of a mosaic fiber Ad5 vector that uses both the Ad3 and serotype 3 Dearing reovirus receptors, which are independent of native Ad5 tropism. This vector was based on our previously reported mosaic fiber Ad5 vector that incorporates two distinct fibers: the Ad5 fiber and a reovirus chimeric fiber. The current mosaic fiber Ad5 vector was generated by substituting the Ad3 knob in place of the Ad5 knob domain. We constructed an Ad5 genome using a tandem fiber cassette, which resulted in an Ad5 vector that expressed both the Ad5/3 and σ1 chimeric fibers. We confirmed that these virions incorporated both fibers by Western blot analysis and by the functional ability of both fibers to use the appropriate receptor(s) for viral transduction. This is the first vector that contains non–CAR-binding fibers from two different virus families to replace Ad5 tropism.
We further confirmed that the addition of the σ1 chimeric fiber contributed to augmentation of gene transfer compared with adenovirus vectors with single fiber in cells lacking the Ad5 and Ad3 receptors. Ad5/3-σ1 provided a 57-fold increase in gene transfer relative to the wild-type fiber control virus, the Ad5Luc1, and the single Ad5/3 fiber control virus, Ad5/3Luc1, in L929 cells that lack Ad5 and Ad3 receptor expression.
Consistent with our hypothesis of enhanced infectivity, we observed augmented gene transfer with Ad5/3-σ1 in all ovarian cancer cell lines tested, ranging from 33-fold to 62-fold, when compared with the wild-type fiber control virus. However, compared with Ad5/3Luc1, augmented gene transfer on the same cell lines was modest, ranging from 1.74-fold to 3.43-fold.
Our thin-slice tissue culture is an ideal method for preclinical ex vivo infectivity analysis because it allows evaluation of adenovirus in primary tumors derived from cancer patients (7). Importantly, the enhanced infectivity of the Ad5/3-σ1 virus was observed on more stringent clinical substrates, human primary ovarian tumor tissue slices, although the augmentation of gene transfer was variable, ranging from 1.7-fold to 59-fold. This variability in augmentation was likely due to differential receptor expression between tumors from different patients. Clearly, it would be of interest to directly correlate receptor expression with the degree of infectivity in clinical specimens. Unfortunately, this was not possible with the samples studied here because the whole tumor specimen was necessary for analysis of infectivity enhancement. However, we have shown a positive correlation between primary receptor density, infectivity, and oncolysis in our previous studies (16, 19–23).
There are two possible explanations for the modest level of Ad5/3-σ1 gene transfer compared with the single Ad5/3 fiber control virus Ad5/3Luc1. At first, it may be due to reduced levels of the σ1 chimeric fiber incorporation compared with the chimeric Ad5/3 fiber in mosaic fiber Ad5 particles. We previously confirmed that both 4D2 anti-Ad5 tail antibody and 6-His antibody detect fibers with similar band intensity in Western blot when equal numbers of vps are loaded. Figure 2B and C shows the presence of both fibers in Ad5/3-σ1 virions. However, to detect the σ1 chimeric fiber in our mosaic fiber Ad5 vector with the 6-His antibody, we had to load 10 times the number of vps compared with the chimeric Ad5/3 fiber as detected by using 4D2 anti-Ad5 tail antibody. This result suggests that incorporation of the σ1 chimeric fiber is decreased compared with the chimeric Ad5/3 fiber on Ad5/3-σ1. This could explain the limited additivity of transductional efficiency by the σ1 chimeric fiber in ovarian cancer cells even when JAM 1 or sialic acid is expressed. We have previously investigated a series of mosaic fiber adenoviruses that possess two genetically distinct fibers from different origins (13). Although the ratio of virion-incorporated fibers varies, both fiber properties have been functionally confirmed in each mosaic fiber adenovirus. A second explanation is that the Ad3 receptor is widely expressed on ovarian cancer cell surfaces of epithelial origin, and the chimeric Ad5/3 fiber in the Ad5/3-σ1 vector was used preferentially, minimizing the role of the σ1 chimeric fiber in these cells (16).
In a clinical setting of ovarian cancer, adenovirus-based vectors might be administered i.p. It would therefore be critical that vector used in this manner has decreased tropism for normal mesothelial cells or liver. We have not yet evaluated the Ad5/3-σ1 vector in an in vivo model of peritoneal administration. In this regard, however, our group has previously investigated the infectious properties in vivo of an adenovirus expressing a single Ad5/3 fiber (21). We showed that Ad5/3Luc1 had higher transgene expression but fewer virus copies in the liver and less transgene expression in peritoneum in comparison with Ad5Luc1 via i.p. administration into tumor-bearing mice. Additionally, histologic studies revealed that serotype 3 Dearing reovirus infection in vivo was restricted to tumor cells, whereas the surrounding normal tissue remained uninfected (24). Considering these facts, we fully expect that the Ad5/3-σ1 vector will not have increased tissue tropism for liver or other normal tissues.
Thus, we have used the “fiber mosaicism” concept, the use of two separate fibers with distinct receptor recognition, to combine the use of multiple receptors to enhance viral infectivity (4). In this study, the mosaic fiber Ad5/3-σ1 vector provided enhanced infectivity in low CAR-expressing ovarian cancer cell lines. This was the result of multiple non-CAR receptor-binding properties provided by the fiber elements of different virus families. Adenovirus gene therapy vectors with CAR-independent tropism may prove valuable for maximal transduction of low CAR-expressing tumors using minimal vector closes. Furthermore, this study used a preclinical assay that involved primary human ovarian tumor tissue to evaluate the mosaic fiber adenovirus vectors and proved further evidence of a preclinical screening strategy for examining improved gene therapy agents.
Acknowledgments
We thank Dr. Victor Krasnykh for providing plasmids pVK700, pNEB.PK.F5/3, and pNEB.PK.3.6 and Dr. Justin C. Roth for his critical reading of the manuscript.
Footnotes
Grant support: NIH grants CA35675, CA97318, CA104177, and CA83821; Department of Defense grant W81XWH-05-1-0035; and Waxman Foundation for Cancer Research.
References
- 1.Gomez-Navarro J, Curiel DT, Douglas JT. Gene therapy for cancer. Eur J Cancer. 1999;35:867–85. doi: 10.1016/s0959-8049(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 2.Krasnykh V, Dmitriev I, Navarro JG, et al. Advanced generation adenoviral vectors possess augmented gene transfer efficiency based upon coxsackie adenovirus receptor-independent cellular entry capacity. Cancer Res. 2000;60:6784–7. [PubMed] [Google Scholar]
- 3.Glasgow JN, Bauerschmitz GJ, Curiel DT, Hemminski A. Transductional and transcriptional targeting of adenovirus for clinical applications. Curr GeneTher. 2004;4:1–14. doi: 10.2174/1566523044577997. [DOI] [PubMed] [Google Scholar]
- 4.Tsuruta Y, Pereboeva L, Glasgow JN, et al. Reovirus σ1 fiber incorporated into adenovirus serotype 5 enhances infectivity via a CAR-independent pathway. Biochem Biophys Res Commun. 2005;335:205–14. doi: 10.1016/j.bbrc.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 5.Barton ES, Forrest JC, Connolly JL, et al. Junction adhesion molecule is a receptor for reovirus. Cell. 2001;104:441–51. doi: 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- 6.Chappell JD, Gunn VL, Wetzel JD, Baer GS, Dermody TS. Mutations in type 3 reovirus that determine binding to sialic acid are contained in the fibrous tail domain of viral attachment protein σ1. J Virol. 1997;71:1834–41. doi: 10.1128/jvi.71.3.1834-1841.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirby TO, Rivera A, Rein D, et al. A novel ex vivo model system for evaluation of conditionally replicative adenoviruses therapeutic efficacy and toxicity. Clin Cancer Res. 2004;10:8697–703. doi: 10.1158/1078-0432.CCR-04-1166. [DOI] [PubMed] [Google Scholar]
- 8.Krumdieck CL, dos Santos JE, Ho KJ. A new instrument for the rapid preparation of tissue slices . Anal Biochem. 1980;104:118–23. doi: 10.1016/0003-2697(80)90284-5. [DOI] [PubMed] [Google Scholar]
- 9.Krasnykh VN, Mikheeva GV, Douglas JT, Curiel DT. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70:6839–46. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belousova N, Krendelchtchikova V, Curiel DT, Krasnykh V. Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein. J Virol. 2002;76:8621–31. doi: 10.1128/JVI.76.17.8621-8631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham F, Prevec L. Manipulation of adenovirus vectors. In: Murray EJ, Walker JM, editors. Methods in molecular biology. Clifton (NJ): Humana Press; 1991. pp. 109–28. [DOI] [PubMed] [Google Scholar]
- 12.Maizel JV, Jr, White DO, Scharff MD. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968;36:115–25. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- 13.Pereboeva L, Komarova S, Mahasreshti PJ, Curiel DT. Fiber-mosaic adenovirus as a novel approach to design genetically modified adenoviral vectors . Virus Res. 2004;105:35–46. doi: 10.1016/j.virusres.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Breidenbach M, Rein DT, Schondorf T, et al. A new targeting approach for breast cancer gene therapy using the heparanase promoter. Cancer Lett. 2006;240:114–22. doi: 10.1016/j.canlet.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Kelly FJ, Miller CR, Buchsbaum DJ, et al. Selectivity of TAG-72-targeted adenovirus gene transfer to primary ovarian carcinoma cells versus autologous mesothelial cells in vitro. Clin Cancer Res. 2000;6:4323–33. [PubMed] [Google Scholar]
- 16.Kanerva A, Mikheeva GV, Krasnykh V, et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res. 2002;8:275–80. [PubMed] [Google Scholar]
- 17.Kim M, Sumerel LA, Belousova N, et al. The coxsackievirus and adenovirus receptor acts as a tumour suppressor in malignant glioma cells. Br J Cancer. 2003;88:1411–6. doi: 10.1038/sj.bjc.6600932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cripe TP, Dunphy EJ, Holub AD, et al. Fiber knob modifications overcome low, heterogeneous expression of the coxsackievirus-adenovirus receptor that limits adenovirus gene transfer and oncolysis for human rhabdomyosarcoma cells. Cancer Res. 2001;61:2953–60. [PubMed] [Google Scholar]
- 19.Yamamoto M, Davydova J, Wang M, et al. Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology. 2003;125:1203–18. doi: 10.1016/s0016-5085(03)01196-x. [DOI] [PubMed] [Google Scholar]
- 20.Kanerva A, Zinn KR, Chaudhuri TR, et al. Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus. Mol Ther. 2003;8:449–58. doi: 10.1016/s1525-0016(03)00200-4. [DOI] [PubMed] [Google Scholar]
- 21.Kanerva A, Wang M, Bauerschmitz GJ, et al. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol Ther. 2002;5:695–704. doi: 10.1006/mthe.2002.0599. [DOI] [PubMed] [Google Scholar]
- 22.Douglas JT, Kim M, Sumerel LA, Carey DE, Curiel DT. Efficient oncolysis by a replicating adenovirus (ad) in vivo is critically dependent on tumor expression of primary ad receptors. Cancer Res. 2001;61:813–7. [PubMed] [Google Scholar]
- 23.Hemminki A, Dmitriev I, Liu B, Desmond RA, Alemany R, Curiel DT. Targeting oncolytic adenoviral agents to the epidermal growth factor pathway with a secretory fusion molecule. Cancer Res. 2001;61:6377–81. [PubMed] [Google Scholar]
- 24.Hirasawa K, Nishikawa SG, Norman KL, Alain T, Kossakowska A, Lee PW. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 2002;62:1696–701. [PubMed] [Google Scholar]


